Apparent Interfacial Tension Effects in Protein Stabilized Emulsions Prepared with Microstructured Systems
Abstract
:1. Introduction
- Localized shear forces: break-up due to the shear forces exerted on a droplet coming close to a branching channel, or due to divergent flow in both legs of a branching.
- Interfacial tension effects: break-up due to deformation inside the channel because of the channel geometry.
- Steric hindrance between the droplets: droplets accumulating before the membrane can influence each other and induce break-up.
2. Materials and Methods
2.1. Premix Membrane Emulsification
2.1.1. Materials
2.1.2. Membranes and Membrane Characterization
2.1.3. Experimental Set-Up and Procedure
2.1.4. Emulsion Characterization
2.2. Microfluidic Y-Junctions
2.2.1. Materials
2.2.2. Experimental Setup
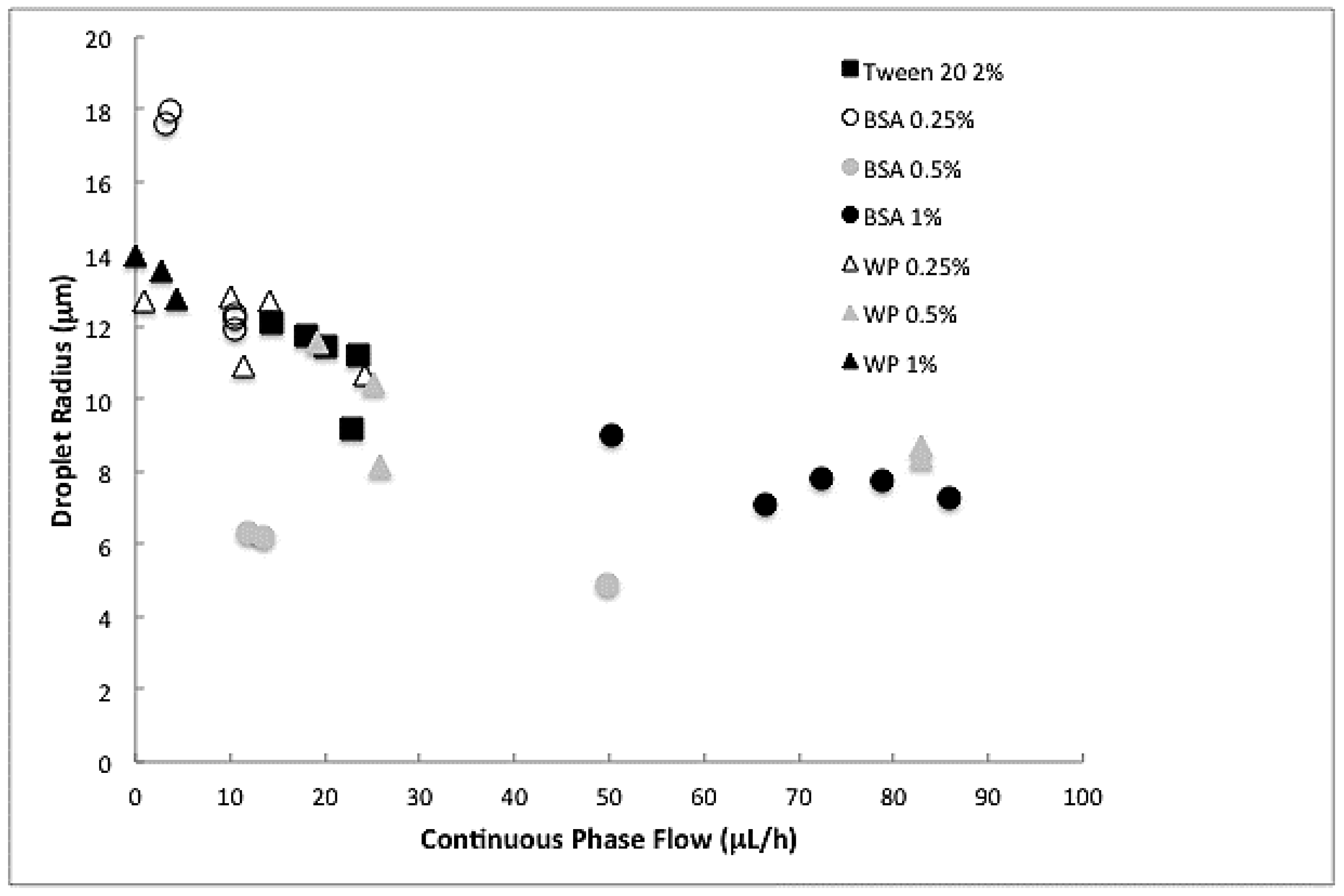
2.2.3. Analysis
3. Results and Discussion
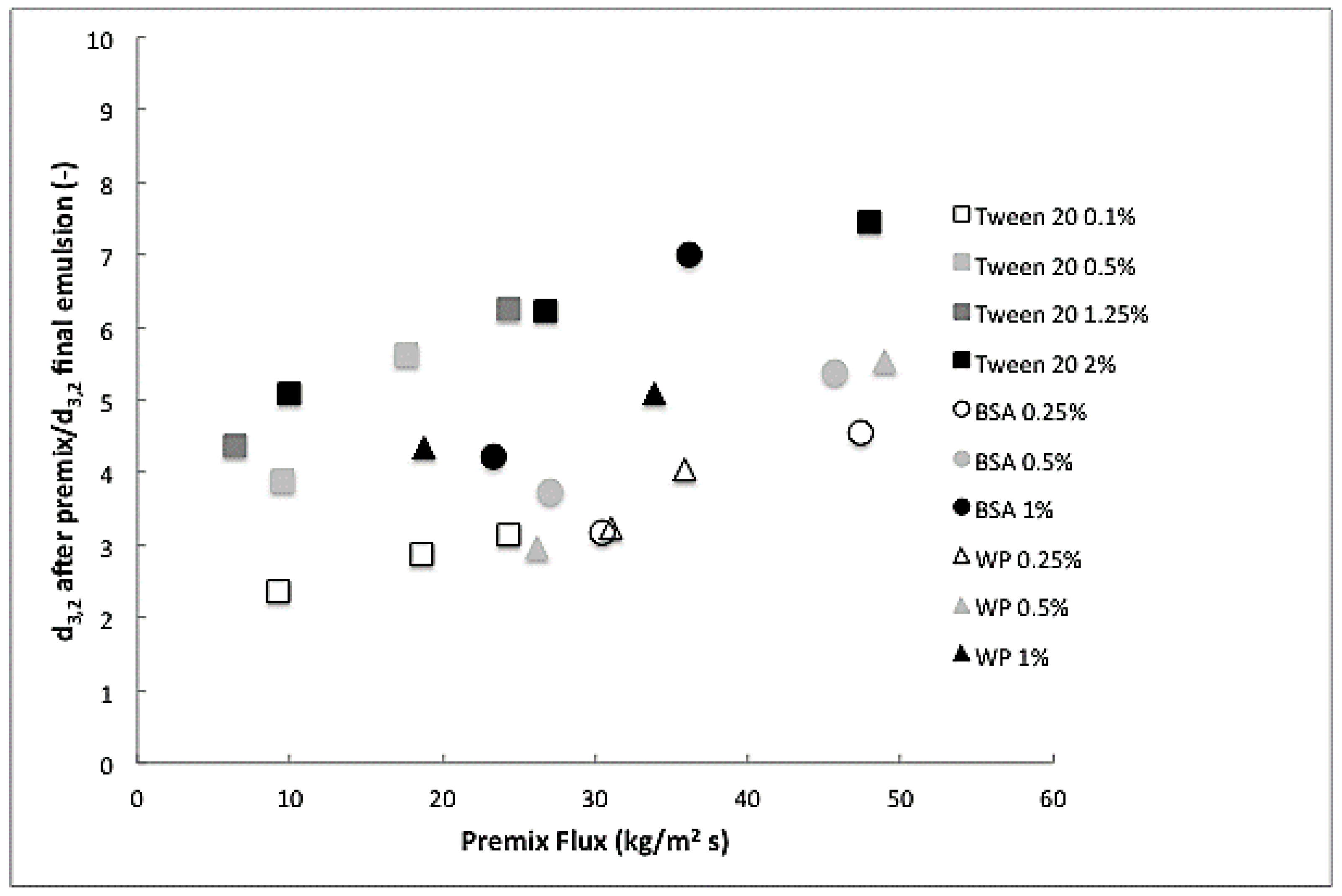
3.1. Droplet Size Reduction after Premix Membrane Emulsification
