Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation
Abstract
:1. Introduction
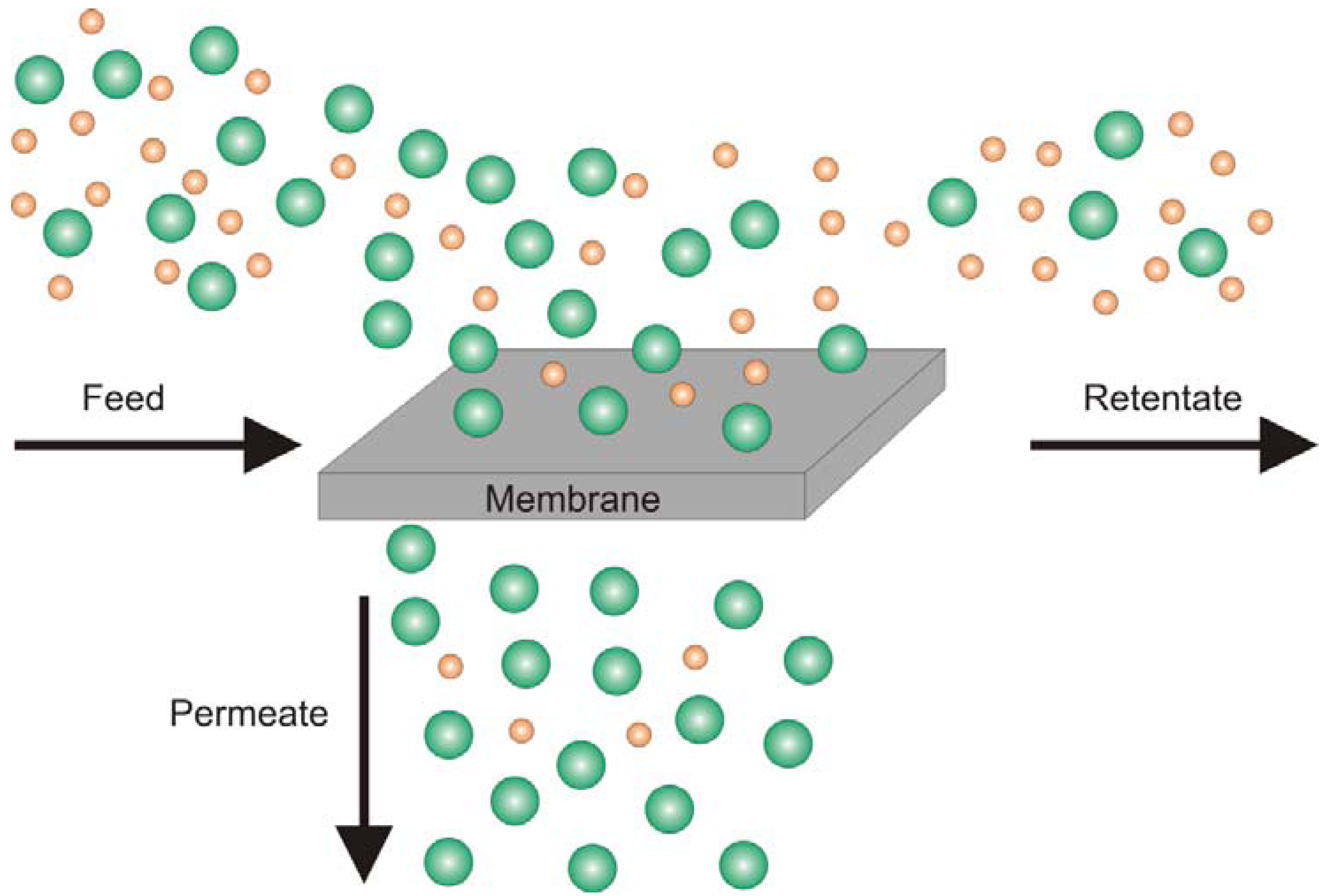

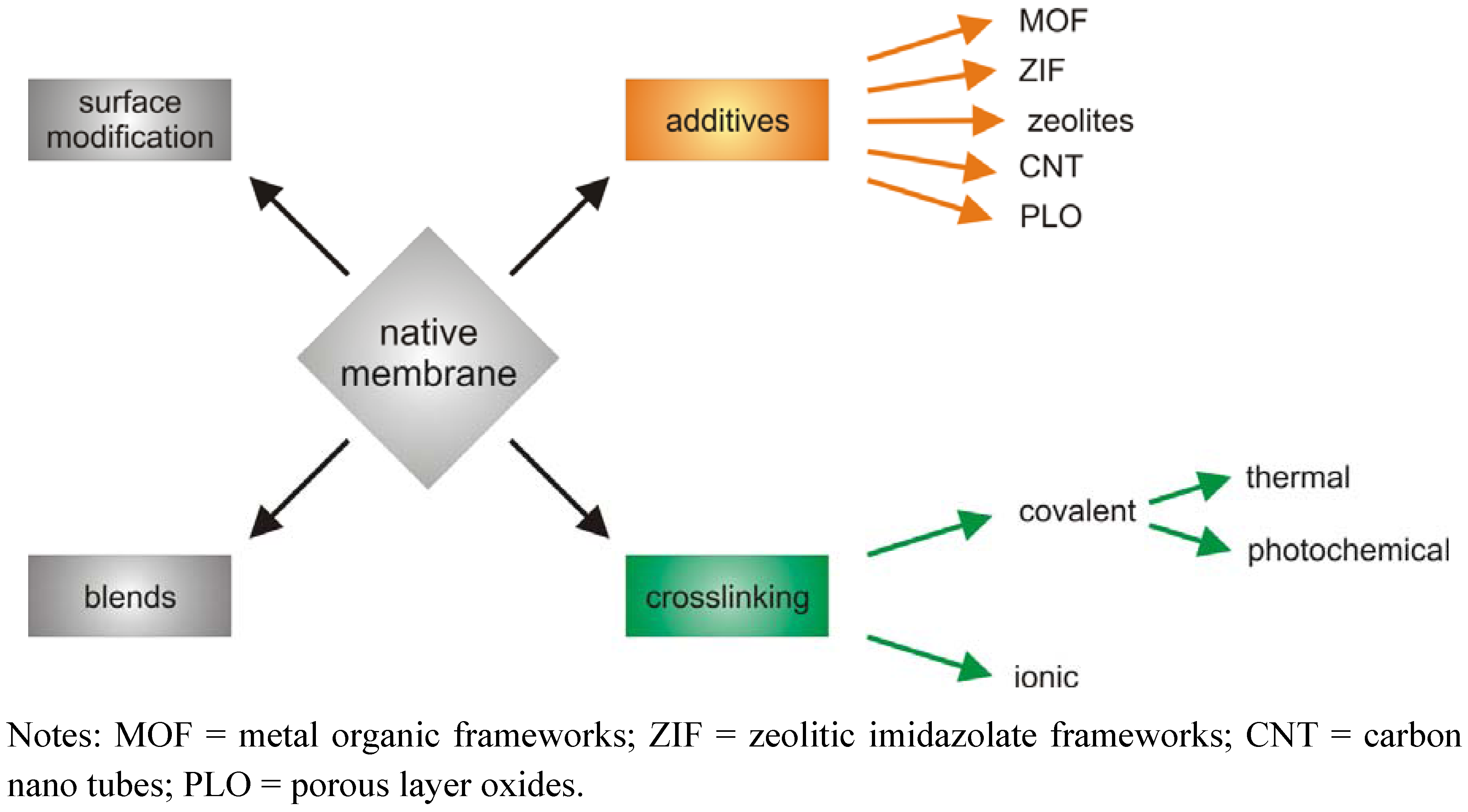
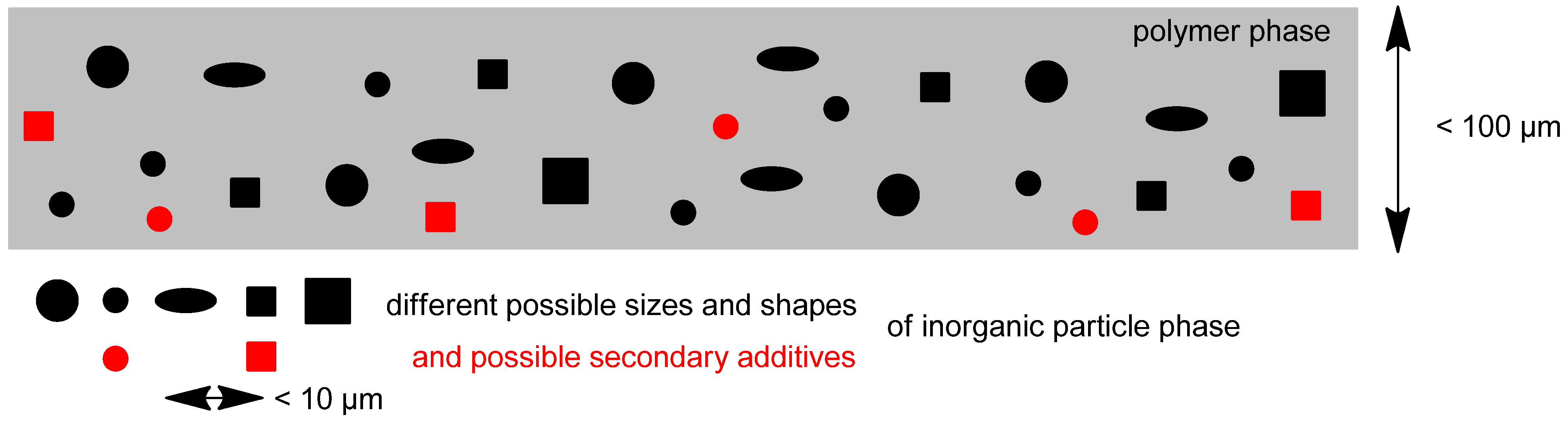
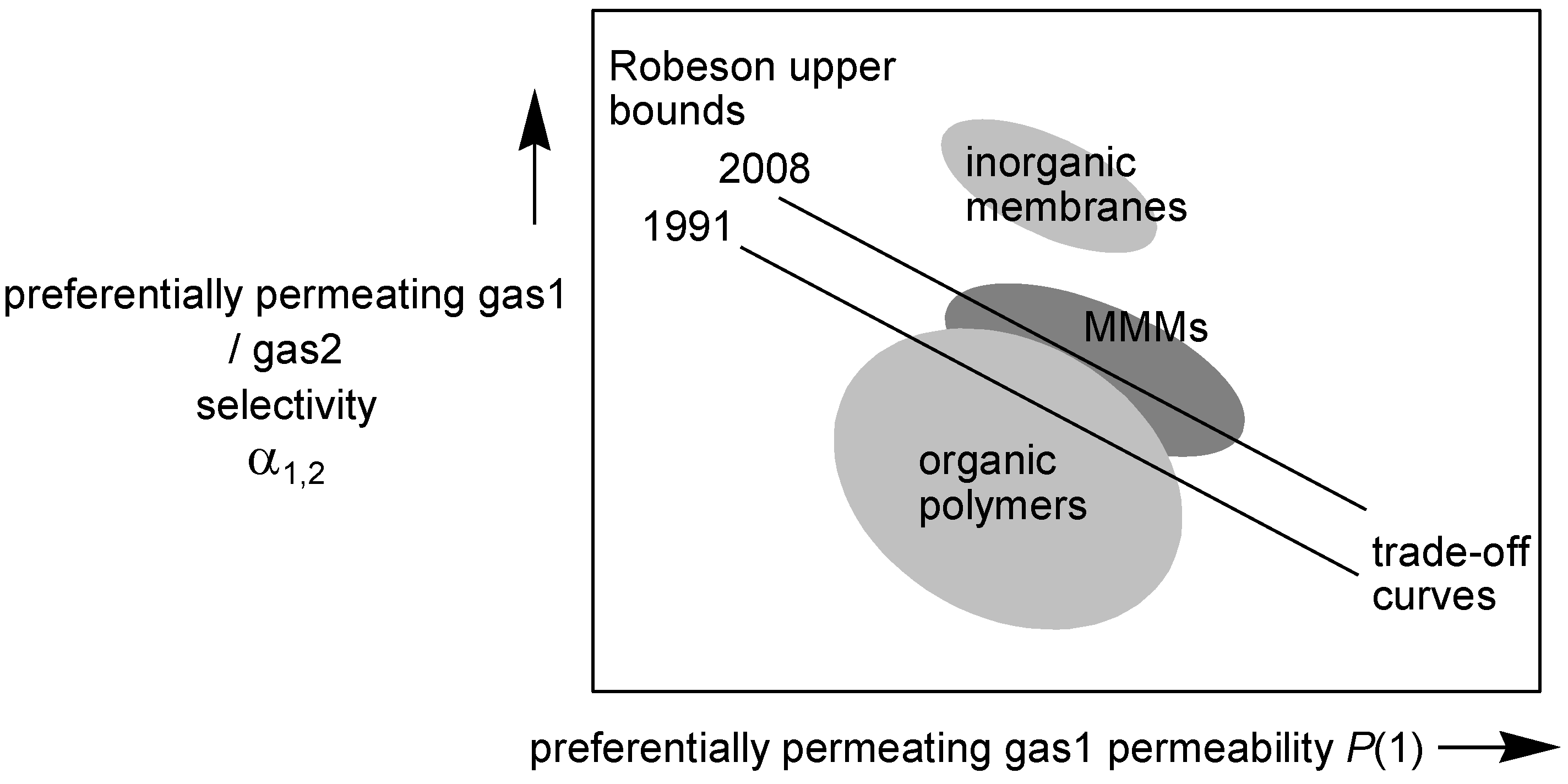
2. Crosslinking
2.1. Ionic Crosslinking
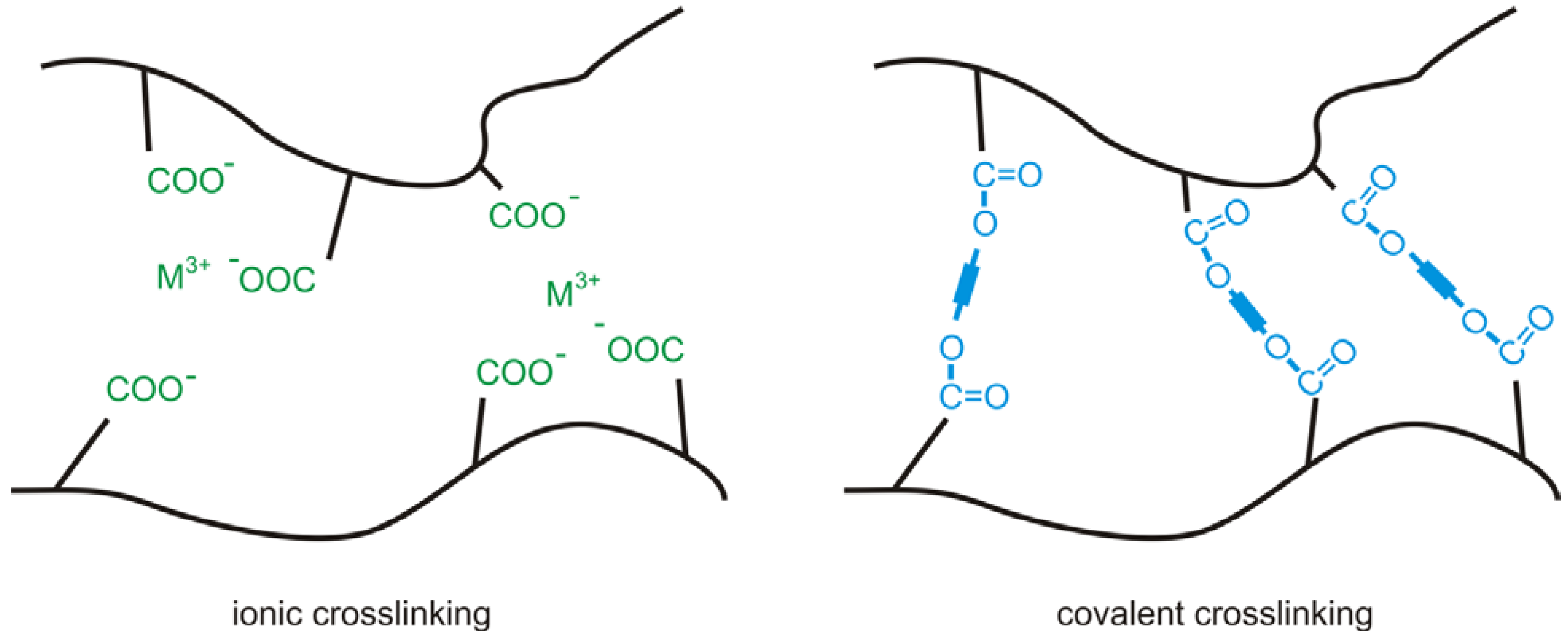
2.2. Thermal Crosslinking
2.3. Photocrosslinking
2.3.1. Mechanistic Studies of Photocrosslinking
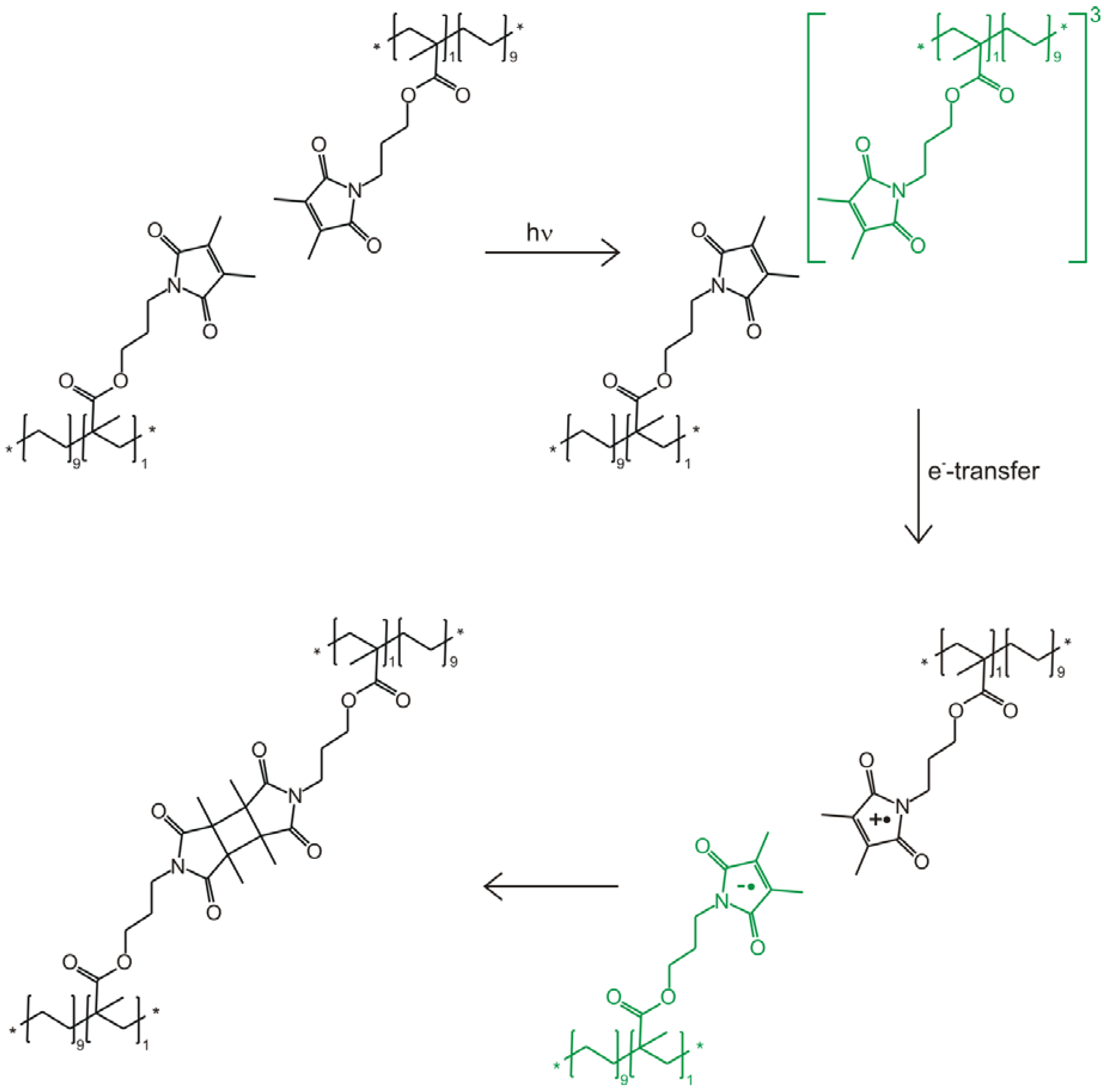
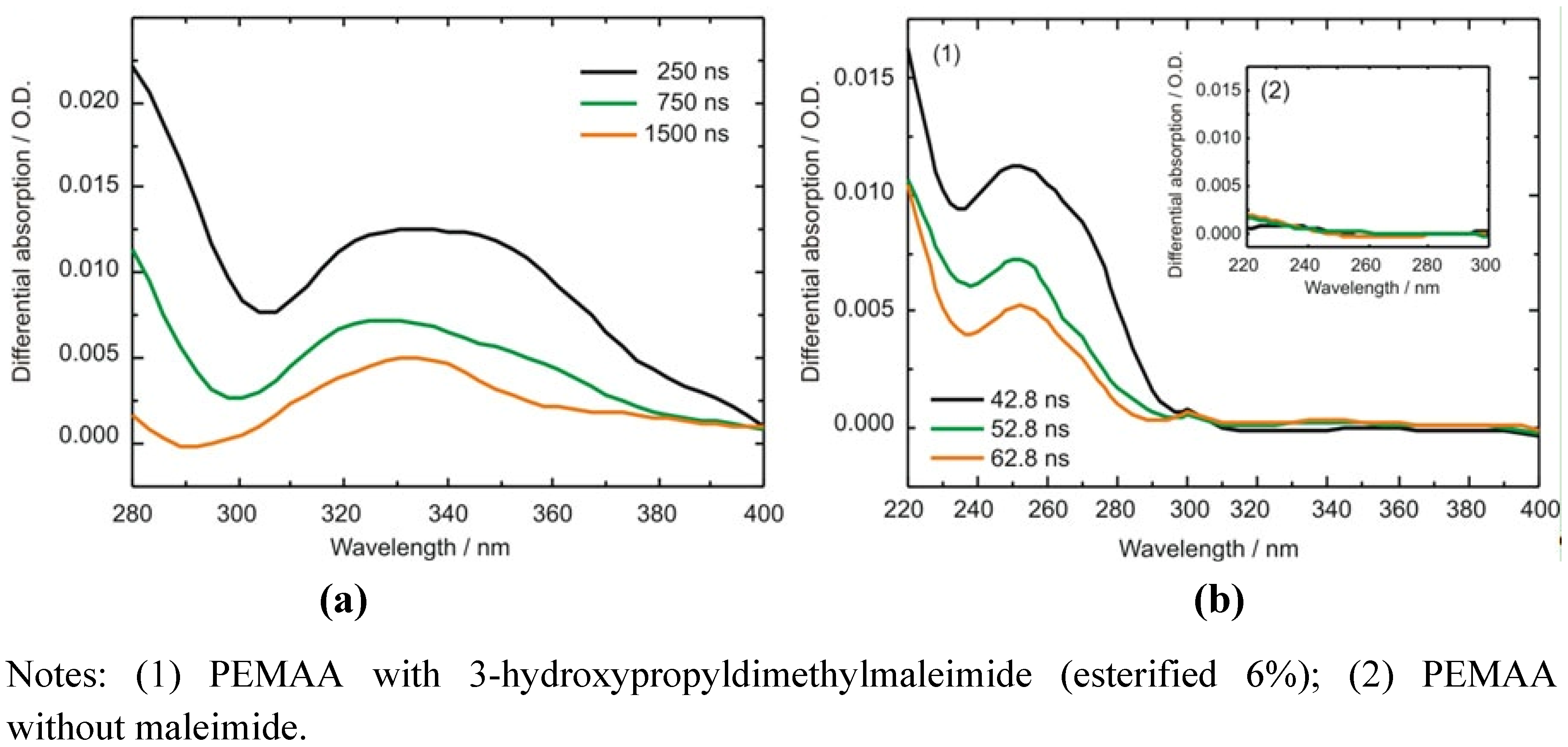
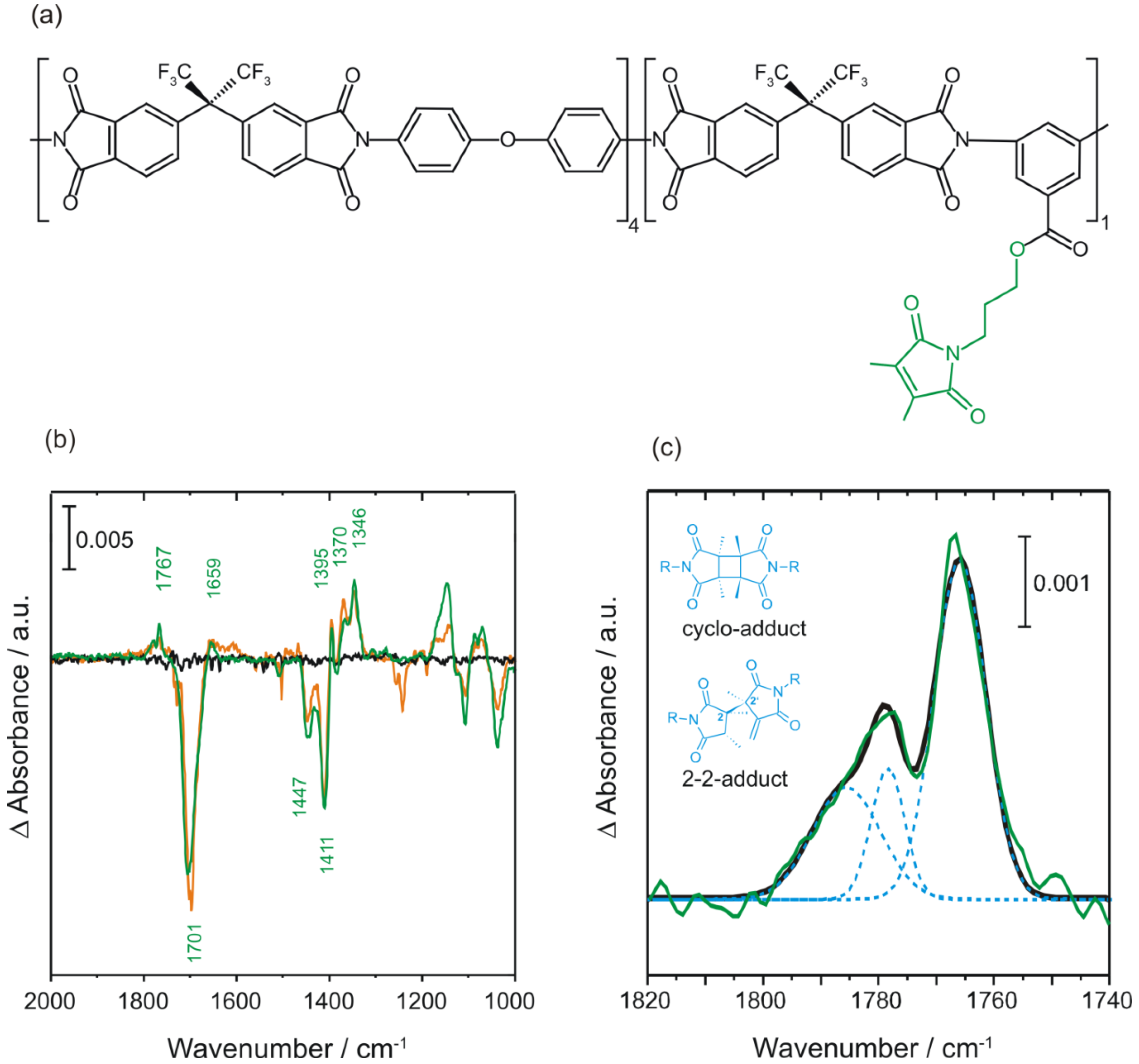
2.3.2. Photocrosslinking Yield
2.4. Pervaporation and Gas Separation studies of Crosslinked Membranes
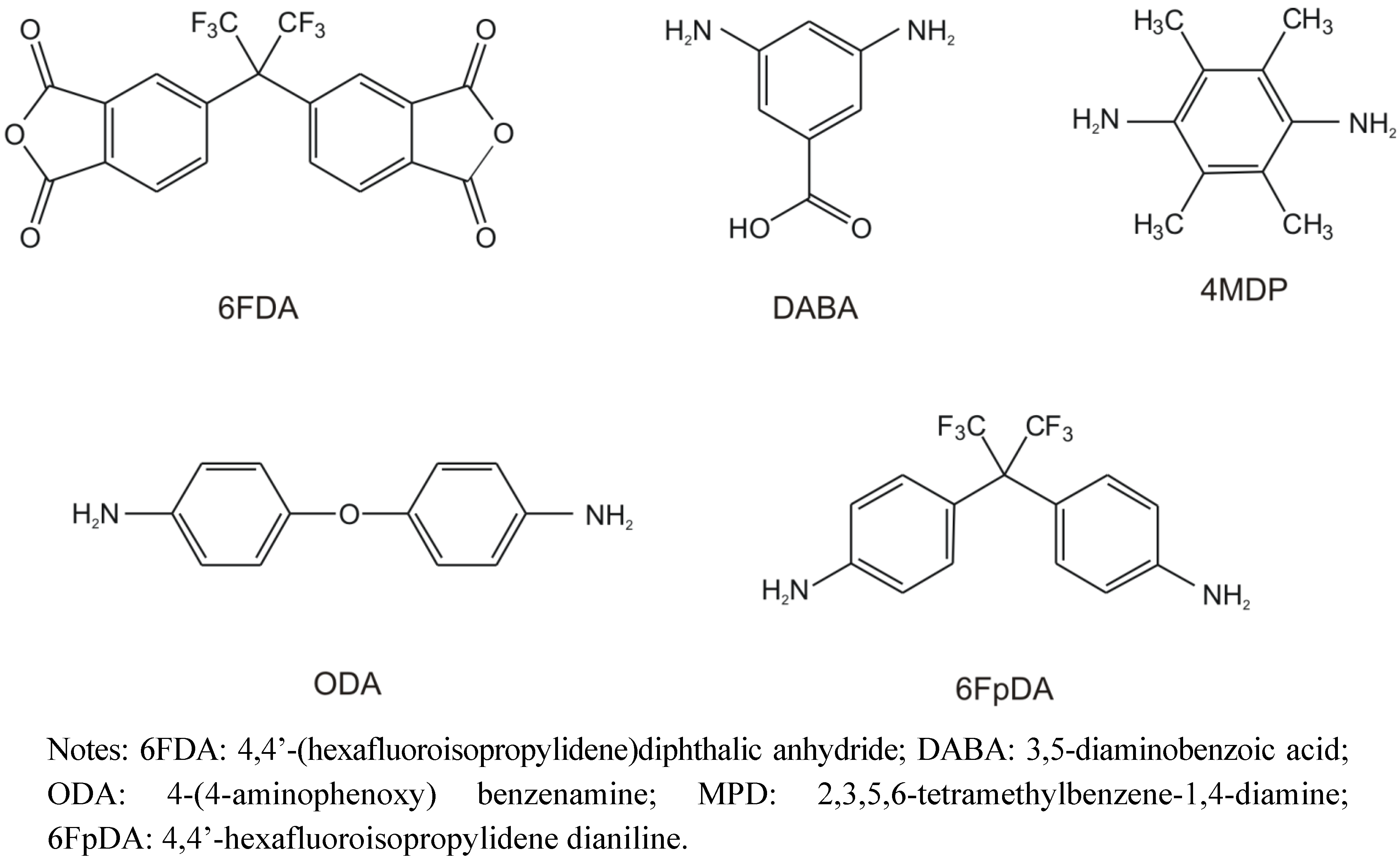
2.4.1. Aromatic/Aliphatic Separation
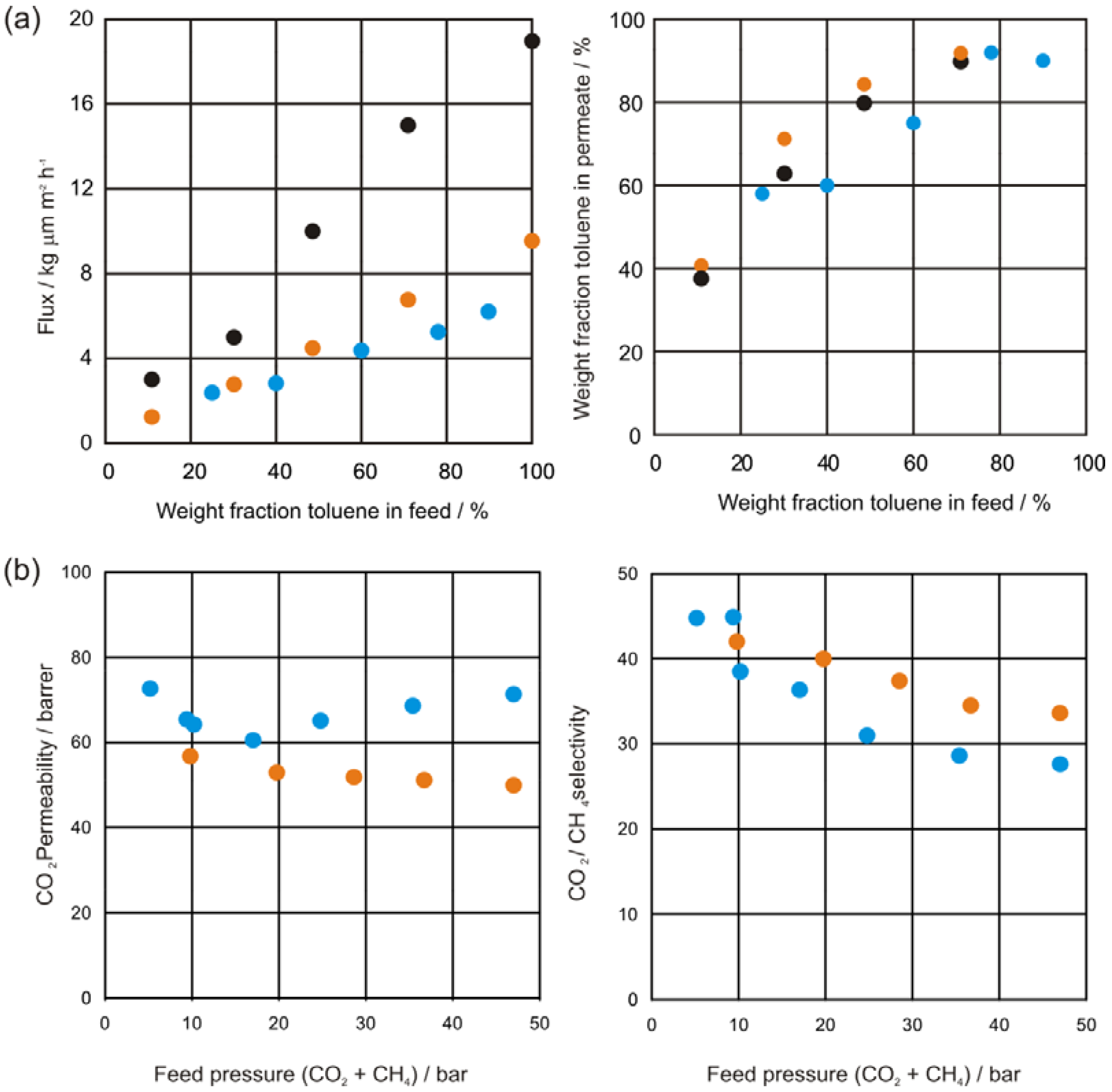
2.4.2. Natural Gas Treatment
3. Metal-organic Frameworks in Mixed Matrix Membranes
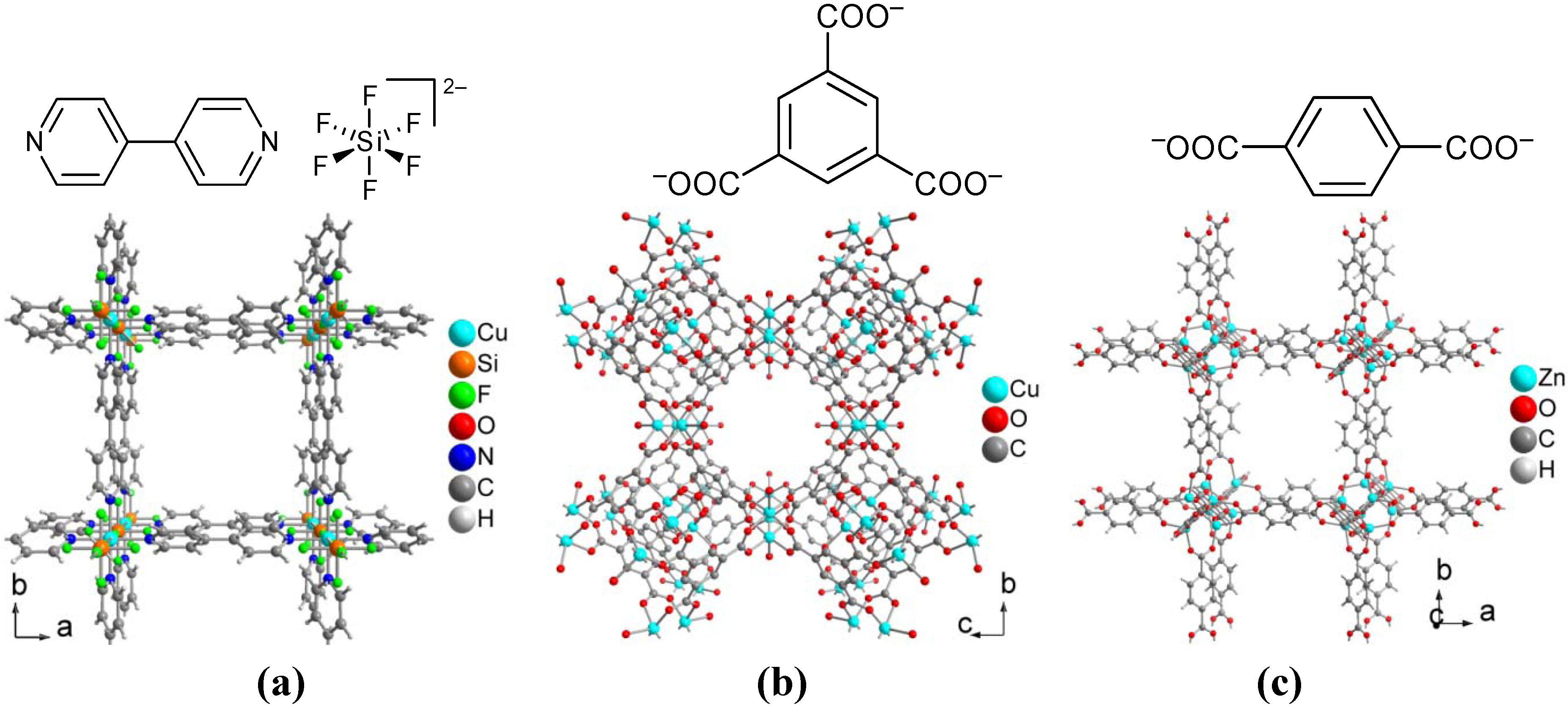
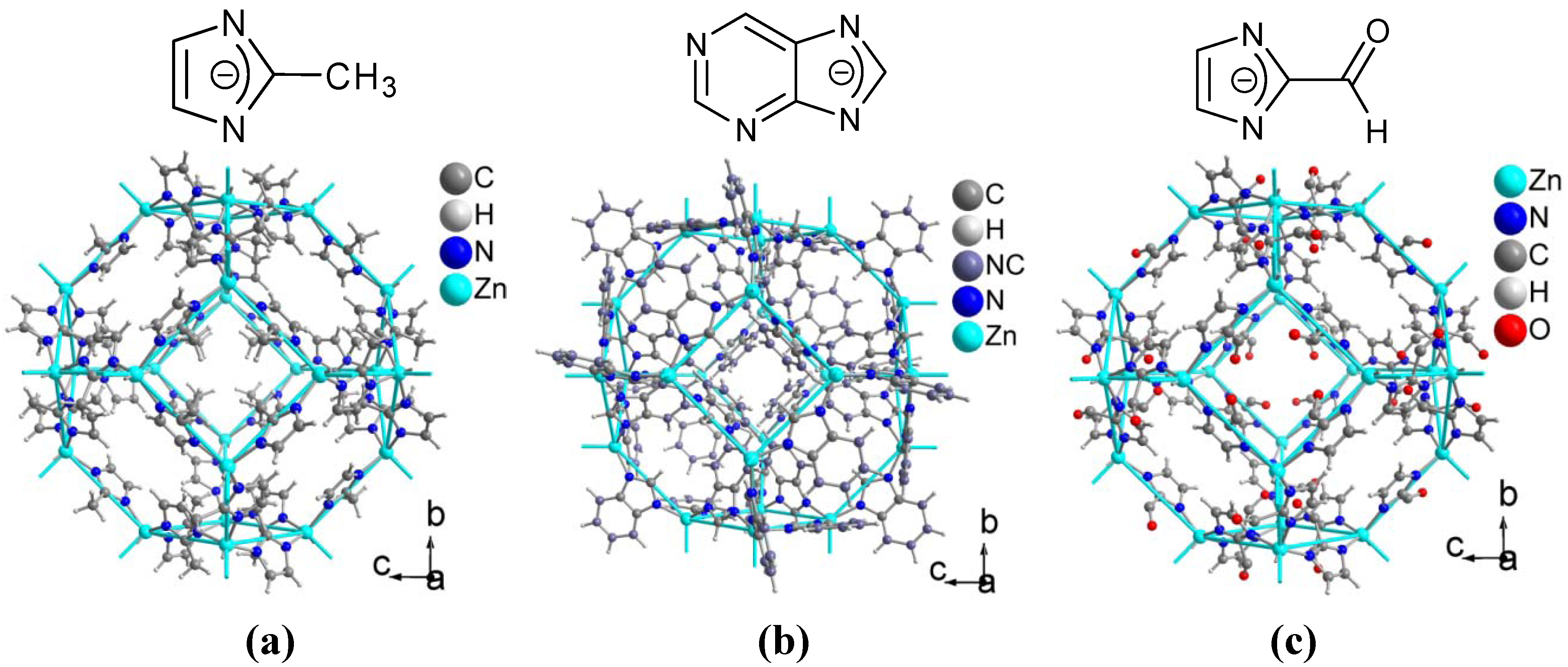

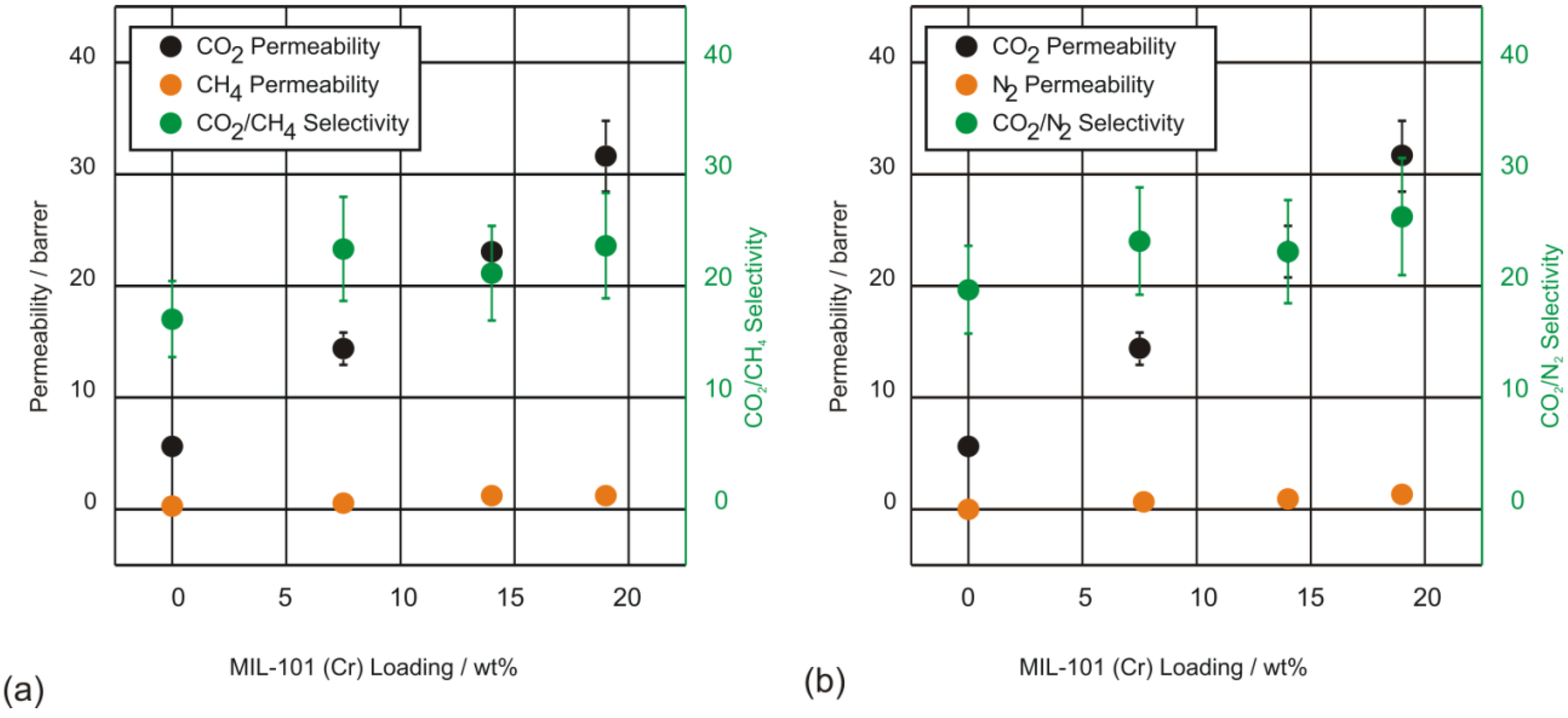
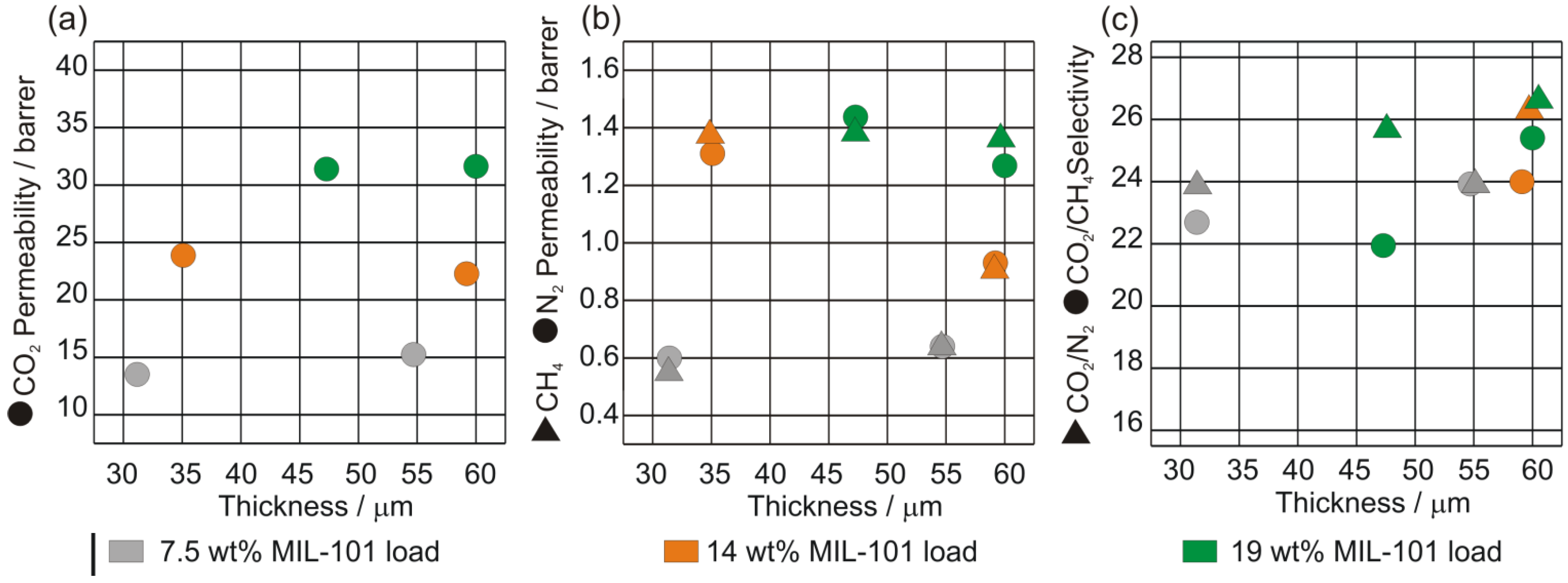
4. Conclusions
Acknowledgments
References
