Strong Static Magnetic Fields Increase the Gel Signal in Partially Hydrated DPPC/DMPC Membranes
Abstract
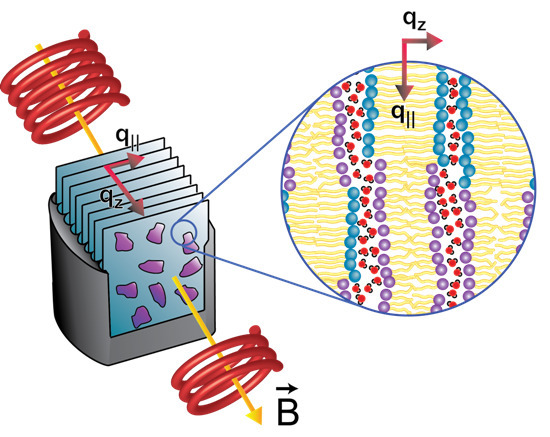
:1. Introduction

2. Results
2.1. Membrane Out-of-Plane Structure
| Lipid Composition | Relative Humidity | Magnetic Field Strength (T) | Spacing | Gel Phase Lipid Area (Å) | |||||
|---|---|---|---|---|---|---|---|---|---|
| % | Salt | 0 | 1 | 2.5 | 3.5 | (Å) | 0 T Field | 3.5 T Field | |
| DMPC/DPPC (1:1) | 97 | KSO | x | – | – | x | 43.04 | 42.98 | |
| 75 | NaCl | x | – | – | x | 43.24 | 43.03 | ||
| 50 | Mg(NO) | x | – | – | x | 42.70 | 42.70 | ||
| 43 | KCO | x | x | x | x | 42.51 | 42.51 | ||
| 25 | CHCOOK | x | – | – | x | 42.23 | 42.27 | ||
| 10 | LiCl | x | x | 42.30 | 42.21 | ||||

2.2. Membrane In-Plane Structure




| 97% | 75% | 50% | 43% | 25% | 10% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field (T) | ord | gel | fluid | ord | gel | fluid | ord | gel | fluid | ord | gel | fluid | ord | gel | fluid | ord | gel | fluid |
| 0 | 1 | 161 ± 11 | 170 ± 27 | 1 | 372 ± 15 | 86 ± 30 | 1 | 691 ± 9 | 33 ± 39 | 1 | 648 ± 7 | 0 | 1 | 1029 ± 14 | 0 | 1 | 921 ± 12 | 0 |
| 3 | 384 ± 8 | 89 ± 10 | 5 | 691 ± 7 | 0 | |||||||||||||
| 1 | 4 | 691 ± 8 | 0 | |||||||||||||||
| 2.5 | 3 | 680 ± 8 | 0 | |||||||||||||||
| 3.5 | 2 | 160 ± 10 | 179 ± 24 | 2 | 383 ± 8 | 88 ± 11 | 2 | 688 ± 9 | 43 ± 39 | 2 | 675 ± 8 | 0 | 2 | 1033 ± 14 | 0 | 2 | 911 ± 13 | 0 |
| 6 | 691 ± 8 | 0 | ||||||||||||||||
3. Discussion
3.1. Magnetic Fields Have an Effect on Dehydrated Lipid Membranes
3.2. A Potential Mechanism for Lipid Tails to Interact with Magnetic Fields