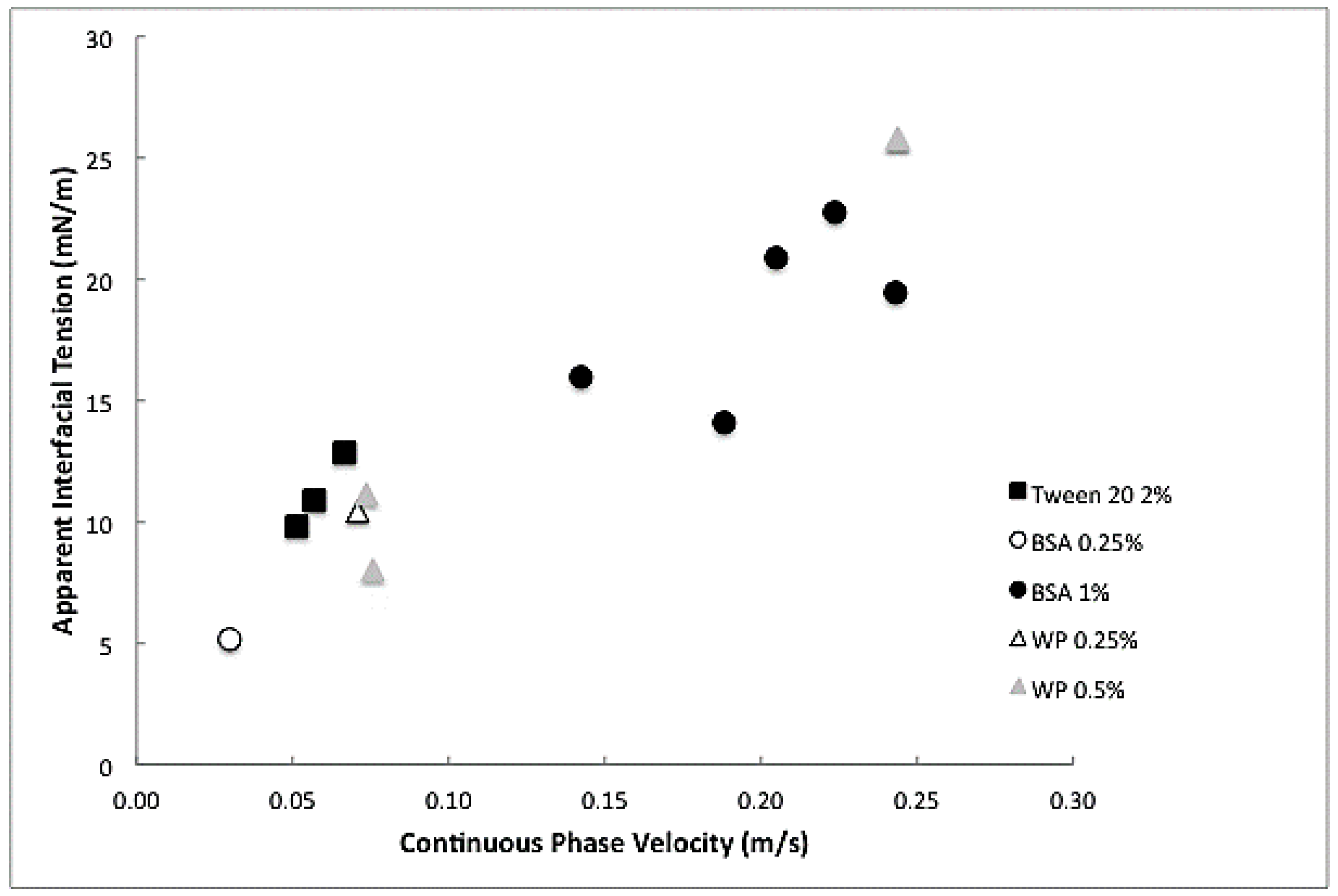
3.2. Apparent Interfacial Tension Measurement
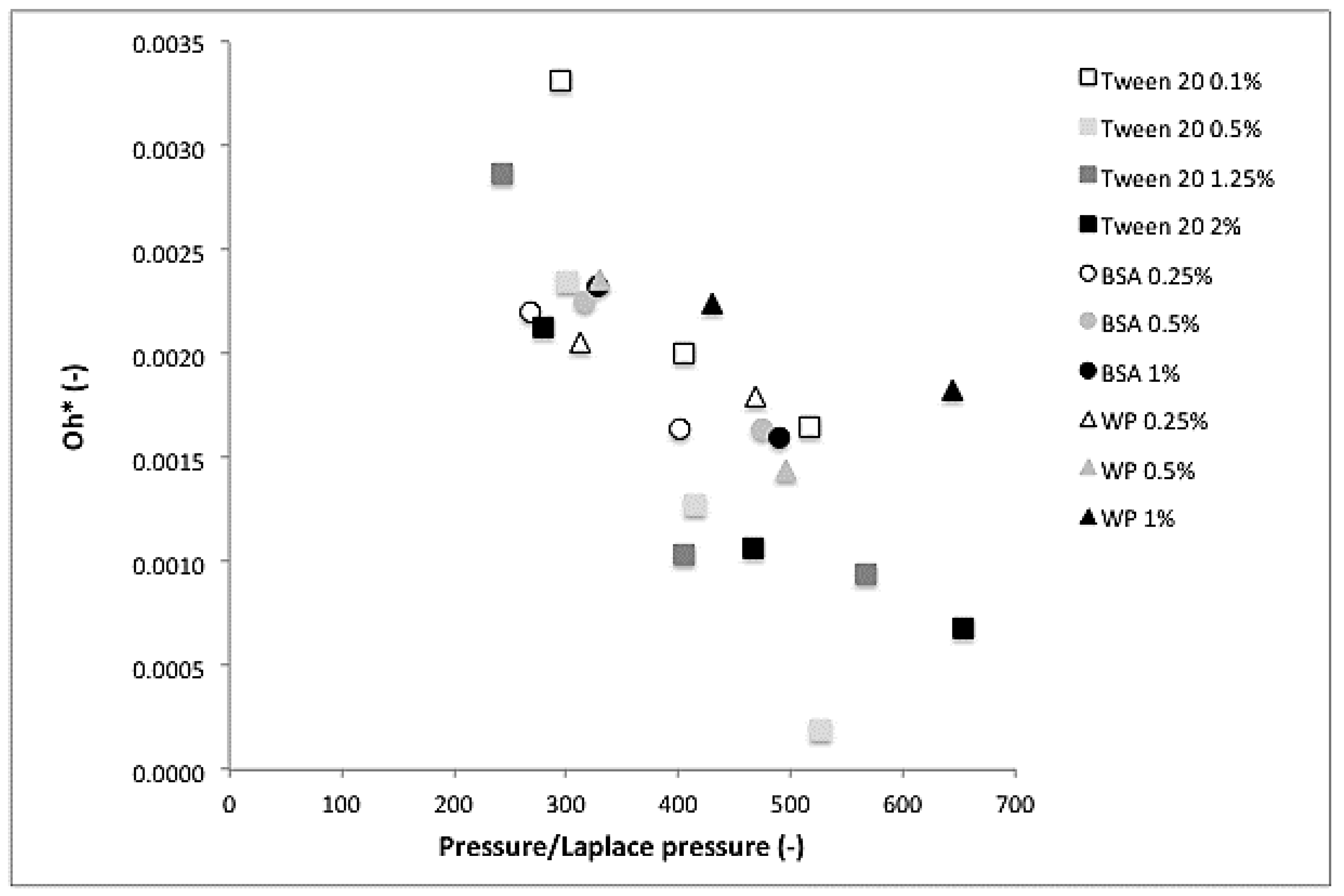
3.3. Dimensionless Numbers
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Charcosset, C. Preparation of emulsions and particles by membrane emulsification for the food processing industry. J. Food Eng. 2009, 92, 241–249. [Google Scholar] [CrossRef]
- Schröder, V.; Schubert, H. Production of emulsions using microporous, ceramic membranes. Colloids Surf. Physicochem. Eng. Asp. 1999, 152, 103–109. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Kobayashi, I.; Nakajima, M. Production of uniform droplets using membrane, microchannel and microfluidic emulsification devices. Microfluid. Nanofluid. 2012, 13, 151–178. [Google Scholar] [CrossRef]
- Schroën, K.; Bliznyuk, O.; Muijlwijk, K.; Sahin, S.; Berton-Carabin, C.C. Microfluidic emulsification devices: From micrometer insights to large-scale food emulsion production. Curr. Opin. Food Sci. 2015, 3, 33–40. [Google Scholar] [CrossRef]
