Co-Utilization of Glucose and Xylose for Enhanced Lignocellulosic Ethanol Production with Reverse Membrane Bioreactors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Performance of the Integrated Permeate Channel (IPC) Flat Sheet Membrane as rMBRs for Ethanol Production
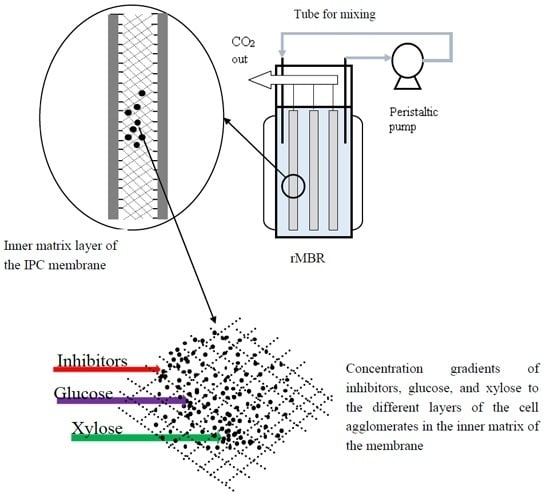
2.2. Simultaneous Utilization of Glucose and Xylose in a Synthetic Medium with the rMBR



2.3. Co-Utilization of Sugars and In Situ Detoxification Using the Liquid Fraction of the Lignocellulosic Hydrolyzate with the rMBR


3. Experimental Section
3.1. Lignocellulosic Material
| Component | Concentration (g/L) | |
|---|---|---|
| Monomeric sugars | Xylose | 33.4 |
| Glucose | 8.5 | |
| Mannose | 1.5 | |
| Arabinose | 4.9 | |
| Galactose | 3.1 | |
| Inhibitors | Acetic acid | 8.9 |
| HMF | 1.1 | |
| Furfural | 9.2 | |
3.2. Enzymes and Yeast Strain
3.3. Cell Cultivation for the rMBR
3.4. Flat Sheet Integrated Permeate Channels (IPC) as the rMBR
3.5. Configuration of the Reverse Membrane Bioreactor (rMBR)
3.6. Synthetic Medium Fermentation with the rMBR
3.7. Fermentation of the Liquid Fraction of the Hydrolyzed Pretreated Wheat Straw with the rMBR
3.8. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farrell, A.E.; Plevin, R.J.; Turner, B.T.; Jones, A.D.; O’Hare, M.; Kammen, D.M. Ethanol can contribute to energy and environmental goals. Science 2006, 311, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Bertilsson, M.; Liden, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Ishola, M.M.; Babapour, A.B.; Gavitar, M.N.; Brandberg, T.; Taherzadeh, M.J. Effect of high solids loading on bacterial contamination in lignocellulosic ethanol production. Bioresources 2013, 8, 4429–4439. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. An overview of existing individual unit operations. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels, 1st ed.; Qureshi, N., Hodge, D., Vertes, A.A., Eds.; Elsevier: Cambridge, UK, 2014; pp. 3–36. [Google Scholar]
- Ishola, M.M.; Taherzadeh, M.J. Effect of fungal and phosphoric acid pretreatment on ethanol production from oil palm empty fruit bunches (OPEFB). Bioresour. Technol. 2014, 165, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Zaldivar, J.; Nielsen, J.; Olsson, L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001, 56, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, M.; Olofsson, K.; Liden, G. Prefermentation improves xylose utilization in simultaneous saccharification and co-fermentation of pretreated spruce. Biotechnol. Biofuels 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Ishola, M.M.; Branbdberg, T.; Taherzadeh, M.J. Simultaneous glucose and xylose utilization for improved ethanol production from lignocellulosic biomass through SSFF with encapsulated yeast. Biomass Bioenergy 2015, 77, 192–199. [Google Scholar] [CrossRef]
- Kotter, P.; Ciriacy, M. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 776–783. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Karhumaa, K.; Fonseca, C.; Spencer-Martins, I.; Gorwa-Grauslund, M.F. Towards industrial pentose-fermenting yeast strains. Appl. Microbiol. Biotechnol. 2007, 74, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Park, YC.; Jin, YS.; Seo, J.H. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism. Biotechnol. Adv. 2013, 31, 51–861. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.; Bonander, N.; Taherzadeh, M.; Franzen, C. Improved sugar co-utilisation by encapsulation of a recombinant Saccharomyces cerevisiae strain in alginate-chitosan capsules. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.O.; Manikondu, R.B.; Franzén, C.J.; Taherzadeh, M.J. Encapsulation-induced stress helps saccharomyces cerevisiae resist convertible lignocellulose derived inhibitors. Int. J. Mol. Sci. 2012, 13, 11881–11894. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.O. Ethanol Production from Lignocellulose Using High Local Cell Density Yeast Cultures. Investigations of Flocculation and encapsulated Saccharomyces cerevisiae. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 19 February 2014. [Google Scholar]