4. Conclusions
5. Materials and Methods
5.1. Neutron Diffraction

5.2. Membrane Preparation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ritz, T.; Thalau, P.; Phillips, J.B.; Wiltschko, R.; Wiltschko, W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 2004, 429, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.; Lohmann, K.J. The physics and neurobiology of magnetoreception. Nat. Rev. Neurosci. 2005, 6, 703–712. [Google Scholar] [PubMed]
- Maeda, K.; Henbest, K.B.; Cintolesi, F.; Kuprov, I.; Rodgers, C.T.; Liddell, P.A.; Gust, D.; Timmel, C.R.; Hore, P.J. Chemical compass model of avian magnetoreception. Nature 2008, 453, 387–390. [Google Scholar] [CrossRef]
- Treiber, C.D.; Salzer, M.C.; Riegler, J.; Edelman, N.; Sugar, C.; Breuss, M.; Pichler, P.; Cadiou, H.; Saunders, M.; Lythgoe, M.; et al. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 2012, 484, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Faivre, D.; Schüler, D. Magnetotactic Bacteria and Magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R.P. Magnetotactic Bacteria. Ann. Rev. Microbiol. 1982, 36, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Schenck, J.F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996, 23, 815–850. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.V.; Geim, A.K. Of flying frogs and levitrons. Eur. J. Phys. 1997, 18, 307–313. [Google Scholar] [CrossRef]
- Pacini, S.; Gulisano, M.; Peruzzi, B.; Sgambati, E.; Gheri, G.; Gheri Bryk, S.; Vannucchi, S.; Polli, G.; Ruggiero, M. Effects of a 0.2T static magnetic field on human skin fibroblasts. Cancer Detect. Prev. 2003, 27, 327–332. [Google Scholar] [CrossRef]
- Tan, C.; Fung, B.; Cho, G. Phospholipid bicelles that align with their normals parallel to the magnetic field. J. Am. Chem. Soc. 2002, 124, 11827–11832. [Google Scholar] [CrossRef]
- Luchette, P.A.; Vetman, T.N.; Prosser, R.S.; Hancock, R.E.; Nieh, M.P.; Glinka, C.J.; Krueger, S.; Katsaras, J. Morphology of fast-tumbling bicelles: A small angle neutron scattering and NMR study. Biochim. Biophys. Acta 2001, 1513, 83–94. [Google Scholar] [CrossRef]
- Nieh, M.P.; Raghunathan, V.; Glinka, C.J.; Harroun, T.A.; Pabst, G.; Katsaras, J. Magnetically alignable phase of phospholipid “bicelle” mixtures is a chiral nematic made up of wormlike micelles. Langmuir 2004, 20, 7893–7897. [Google Scholar] [CrossRef] [PubMed]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef]
- Galland, P.; Pazur, A. Magnetoreception in plants. J. Plant Res. 2005, 118, 371–389. [Google Scholar] [CrossRef]
- Harris, S.; Henbest, K.; Maeda, K.; Pannell, J.; Timmel, C.; Hore, P.; Okamoto, H. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 2009, 6, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, A.; Nagarajan, S. Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.). Bioelectromagentics 2008, 29, 571–578. [Google Scholar] [CrossRef]
- Poinapen, D.; Toppozini, L.; Dies, H.; Brown, D.C.; Rheinstädter, M.C. Static magnetic fields enhance lipid order in native plant plasma membrane. Soft Matter 2013, 9, 6804–6813. [Google Scholar] [CrossRef]
- Weik, M.; Lehnert, U.; Zaccai, G. Liquid-Like Water Confined in Stacks of Biological Membranes at 200 K and Its Relation to Protein Dynamics. Biophys. J. 2005, 89, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Trapp, Marcus and Gutberlet, Thomas and Juranyi, Fanni and Unruh, Tobias and Demé, Bruno and Tehei, Moeava and Peters, Judith Hydration dependent studies of highly aligned multilayer lipid membranes by neutron scattering. J. Chem. Phys. 2010, 133, 164505.
- Tristram-Nagle, S.; Liu, Y.; Legleiter, J.; Nagle, J.F. Structure of Gel Phase DMPC Determined by X-ray Diffraction. Biophys. J. 2002, 83, 3324–3335. [Google Scholar] [CrossRef]
- Katsaras, J.; Raghunathan, V.A.; Dufourc, E.J.; Dufourcq, J. Evidence for a Two-Dimensional Molecular Lattice in Subgel Phase DPPC Bilayers. Biochemistry 1995, 34, 4684–4688. [Google Scholar] [CrossRef] [PubMed]
- Rheinstädter, M.C.; Ollinger, C.; Fragneto, G.; Demmel, F.; Salditt, T. Collective dynamics of lipid membranes studied by inelastic neutron scattering. Phys. Rev. Lett. 2004, 93, 108107. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Marquardt, D.; Dies, H.; Kučerka, N.; Yamani, Z.; Harroun, T.A.; Katsaras, J.; Shi, A.C.; Rheinstädter, M.C. The Observation of Highly Ordered Domains in Membranes with Cholesterol. PLoS ONE 2013, 8, e66162. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.T.; Toombes, G.E.S.; Tristram-Nagle, S.; Smilgies, D.M.; Feigenson, G.W.; Nagle, J.F. Order Parameters and Areas in Fluid-Phase Oriented Lipid Membranes Using Wide Angle X-ray Scattering. Biophys. J. 2008, 95, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.A.; Zheng, S.; Roshankar, G.; Alsop, R.J.; Belanger, R.K.; Huynh, C.; Kučerka, N.; Rheinstädter, M.C. Interaction of Aspirin (Acetylsalicylic Acid) with Lipid Membranes. PLoS ONE 2012, 7, e34357. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Zheng, S.; Toppozini, L.; Alsop, R.; Dies, H.; Wang, A.; Jago, N.; Moore, M.; Rheinstädter, M. Solubility of cholesterol in lipid membranes and the formation of immiscible cholesterol plaques at High cholesterol concentrations. Soft Matter 2013, 9, 9342–9351. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Barrett, M.A.; Toppozini, L.; Kučerka, N.; Yamani, Z.; Katsaras, J.; Fragneto, G.; Rheinstädter, M.C. Co-existence of gel and fluid domains in single-component phospholipid membranes. Soft Matter 2012, 8, 4687–4694. [Google Scholar] [CrossRef]
- Rheinstädter, M.C.; Mouritsen, O.G. Small-scale structures in fluid cholesterol-lipid bilayers. Curr. Opin. Colloid Interface Sci. 2013, 18, 440–447. [Google Scholar] [CrossRef]
- Toppozini, L.; Meinhardt, S.; Armstrong, C.L.; Yamani, Z.; Kučerka, N.; Schmid, F.; Rheinstädter, M.C. Structure of cholesterol in lipid rafts. Phys. Rev. Lett. 2014, 113, 228101. [Google Scholar] [CrossRef] [PubMed]
- Alsop, R.J.; Toppozini, L.; Marquardt, D.; Kučerka, N.; Harroun, T.A.; Rheinstädter, M.C. Aspirin inhibits formation of cholesterol rafts in fluid lipid membranes. Biochim. Biophys. Acta 2015, 1848, 805–812. [Google Scholar] [PubMed]
- Hristova, K.; White, S.H. Determination of the Hydrocarbon Core Structure of Fluid Dioleoylphosphocholine (DOPC) Bilayers by X-ray Diffraction Using Specific Bromination of the Double-Bonds: Effect of Hydration. Biophys. J. 1998, 74, 2419–2433. [Google Scholar] [PubMed]
- Ram, P.; Prestegard, J. Magnetic field induced ordering of bile salt/phospholipid micelles: New media for NMR structural investigations. Biochim. Biophys. Acta 1988, 940, 289–294. [Google Scholar] [CrossRef]
- Vogel, V.; Möbius, D. Local surface potentials and electric dipole moments of lipid monolayers: Contributions of the water/lipid and the lipid/air interfaces. J. Colloid Interface Sci. 1988, 126, 408–420. [Google Scholar] [CrossRef]
- Mingins, J.; Stigter, D.; Dill, K.A. Phospholipid interactions in model membrane systems. I. Experiments on monolayers. Biophys. J. 1992, 61, 1603. [Google Scholar] [CrossRef]
- Brockman, H. Dipole potential of lipid membranes. Chem. Phys. Lipids 1994, 73, 57–79. [Google Scholar] [CrossRef]
- Pohl, E.E. Dipole potential of bilayer membranes. Adv. Planar Lipid Bilayers Liposomes 2005, 1, 77–100. [Google Scholar]
- Peterson, U.; Mannock, D.A.; Lewis, R.N.; Pohl, P.; McElhaney, R.N.; Pohl, E.E. Origin of membrane dipole potential: Contribution of the phospholipid fatty acid chains. Chem. Phys. Lipids 2002, 117, 19–27. [Google Scholar] [CrossRef]
- Armstrong, CL and Trapp, M and Peters, J and Seydel, T and Rheinstädter, MC Short range ballistic motion in fluid lipid bilayers studied by quasi-elastic neutron scattering. Soft Matter 2011, 18, 8358–8362.
- Rheinstädter, M.C.; Seydel, T.; Demmel, F.; Salditt, T. Molecular motions in lipid bilayers studied by the neutron backscattering technique. Phys. Rev. E 2005, 71, 061908. [Google Scholar] [CrossRef]
- Rheinstädter, M.C.; Ollinger, C.; Fragneto, G.; Salditt, T. Collective dynamics in phospholipid bilayers investigated by inelastic neutron scattering: Exploring the dynamics of biological membranes with neutrons. Physica B 2004, 350, 136–139. [Google Scholar] [CrossRef]
- Katsaras, J.; Epand, R.F.; Epand, R.M. Absence of chiral domains in mixtures of dipalmitoylphosphatidylcholine molecules of opposite chirality. Phys. Rev. E 1997, 55, 3751–3753. [Google Scholar] [CrossRef]
- Mabrey, S.; Sturtevant, J. Investigation of phase transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA 1976, 73, 3862–3866. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Alsop, R.J.; Schmalzl, K.; Epand, R.M.; Rheinstädter, M.C. Strong Static Magnetic Fields Increase the Gel Signal in Partially Hydrated DPPC/DMPC Membranes. Membranes 2015, 5, 532-552. https://doi.org/10.3390/membranes5040532
Tang J, Alsop RJ, Schmalzl K, Epand RM, Rheinstädter MC. Strong Static Magnetic Fields Increase the Gel Signal in Partially Hydrated DPPC/DMPC Membranes. Membranes. 2015; 5(4):532-552. https://doi.org/10.3390/membranes5040532
Chicago/Turabian StyleTang, Jennifer, Richard J. Alsop, Karin Schmalzl, Richard M. Epand, and Maikel C. Rheinstädter. 2015. "Strong Static Magnetic Fields Increase the Gel Signal in Partially Hydrated DPPC/DMPC Membranes" Membranes 5, no. 4: 532-552. https://doi.org/10.3390/membranes5040532
APA StyleTang, J., Alsop, R. J., Schmalzl, K., Epand, R. M., & Rheinstädter, M. C. (2015). Strong Static Magnetic Fields Increase the Gel Signal in Partially Hydrated DPPC/DMPC Membranes. Membranes, 5(4), 532-552. https://doi.org/10.3390/membranes5040532