- King, C.J. Separation Processes, Introduction. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Davis, J.C.; Valus, R.J.; Eshraghi, R.; Velikoff, A.E. Facilitated transport membrane hybrid systems for olefin purification. Sep. Sci. Tec. 1993, 28, 463–476. [Google Scholar] [CrossRef]
- Kondo, M.; Komori, M.; Kita, H.; Okamoto, K. Tubular-type pervaporation module with zeolite NaA membrane. J. Membrane Sci. 1997, 133, 133–141. [Google Scholar] [CrossRef]
- Holmes, S.M.; Schmitt, M.; Markert, C.; Plaisted, R.J.; Forrest, J.O.; Sharratt, P.N.; Garforth, A.A.; Cundy, C.S.; Dwyer, J. Zeolite A membranes for use in alcohol/water separations—Part I: Experimental investigation. Chem. Eng. Res. Des. 2000, 78, 1084–1088. [Google Scholar] [CrossRef]
- Van den Berg, A.W.C.; Gora, L.; Jansen, J.C.; Makkee, M.; Maschmeyer, T. Zeolite A membranes synthesized on a UV-irradiated TiO2 coated metal support: The high pervaporation performance. J. Membrane Sci. 2003, 224, 29–37. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa. J. Chem. Soc. 1867, 20, 235–288. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membrane Sci. 1993, 83, 1–80. [Google Scholar]