- Ishola, M.M. Novel Application of Membrane Bioreactors in Lignocellulosic Ethanol Production: Simultaneous Saccharification, Filtration and Fermentation (SSFF). Ph.D. Thesis, University of Borås, Borås, Sweden, 31 October 2014. [Google Scholar]
- Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Ethanol production at elevated temperatures using encapsulation of yeast. J. Biotechnol. 2011, 156, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.; Judd, C. The MBR book. In Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2011. [Google Scholar]
- Guglielmi, G.; Andreottola, G. Selection and Design of Membrane Bioreactors in Environmental Bioengineering. In Environmental Biotechnology; Wang, L.K., Ivanov, V., Tay, J.H., Eds.; Humana Press: New York, NY, USA, 2010; Volume 10, pp. 439–516. [Google Scholar]
- Ylitervo, P.; Franzen, C.J.; Taherzadeh, M.J. Impact of furfural on rapid ethanol production using a membrane bioreactor. Energies 2013, 6, 1604–1617. [Google Scholar] [CrossRef]
- Ylitervo, P.; Franzen, J.C.; Taherzadeh, M.J. Continuous ethanol production with a membrane bioreactor at high acetic acid concentration. Membranes 2014, 4, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Ishola, M.M.; Jahandideh, A.; Haidarian, B.; Brandberg, T.; Taherzadeh, M.J. Simultaneous saccharification, filtration and fermentation (SSFF): A novel method for bioethanol production from lignocellulosic biomass. Bioresour. Technol. 2013, 133, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Luyten, K.; Albertyn, J.; Skibbe, W.F.; Prior, B.A.; Ramos, J.; Thevelein, J.M.; Hohmann, S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995, 14, 1360–1371. [Google Scholar] [PubMed]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Niklasson, C.; Liden, G. Acetic acid—Friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem. Eng. Sci. 1997, 52, 2653–2659. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Taherzadeh, M.J. Ethanol from Lignocellulose: Physiological Effects of Inhibitors and Fermentation Strategies. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 28 May 1999. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities. In Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Doyen, W.; Mues, W.; Molenberghs, B.; Cobben, B. Spacer fabric supported flat sheet membranes: A new era of flat-sheet membrane technology. Desalination 2010, 250, 1078–1082. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishola, M.M.; Ylitervo, P.; Taherzadeh, M.J. Co-Utilization of Glucose and Xylose for Enhanced Lignocellulosic Ethanol Production with Reverse Membrane Bioreactors. Membranes 2015, 5, 844-856. https://doi.org/10.3390/membranes5040844
Ishola MM, Ylitervo P, Taherzadeh MJ. Co-Utilization of Glucose and Xylose for Enhanced Lignocellulosic Ethanol Production with Reverse Membrane Bioreactors. Membranes. 2015; 5(4):844-856. https://doi.org/10.3390/membranes5040844
Chicago/Turabian StyleIshola, Mofoluwake M., Päivi Ylitervo, and Mohammad J. Taherzadeh. 2015. "Co-Utilization of Glucose and Xylose for Enhanced Lignocellulosic Ethanol Production with Reverse Membrane Bioreactors" Membranes 5, no. 4: 844-856. https://doi.org/10.3390/membranes5040844