- Schmeling, N.; Konietzny, R.; Sieffert, D.; Rölling, P.; Staudt, C. Functionalized copolyimide membranes for the separation of gaseous and liquid mixtures. Beilstein J. Org. Chem. 2010, 6, 789–800. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed; Wiley: Chichester, UK, 2004. [Google Scholar]
- Paul, D.R.; Yampol’skii, Y.P. Polymeric Gas Separation Membranes; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Mixa, A.; Staudt, C. Membrane-based separation of phenol/water mixtures using ionically and covalently cross-linked ethylene-methacrylic acid copolymers. Int. J. Chem. Eng. 2008, 2008. [Google Scholar] [CrossRef]
- Strathmann, H. Introduction to Membrane Science and Technology; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Saracco, G.; Neomagus, H.W.J.P.; Versteeg, G.F.; van Swaaij, W.P.M. High-temperature membrane reactors: Potential and problems. Chem. Eng. Sci. 1999, 54, 1997–2017. [Google Scholar] [CrossRef]
- Julbe, A. Zeolite Membranes—Synthesis, Characterization and Application. In Introduction to Zeolite Science and Practice, 3rd; Cejka, J., Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2007; p. 181. [Google Scholar]
- Staude, E. Membranen und Membranprozesse; Wiley-VCH: Weinheim, Germany, 1992. [Google Scholar]
- Maier, G. Gas separation with polymer membranes. Angew. Chem. Int. Ed. 1998, 37, 2961–2974. [Google Scholar]
- Lin, L.G.; Zhang, Y.Z.; Kong, Y. Recent advances in sulfur removal from gasoline by pervaporation. Fuel 2009, 88, 1799–1809. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z.L. A review of membrane selection for the dehydration of aqueous ethanol by pervaporation. Chem. Eng. Process. 2011, 50, 227–235. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membrane Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membrane Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Ohlrogge, K.; Stürken, K. The Separation of Organic Vapors from Gas Streams by Means of Membranes. In Membrane Technology; Wiley-VCH: Weinheim, Germany, 2001; pp. 69–94. [Google Scholar]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Spillman, R.W. Economics of gas separation membranes. Chem. Eng. Prog. 1989, 85, 41–62. [Google Scholar]
- Ohya, H.; Kudryavtsev, V.V.; Semenova, S.I. Polyimide Membranes: Applications, Fabrications and Properties; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Du, N.Y.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Gas Purification, 5 ed; Gulf Professional Publishing: Houston, TX, USA, 1997. [Google Scholar]
- Hugman, R.H.; Vidas, E.H.; Springer, P.S. Chemical Composition of Discovered and Undiscovered Natural Gas in the Lower-48 United States. Project Summary. Final report, 1 November 1988–31 March 1990; Technical Report No. PB-91-144600/XAB; Energy and Environmental Analysis, Inc.: Arlington, VA, USA, 1 November 1990.
- Wind, J.D.; Staudt-Bickel, C.; Paul, D.R.; Koros, W.J. The effects of crosslinking chemistry on CO2 plasticization of polyimide gas separation membranes. Ind. Eng. Chem. Res. 2002, 41, 6139–6148. [Google Scholar] [CrossRef]
- Directive 98/70/EC of the European Parliament and of the Council of 13 October 1998 relating to the quality of petrol and diesel fuels and amending Council Directive 93/12/EEC In Official Journal of the European Communities EU, 1998; L 350/358-368. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1998L0070:20031120:EN:PDF (accessed on 18 October 2012).
- Ren, J.Z.; Staudt-Bickel, C.; Lichtenthaler, R.N. Separation of aromatics/aliphatics with crosslinked 6FDA-based copolyimides. Sep. Purif. Tech. 2001, 22–23, 31–43. [Google Scholar]
- Tanihara, N.; Tanaka, K.; Kita, H.; Okamoto, K. Pervaporation of organic liquid-mixtures through membranes of polyimides containing methyl-substituted phenylenediamine moieties. J. Membrane Sci. 1994, 95, 161–169. [Google Scholar] [CrossRef]
- Terada, J.; Hohjoh, T.; Yoshimasu, S.; Ikemi, M.; Shinohara, I. Separation of benzene/cyclohexane azeotropic mixture through polymeric membranes with microphase separated structures. Polym. J. 1982, 14, 347–353. [Google Scholar] [CrossRef]
- Inui, K.; Tsukamoto, K.; Miyata, T.; Uragami, T. Permeation and separation of a benzene/cyclohexane mixture through benzoylchitosan membranes. J. Membrane Sci. 1998, 138, 67–75. [Google Scholar] [CrossRef]
- Inui, K.; Noguchi, T.; Miyata, T.; Uragami, T. Pervaporation characteristics of methyl methacrylate-methacrylic acid copolymer membranes ionically crosslinked with metal ions for a benzene/cyclohexane mixture. J. Appl. Polym. Sci. 1999, 71, 233–241. [Google Scholar]
- Ito, A.; Hwang, S.-T. Permeation of propane and propylene thrugh cellulosic polymer membranes. J. Appl. Polym. Sci. 1989, 38, 483–490. [Google Scholar] [CrossRef]
- Shimazu, A.; Ikeda, K.; Hachisuka, H. Method of Selectively Separating Unsaturated Hydrocarbon. U.S. Patent 5,749,943,12, May 1998. [Google Scholar]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membrane Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Tanaka, K.; Taguchi, A.; Hao, J.; Kita, H.; Okamoto, K. Permeation and separation properties of polyimide membranes to olefins and paraffins. J. Membrane Sci. 1996, 121, 197–207. [Google Scholar] [CrossRef]
- Lee, K.-R.; Hwang, S.-T. Separation of propylene and propane by polyimide hollow-fiber membrane module. J. Membrane Sci. 1992, 73, 37–45. [Google Scholar] [CrossRef]
- Krol, J.J.; Boerrigter, M.; Koops, G.H. Polyimide hollow fiber gas separation membranes: preparation and the suppression of plasticization in propane/propylene environments. J. Membrane Sci. 2001, 184, 275–286. [Google Scholar] [CrossRef]
- Shimazu, A.; Miyazaki, T.; Maeda, M.; Ikeda, K. Relationships between the chemical structures and the solubility, diffusivity, and permselectivity of propylene and propane in 6FDA-based polyimides. J. Polym. Sci. B 2000, 38, 2525–2536. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K.; Jordan, S.M.; Kim, T.H.; Hoehn, H.H. Polymeric membrane materials for solution-diffusion based permeation separations. Prog. Polym. Sci. 1988, 13, 339–401. [Google Scholar] [CrossRef]
- Robeson, L.M.; Burgoyne, W.F.; Langsam, M.; Savoca, A.C.; Tien, C.F. High-performance polymers for membrane separation. Polymer 1994, 35, 4970–4978. [Google Scholar] [CrossRef]
- Tanaka, K.; Kita, H.; Okano, M.; Okamoto, K. Permeability and permselectivity of gases in fluorinated and non-fluorinated polyimides. Polymer 1992, 33, 585–592. [Google Scholar] [CrossRef]
- Stern, S.A. Polymers for gas separations: The next decade. J. Membrane Sci. 1994, 94, 1–65. [Google Scholar] [CrossRef]
- Tanaka, K.; Okano, M.; Kita, H.; Okamoto, K.-i; Nishi, S. Effects of trifluoromethyl side groups on gas permeability and permselectivity in polyimides. Polym. J. 1994, 26, 1186–1189. [Google Scholar] [CrossRef]
- Kochkodan, V.M.; Sharma, V.K. Graft polymerization and plasma treatment of polymer membranes for fouling reduction: A review. J. Environ. Sci. Heal. A 2012, 47, 1713–1727. [Google Scholar]
- Staudt-Bickel, C.; J. Koros, W. Improvement of CO2/CH4 separation characteristics of polyimides by chemical crosslinking. J. Membrane Sci. 1999, 155, 145–154. [Google Scholar] [CrossRef]
- Wessling, M.; Schoeman, S.; van der Boomgaard, T.; Smolders, C.A. Plasticization of gas separation membranes. Gas Sep. Pur. 1991, 5, 222–228. [Google Scholar] [CrossRef]
- Kim, J.H.; Koros, W.J.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranes: Part 1. Without crosslinking. J. Membrane Sci. 2006, 282, 21–31. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membrane Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Schiewe, B.; Staudt-Bickel, C.; Vuin, A.; Wegner, G. Membrane-based gas separation of ethylene/ethylene oxide mixtures for product enrichment in microreactor technology. ChemPhysChem 2001, 2, 211–218. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. High pressure CO2/CH4 separation using carbon molecular sieve hollow fiber membranes. Ind. Eng. Chem. Res. 2001, 41, 367–380. [Google Scholar]
- Pithan, F.; Staudt-Bickel, C.; Hess, S.; Lichtenthaler, R.N. Polymeric membranes for aromatic/aliphatic separation processes. ChemPhysChem 2002, 3, 856–862. [Google Scholar] [CrossRef]
- Cao, B.; Hinode, H.; Kajiuchi, T. Permeation and separation of styrene/ethylbenzene mixtures through cross-linked poly(hexamethylene sebacate) membranes. J. Membrane Sci. 1999, 156, 43–47. [Google Scholar] [CrossRef]
- Matsui, S.; Paul, D.R. Pervaporation separation of aromatic/aliphatic hydrocarbons by crosslinked poly(methyl acrylate-co-acrylic acid) membranes. J. Membrane Sci. 2002, 195, 229–245. [Google Scholar] [CrossRef]
- McCaig, M.S.; Paul, D.R. Effect of UV crosslinking and physical aging on the gas permeability of thin glassy polyarylate films. Polymer 1999, 40, 7209–7225. [Google Scholar] [CrossRef]
- Rezac, M.E.; Schöberl, B. Transport and thermal properties of poly(ether imide)/acetylene-terminated monomer blends. J. Membrane Sci. 1999, 156, 211–222. [Google Scholar] [CrossRef]
- Bos, A.; Punt, I.G.M.; Wessling, M.; Strathmann, H. Plasticization-resistant glassy polyimide membranes for CO2/CH4 separations. Sep. Purif. Technol. 1998, 14, 27–39. [Google Scholar] [CrossRef]
- Bos, A.; Punt, I.G.M.; Wessling, M.; Strathmann, H. Suppression of CO2-plasticization by semiinterpenetrating polymer network formation. J. Polym. Sci. Pt. B Polym. Phys. 1998, 36, 1547–1556. [Google Scholar] [CrossRef]
- Kita, H.; Inada, T.; Tanaka, K.; Okamoto, K.-i. Effect of photocrosslinking on permeability and permselectivity of gases through benzophenone- containing polyimide. J. Membrane Sci. 1994, 87, 139–147. [Google Scholar] [CrossRef]
- Rezac, M.E.; Todd Sorensen, E.; Beckham, H.W. Transport properties of crosslinkable polyimide blends. J. Membrane Sci. 1997, 136, 249–259. [Google Scholar] [CrossRef]
- Wright, C.T.; Paul, D.R. Gas sorption and transport in UV-irradiated polyarylate copolymers based on tetramethyl bisphenol-A and dihydroxybenzophenone. J. Membrane Sci. 1997, 124, 161–174. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef]
- Biradha, K. Introduction to the themed issue “Coordination polymers: Structure and function”. New J. Chem. 2010, 34, 2353–2354. [Google Scholar] [CrossRef]
- Zaworotko, M.J. There is plenty of room in the middle: Crystal clear opportunities abound for coordination polymers. New J. Chem. 2010, 34, 2355–2356. [Google Scholar] [CrossRef]
- Hindson, K. Quo vadis MOFs? Eur. J. Inorg. Chem. 2010, 3683–3683. [Google Scholar] [CrossRef]
- Kitagawa, S.; Natarajan, S. Targeted fabrication of MOFs for hybrid functionality. Eur. J. Inorg. Chem. 2010, 3685–3685. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Skoulidas, A.I.; Sholl, D.S.; Johnson, J.K. Adsorption and diffusion of carbon dioxide and nitrogen through single-walled carbon nanotube membranes. J. Chem. Phys. 2006, 124, 054708–1. [Google Scholar]
- Kang, D.-Y.; Tong, H.M.; Zang, J.; Choudhury, R.P.; Sholl, D.S.; Beckham, H.W.; Jones, C.W.; Nair, S. Single-walled aluminosilicate nanotube/poly(vinyl alcohol) nanocomposite membranes. ACS Appl. Mater. Interfaces 2012, 4, 965–976. [Google Scholar] [CrossRef]
- Fonseca, A.; Reijerkerk, S.; Potreck, J.; Nijmeijer, K.; Mekhalif, Z.; Delhalle, J. Very short functionalized carbon nanotubes for membrane applications. Desalination 2010, 250, 1150–1154. [Google Scholar]
- Kim, S.; Pechar, T.W.; Marand, E. Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 2006, 192, 330–339. [Google Scholar] [CrossRef]
- Liu, T.; Tong, Y.; Zhang, W.-D. Preparation and characterization of carbon nanotube/polyetherimide nanocomposite films. Compos. Sci. Technol. 2007, 67, 406–412. [Google Scholar] [CrossRef]
- Pantano, A.; Modica, G.; Cappello, F. Multiwalled carbon nanotube reinforced polymer composites. Mater. Sci. Eng. A 2008, 486, 222–227. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Ismail, A.F.; Aziz, M.; Hayashi, Y. Pre-treatment of multi-walled carbon nanotubes for polyetherimide mixed matrix hollow fiber membranes. J. Colloid Interface Sci. 2012, 386, 80–87. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Jones, C.W.; Nair, S. Modeling molecular transport in composite membranes with tubular fillers. J. Membrane Sci. 2011, 381, 50–63. [Google Scholar] [CrossRef]
- Cong, H.; Zhang, J.; Radosz, M.; Shen, Y. Carbon nanotube composite membranes of brominated poly(2,6-diphenyl-1,4-phenylene oxide) for gas separation. J. Membrane Sci. 2007, 294, 178–185. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Krych, W.; Ramanan, H.; Nair, S.; Marand, E.; Tsapatsis, M. Fabrication of polymer/selective-flake nanocomposite membranes and their use in gas separation. Chem. Mater. 2004, 16, 3838–3845. [Google Scholar] [CrossRef]
- Johnson, J.R.; Koros, W.J. Utilization of nanoplatelets in organic-inorganic hybrid separation materials: Separation advantages and formation challenges. Taiwan Inst. Chem. Eng 2009, 40, 268–275. [Google Scholar] [CrossRef]
- Yang, C.; Smyrl, W.H.; Cussler, E.L. Flake alignment in composite coatings. J. Membrane Sci. 2004, 231, 1–12. [Google Scholar] [CrossRef]
- Sheffel, J.A.; Tsapatsis, M. A model for the performance of microporous mixed matrix membranes with oriented selective flakes. J. Membrane Sci. 2007, 295, 50–70. [Google Scholar] [CrossRef]
- Choi, J.; Tsapatsis, M. MCM-22/silica selective flake nanocomposite membranes for hydrogen separations. J. Am. Chem. Soc. 2009, 132, 448–449. [Google Scholar]
- Rubio, C.; Casado, C.; Gorgojo, P.; Etayo, F.; Uriel, S.; Téllez, C.; Coronas, J. Exfoliated titanosilicate material UZAR-S1 obtained from JDF-L1. Eur. J. Inorg. Chem. 2010, 2010, 159–163. [Google Scholar]
- Galve, A.; Sieffert, D.; Vispe, E.; Téllez, C.; Coronas, J.; Staudt, C. Copolyimide mixed matrix membranes with oriented microporous titanosilicate JDF-L1 sheet particles. J. Membrane Sci. 2011, 370, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Marand, E.; Dhingra, S.; Fritsch, D.; Wen, J.; Wilkes, G. Poly(amide-imide)/TiO2 nano-composite gas separation membranes: Fabrication and characterization. J. Membrane Sci. 1997, 135, 65–79. [Google Scholar] [CrossRef]
- Zimmerman, C.M.; Singh, A.; Koros, W.J. Tailoring mixed matrix composite membranes for gas separations. J. Membrane Sci. 1997, 137, 145–154. [Google Scholar] [CrossRef]
- Mahajan, R.; Koros, W.J. Factors controlling successful formation of mixed-matrix gas separation materials. Ind. Eng. Chem. Res. 2000, 39, 2692–2696. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Chung, T.S.; Cao, C.; Huang, Z.; Kulprathipanja, S. Fundamental understanding of nano-sized zeolite distribution in the formation of the mixed matrix single- and dual-layer asymmetric hollow fiber membranes. J. Membrane Sci. 2005, 252, 89–100. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, reverse-selective nanocomposite membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Moore, T.T.; Mahajan, R.; Vu, D.Q.; Koros, W.J. Hybrid membrane materials comprising organic polymers with rigid dispersed phases. Angew. Chem. Int. Ed. 2004, 50, 311–321. [Google Scholar]
- Langhendries, G.; Baron, G.V. Mass transfer in composite polymer zeolite catalytic membranes. J. Membrane Sci. 1998, 141, 265–275. [Google Scholar] [CrossRef]
- Gorgojo, P.; Sieffert, D.; Staudt, C.; Tellez, C.; Coronas, J. Exfoliated zeolite Nu-6(2) as filler for 6FDA-based copolyimide mixed matrix membranes. J. Membrane Sci. 2012, 411, 146–152. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic-inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar]
- Mahajan, R.; Koros, W.J. Mixed matrix membrane materials with glassy polymers. Part 1. Polym. Eng. Sci. 2002, 42, 1420–1431. [Google Scholar]
- Duval, J.M.; Kemperman, A.J.B.; Folkers, B.; Mulder, M.H.V.; Desgrandchamps, G.; Smolders, C.A. Preparation of zeolite filled glassy polymer membranes. J. Appl. Polym. Sci. 1994, 54, 409–418. [Google Scholar] [CrossRef]
- Pechar, T.W.; Kim, S.; Vaughan, B.; Marand, E.; Tsapatsis, M.; Jeong, H.K.; Cornelius, C.J. Fabrication and characterization of polyimide-zeolite L mixed matrix membranes for gas separations. J. Membrane Sci. 2006, 277, 195–202. [Google Scholar] [CrossRef]
- Moaddeb, M.; Koros, W.J. Gas transport properties of thin polymeric membranes in the presence of silicon dioxide particles. J. Membrane Sci. 1997, 125, 143–163. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Guiver, M.D. Poly sulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membrane Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Merkel, T.C.; He, Z.J.; Pinnau, I.; Freeman, B.D.; Meakin, P.; Hill, A.J. Sorption and transport in poly(2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole-co-tetrafluoroeth ylene) containing nanoscale fumed silica. Macromolecules 2003, 36, 8406–8414. [Google Scholar] [CrossRef]
- Yan, F.; Goedel, W.A. Polymer membranes with two-dimensionally arranged pores derived from monolayers of silica particles. Chem. Mater. 2004, 16, 1622–1626. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z.L. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Peter, S.; Hese, N.; Stefan, R. Phenol-selective, highly resistant RO-membranes made from PVA for the purification of toxic industrial wastes. Desalination 1976, 19, 161–167. [Google Scholar] [CrossRef]
- Hickey, A.S.; Peppas, N.A. Mesh size and diffusive characteristics of semicrystalline poly(vinyl alcohol) membranes prepared by freezing/thawing techniques. J. Membrane Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Ofstead, R.F.; Poser, C.I. Semicrystalline poly (vinyl alcohol) hydrogels. In Polymers in Aqueous Media: Performance through Association; Glass, J.E., Ed.; American Chemical Society: Washington, DC, USA, 1989; pp. 61–72. [Google Scholar]
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Xianda, Y.; Anlai, W.; Suqin, C. Water-vapor permeability of polyvinyl alcohol films. Desalination 1987, 62, 293–297. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Immelman, E.; Bezuidenhout, D.; Jacobs, E.P.; Vanreenen, A.J. Polyvinyl alcohol and modified polyvinyl alcohol reverse osmosis membranes. Desalination 1993, 90, 15–29. [Google Scholar] [CrossRef]
- Immelman, E.; Bezuidenhout, D.; Sanderson, R.D.; Jacobs, E.P.; Vanreenen, A.J. Poly(vinyl alcohol) gel sublayers for reverse-osmosis membranes. III. Insolubilization by cross-linking with potassium peroxodisulfate. Desalination 1993, 94, 115–132. [Google Scholar] [CrossRef]
- Konietzny, R.; Bettermann, I.; Staudt, C. Removal of sulfur aromatics from jet fuel using membrane technology. Annu. B. Aust. Inst. High En. Mater. 2010, 1, 57–62. [Google Scholar]
- Sieffert, D.; Staudt, C. Preparation of hybrid materials containing copolyimides covalently linked with carbon nanotubes. Sep. Pur. Technol. 2011, 77, 99–103. [Google Scholar] [CrossRef]
- Sterescu, D.M.; Stamatialis, D.F.; Mendes, E.; Wubbenhorst, M.; Wessling, M. Fullerene-modified poly(2,6-dimethyl-1,4-phenylene oxide) gas separation membranes: Why binding is better than dispersing. Macromolecules 2006, 39, 9234–9242. [Google Scholar] [CrossRef]
- Peng, F.; Hu, C.; Jiang, Z. Novel poly(vinyl alcohol)/carbon nanotube hybrid membranes for pervaporation separation of benzene/cyclohexane mixtures. J. Membrane Sci. 2007, 297, 236–242. [Google Scholar] [CrossRef]
- Peng, F.; Pan, F.; Sun, H.; Lu, L.; Jiang, Z. Novel nanocomposite pervaporation membranes composed of poly(vinyl alcohol) and chitosan-wrapped carbon nanotube. J. Membrane Sci. 2007, 300, 13–19. [Google Scholar] [CrossRef]
- Srivastava, R.; Banerjee, S.; Jehnichen, D.; Voit, B.; Böhme, F. In situ Preparation of polyimide composites based on functionalized carbon nanotubes. Macromol. Mat. Eng. 2009, 294, 96–102. [Google Scholar] [CrossRef]
- Han, B.; Li, J.; Chen, C.; Xu, C.; Wickramasinghe, S.R. Effects of degree of formaldehyde acetal treatment and maleic acid crosslinking on solubility and diffusivity of water in PVA membranes. Chem. Eng. Res. Design 2003, 81, 1385–1392. [Google Scholar] [CrossRef]
- Chen, C.T.; Chang, Y.J.; Chen, M.C.; Tobolsky, A.V. Formalized poly(vinyl alcohol) membranes for reverse osmosis. J. Appl. Polym. Sci. 1973, 17, 789–796. [Google Scholar] [CrossRef]
- Durmaz-Hilmioglu, N.; Yildirim, A.E.; Sakaoglu, A.S.; Tulbentci, S. Acetic acid dehydration by pervaporation. Chem. Eng. Process. 2001, 40, 263–267. [Google Scholar] [CrossRef]
- Jian, S.; Ming, S.X. Crosslinked PVA-PS thin-film composite membrane for reverse osmosis. Desalination 1987, 62, 395–403. [Google Scholar] [CrossRef]
- Peters, T.A.; Poeth, C.H.S.; Benes, N.E.; Buijs, H.C.W.M.; Vercauteren, F.F.; Keurentjes, J.T.F. Ceramic-supported thin PVA pervaporation membranes combining high flux and high selectivity; contradicting the flux-selectivity paradigm. J. Membrane Sci. 2006, 276, 42–50. [Google Scholar] [CrossRef]
- Ungerank, M.; Baumgarten, G. Polyimide Membranes Made of Polymerization Solutions. U.S.2012/012079 A1 2012. [Google Scholar]
- Decker, C.; Bianchi, C. Photocrosslinking of a maleimide functionalized polymethacrylate. Polym. Int. 2003, 52, 722–732. [Google Scholar] [CrossRef]
- Decker, C.; Bianchi, C.; Jonsson, S. Light-induced crosslinking polymerization of a novel N-substituted bis-maleimide monomer. Polymer 2004, 45, 5803–5811. [Google Scholar] [CrossRef]
- Mattson, G.; Conklin, E.; Desai, S.; Nielander, G.; Savage, M.D.; Morgensen, S. A practical approach to crosslinking. Mol. Biol. Rep. 1993, 17, 167–183. [Google Scholar] [CrossRef]
- Pfeifer, S.; Lutz, J.F. Development of a library of N-substituted maleimides for the local functionalization of linear polymer chains. Chem. Eur. J. 2008, 14, 10949–10957. [Google Scholar] [CrossRef]
- Stevens, M.P.; Jenkins, A.D. Crosslinking of polystyrene via pendant maleimide groups. J. Polym. Sci. Polym. Chem. 1979, 17, 3675–3685. [Google Scholar]
- Nagarathinam, M.; Vittal, J.J. A rational approach to crosslinking of coordination polymers using the photochemical [2+2] cycloaddition reaction. Macromol. Rapid Commun. 2006, 27, 1091–1099. [Google Scholar] [CrossRef]
- Beyer, U.; Krüger, M.; Schumacher, P.; Unger, C.; Kratz, F. Synthese von neuen bifunktionellen Maleinimidverbindungen zur Herstellung von Chemoimmunokonjugaten. Monatshefte für Chemie / Chemical Monthly 1997, 128, 91–102. [Google Scholar]
- Delzenne, G.A. Synthesis and photocrosslinking of light-sensitive polymers. Eur. Polym. J. 1969, 5, 55–91. [Google Scholar] [CrossRef]
- Wind, J.D.; Staudt-Bickel, C.; Paul, D.R.; Koros, W.J. Solid-state covalent cross-linking of polyimide membranes for carbon dioxide plasticization reduction. Macromolecules 2003, 36, 1882–1888. [Google Scholar] [CrossRef]
- Hillock, A.M.W.; Koros, W.J. Cross-linkable polyimide membrane for natural gas purification and carbon dioxide plasticization reduction. Macromolecules 2007, 40, 583–587. [Google Scholar] [CrossRef]
- Kim, J.H.; Koros, W.J.; Paul, D.R. Effects of CO2 exposure and physical aging on the gas permeability of thin 6FDA-based polyimide membranes: Part 2. with crosslinking. J. Membrane Sci. 2006, 282, 32–43. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Kim, W.S.; Um, K.H.; Shin, S.C.; Lee, Y.J.; Hong, K.H. Synthesis and properties of photocrosslinkable polymers with maleimide moiety. Polym.-Korea 1999, 23, 502–506. [Google Scholar]
- Kim, W.S.; Seo, K.H.; Chang, W.S. Synthesis and photocrosslinking of maleimide-type polymers. Macromol. Rapid Commun. 1996, 17, 835–841. [Google Scholar] [CrossRef]
- He, D.M.; Susanto, H.; Ulbricht, M. Photo-irradiation for preparation, modification and stimulation of polymeric membranes. Prog. Polym. Sci. 2009, 34, 62–98. [Google Scholar]
- Kang, J.S.; Won, J.; Park, H.C.; Kim, U.Y.; Kang, Y.S.; Lee, Y.M. Morphology control of asymmetric membranes by UV irradiation on polyimide dope solution. J. Membrane Sci. 2000, 169, 229–235. [Google Scholar] [CrossRef]
- Guthrie, J.; Jeganathan, M.B.; Otterburn, M.S.; Woods, J. Light screening effects of photoinitiators in UV curable systems. Polym. Bull. 1986, 15, 51–58. [Google Scholar]
- Dudley, C.N.; Schöberl, B.; Sturgill, G.K.; Beckham, H.W.; Rezac, M.E. Influence of crosslinking technique on the physical and transport properties of ethynyl-terminated monomer/polyetherimide asymmetric membranes. J. Membrane Sci. 2001, 191, 1–11. [Google Scholar] [CrossRef]
- Lin, A.A.; Sastri, V.R.; Tesoro, G.; Reiser, A.; Eachus, R. On the cross-linking mechanism of benzophenone-containing polyimides. Macromolecules 1988, 21, 1165–1169. [Google Scholar] [CrossRef]
- Schmeling, N.; Hunger, K.; Engler, G.; Breiten, B.; Rölling, P.; Mixa, A.; Staudt, C.; Kleinermanns, K. Photo-crosslinking of poly[ethene-stat-(methacrylic acid)] functionalised with maleimide side groups. Polym. Int. 2009, 58, 720–727. [Google Scholar] [CrossRef]
- Put, J.; De Schryver, F.C. Photochemistry of nonconjugated bichromophoric systems. intramolecular photocycloaddition of N,N'-alkylenedimaleimides in solution. J. Am. Chem. Soc. 1973, 95, 137–145. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Tedaldi, L.M.; Aliev, A.E.; Baker, J.R. [2 + 2] Photocycloadditions of thiomaleimides. Chem. Commun. 2012, 48, 4725–4727. [Google Scholar] [CrossRef]
- Braun, D.; Cherdron, H.; Ritter, H. Praktikum der Makromolekularen Stoffe; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Tieke, B. Makromolekulare Chemie: Eine Einführung; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Katarzynski, D. Pervaporation komplexer Aromaten am Beispiel von Naphthalin/n-Decan-Mischungen. Ph.D. Thesis, Heinrich-Heine-University, Düsseldorf, Germany, July 2008. [Google Scholar]
- Talrose, V.; Yermakov, A.N.; Usov, A.A.; Goncharova, A.A.; Leskin, A.N.; Messineva, N.A.; Trusova, N.V.; Efimkina, M.V. UV/Visible Spectra. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Von Sonntag, J.; Knolle, W. Maleimides as electron-transfer photoinitiators: Quantum yields of triplet states and radical-ion formation. J. Photochem. Photobiol. A 2000, 136, 133–139. [Google Scholar] [CrossRef]
- Von Sonntag, J.; Knolle, W.; Naumov, S.; Mehnert, R. Deprotonation and dimerization of maleimide in the triplet state: A laser flash photolysis study with optical and conductometric detection. Chem. Eur. J. 2002, 8, 4199–4209. [Google Scholar] [CrossRef]
- Hunger, K.; Buschhaus, L.; Schmeling, N.; Staudt, C.; Pfeifer, A.; Kleinermanns, K. Characterization of maleimide dimers in photo-cross-linked copolyimide films. Phys. Chem. Chem. Phys. 2012, 14, 4538–4547. [Google Scholar]
- Marks, M.J.; Scott, D.C.; Guilbeaux, B.R.; Bales, S.E. Synthesis and thermochemistry of phenylmaleimide- and phenylnadimide-terminated bisphenol A polycarbonates. J. Polym. Sci. Polym. Chem. 1997, 35, 385–390. [Google Scholar]
- Chen, Y.; Tsay, C.J. Preparation and photo-cross-linking behaviors of polyesters derived from trans-2,2'-dihydroxystilbene. J. Polym. Sci. Polym. Chem. 1995, 33, 1319–1327. [Google Scholar]
- Vlad, C.D.; Hulubei, C. Crosslinked copolymers based on N-(p-Carboxyphenyl)-maleimide. High Perform. Polym. 2002, 14, 31–40. [Google Scholar]
- Seiffert, S.; Oppermann, W.; Saalwächter, K. Hydrogel formation by photocrosslinking of dimethylmaleimide functionalized polyacrylamide. Polymer 2007, 48, 5599–5611. [Google Scholar] [CrossRef]
- Schinner, R.; Wolff, T. Defined photo-cross-linking and viscometric data II. Photo-cross-linking versus coordinative cross-linking. Colloid Polym. Sci. 2001, 279, 1225–1230. [Google Scholar] [CrossRef]
- Yu, X.; Corten, C.; Görner, H.; Wolff, T.; Kuckling, D. Photodimers of N-alkyl-3,4-dimethylmaleimides—Product ratios and reaction mechanism. J. Photochem. Photobiol. A 2008, 198, 34–44. [Google Scholar] [CrossRef]
- Büchi, G.; Inman, C.G.; Lipinsky, E.S. Light-catalyzed organic reactions. I. The reaction of carbonyl compounds with 2-methyl-2-butene in the presence of ultraviolet light. J. Am. Chem. Soc. 1954, 76, 4327–4331. [Google Scholar] [CrossRef]
- Masson, F.; Decker, C.; Jaworek, T.; Schwalm, R. UV-radiation curing of waterbased urethane-acrylate coatings. Prog. Org. Coat. 2000, 39, 115–126. [Google Scholar]
- Schmeling, N. Vernetzbare Polymermaterialien für die Pervaporation. Ph.D. Thesis, Heinrich-Heine-University, Düsseldorf, Germany, December 2012. [Google Scholar]
- Lin, L.; Kong, Y.; Yang, J.; Shi, D.; Xie, K.; Zhang, Y. Scale-up of pervaporation for gasoline desulphurization: Part 1. Simulation and design. J. Membrane Sci. 2007, 298, 1–13. [Google Scholar] [CrossRef]
- Noble, R.D. Perspectives on mixed matrix membranes. J. Membrane Sci. 2011, 378, 393–397. [Google Scholar] [CrossRef]
- Li, J.R.; Ma, Y.G.; McCarthy, M.C.; Sculley, J.; Yu, J.M.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar]
- Liu, D.H.; Zhong, C.L. Understanding gas separation in metal-organic frameworks using computer modeling. J. Mater. Chem. 2010, 20, 10308–10318. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef]
- Bae, T.H.; Lee, J.S.; Qiu, W.L.; Koros, W.J.; Jones, C.W.; Nair, S. A high-performance gas-separation membrane containing submicrometer-sized metal-organic framework crystals. Angew. Chem. Int. Ed. 2010, 49, 9863–9866. [Google Scholar]
- Zhang, Y.F.; Musseman, I.H.; Ferraris, J.P.; Balkus, K.J. Gas permeability properties of Matrimid® membranes containing the metal-organic framework Cu-BPY-HFS. J. Membrane Sci. 2008, 313, 170–181. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Matrimid®/[Cu3(BTC)2] mixed-matrix membranes for gas separations. J. Membrane Sci. 2010, 362, 478–487. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Hu, J.; Cai, H.P.; Ren, H.Q.; Wei, Y.M.; Xu, Z.L.; Liu, H.L.; Hu, Y. Mixed-matrix membrane hollow fibers of Cu3(BTC)2 MOF and polyimide for gas separation and adsorption. Ind. Eng. Chem. Res. 2010, 49, 12605–12612. [Google Scholar] [CrossRef]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Tellez, C.; Coronas, J. Combination of MOFs and zeolites for mixed-matrix membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Mixed-matrix membranes containing MOF-5 for gas separations. J. Membrane Sci. 2009, 328, 165–173. [Google Scholar] [CrossRef]
- Ordonez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/ Matrimid® mixed-matrix membranes. J. Membrane Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Diaz, K.; Garrido, L.; Lopez-Gonzalez, M.; del Castillo, L.F.; Riande, E. CO2 transport in polysulfone membranes containing zeolitic imidazolate frameworks as determined by permeation and PFG NMR techniques. Macromolecules 2010, 43, 316–325. [Google Scholar] [CrossRef]
- Thompson, J.A.; Chapman, K.W.; Koros, W.J.; Jones, C.W.; Nair, S. Sonication-induced Ostwald ripening of ZIF-8 nanoparticles and formation of ZIF-8/polymer composite membranes. Micropor. Mesopor. Mater. 2012, 158, 292–299. [Google Scholar] [CrossRef]
- Seoane, B.; Zamaro, J.M.; Tellez, C.; Coronas, J. Insight into the crystal synthesis, activation and application of ZIF-20. RSC Adv. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Peinemann, K.-V. Hybrid membrane materials with different metal-organic frameworks (MOFs) for gas separation. Desalination 2006, 200, 424–426. [Google Scholar] [CrossRef]
- Zornoza, B.; Martinez-Joaristi, A.; Serra-Crespo, P.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Functionalized flexible MOFs as fillers in mixed matrix membranes for highly selective separation of CO2 from CH4 at elevated pressures. Chem. Commun. 2011, 47, 9522–9524. [Google Scholar]
- Jeazet, H.B.T.; Staudt, C.; Janiak, C. A method for increasing permeability in O2/N2 separation with mixed-matrix membranes made of water-stable MIL-101 and polysulfone. Chem. Commun. 2012, 48, 2140–2142. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Janiak, C. MOFs for use in adsorption heat pump processes. Eur. J. Inorg. Chem. 2012, 2625–2634. [Google Scholar]
- Jeremias, F.; Khutia, A.; Henninger, S.K.; Janiak, C. MIL-100(Al, Fe) as water adsorbents for heat transformation purposes—A promising application. J. Mater. Chem. 2012, 22, 10148–10151. [Google Scholar]
- Ehrenmann, J.; Henninger, S.K.; Janiak, C. Water adsorption characteristics of MIL-101 for heat-transformation applications of MOFs. Eur. J. Inorg. Chem. 2011, 471–474. [Google Scholar]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual high throughput screening confirmed experimentally: Porous coordination polymer hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Aguado, S.; Canivet, J.; Farrusseng, D. Engineering structured MOF at nano and macroscales for catalysis and separation. J. Mat. Chem. 2011, 21, 7582–7588. [Google Scholar] [CrossRef]
- Aguado, S.; Nicolas, C.-H.; Moizan-Basle, V.; Nieto, C.; Amrouche, H.; Bats, N.; Audebrand, N.; Farrusseng, D. Facile synthesis of an ultramicroporous MOF tubular membrane with selectivity towards CO2. New J. Chem. 2011, 35, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Gascon, J.; Kapteijn, F. Metal-organic framework membranes-high potential, bright future? Angew. Chem. Int. Ed. 2010, 49, 1530–1532. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- Li, Y.-S.; Liang, F.-Y.; Bux, H.; Feldhoff, A.; Yang, W.-S.; Caro, J. Molecular sieve membrane: supported metal-organic framework with high hydrogen selectivity. Angew. Chem. Int. Ed. 2010, 49, 548–551. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hunger, K.; Schmeling, N.; Jeazet, H.B.T.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation. Membranes 2012, 2, 727-763. https://doi.org/10.3390/membranes2040727
Hunger K, Schmeling N, Jeazet HBT, Janiak C, Staudt C, Kleinermanns K. Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation. Membranes. 2012; 2(4):727-763. https://doi.org/10.3390/membranes2040727
Chicago/Turabian StyleHunger, Katharina, Nadine Schmeling, Harold B. Tanh Jeazet, Christoph Janiak, Claudia Staudt, and Karl Kleinermanns. 2012. "Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation" Membranes 2, no. 4: 727-763. https://doi.org/10.3390/membranes2040727
APA StyleHunger, K., Schmeling, N., Jeazet, H. B. T., Janiak, C., Staudt, C., & Kleinermanns, K. (2012). Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation. Membranes, 2(4), 727-763. https://doi.org/10.3390/membranes2040727