- Van der Graaf, S.; Steegmans, M.L.J.; van der Sman, R.G.M.; Schroën, C.G.P.H.; Boom, R.M. Droplet formation in a T-shaped microchannel junction: A model system for membrane emulsification. Colloids Surf. Physicochem. Eng. Asp. 2005, 266, 106–116. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Williams, R.A. Recent developments in manufacturing emulsions and particulate products using membranes. Adv. Colloid Interface Sci. 2005, 113, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vladisavljevic, G.T.; Shimizu, M.; Nakashima, T. Production of multiple emulsions for drug delivery systems by repeated SPG membrane homogenization: Influence of mean pore size, interfacial tension and continuous phase viscosity. J. Membr. Sci. 2006, 284, 373–383. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix emulsification: A review. J. Membr. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Güell, C.; Ferrando, M.; Schroën, K. Membranes for enhanced emulsification processes. In Innovative Food Processing Technologies. Extraction, Separation, Component Modification, and Process Intensification; Knoerzer, K., Juliano, P., Smithers, G.W., Eds.; Elsevier Science: San Diego, CA, USA, 2016; pp. 429–454. [Google Scholar]
- De Menech, M.; Garstecki, P.; Jousse, F.; Stone, H. Transition from squeezing to dripping in a microfluidic T-shaped junction. J. Fluid Mech. 2008, 595, 141–161. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic t-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Tice, J.D.; Lyon, A.D.; Ismagilov, R.F. Effects of viscosity on droplet formation and mixing in microfluidic channels. Anal. Chim. Acta 2004, 507, 73–77. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using ‘flow focusing’ in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Garstecki, P.; Gitlin, I.; DiLuzio, W.; Whitesides, G.M.; Kumacheva, E.; Stone, H.A. Formation of monodisperse bubbles in a microfluidic flow-focusing device. Appl. Phys. Lett. 2004, 85, 2649–2651. [Google Scholar] [CrossRef]
- Utada, A.S.; Fernandes-Nieves, A.F.; Stone, H.A.; Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Boom, R.M.; Schroen, K. Influence of the emulsion formulation in premix emulsification using packed beds. Chem. Eng. Sci. 2014, 116, 547–557. [Google Scholar] [CrossRef]
- Van der Zwan, E.A.; Schroën, C.G.P.H.; van Dijke, K.C.; Boom, R.M. Visualization of droplet break-up in pre-mix membrane emulsification using microfluidic devices. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 223–229. [Google Scholar] [CrossRef]
- Janssen, L.; Warmoeskerken, M. Transport Phenomena Data Companion, 2nd ed.; Edward Arnold: London, UK; Delftse Uitgevers Maatschappij: Delft, The Netherlands, 1987. [Google Scholar]
- Muijlwijk, K.; Huang, W.; Vuist, J.-E.; Berton-Carabin, C.; Schroen, K. Convective mass transport dominates surfactant adsorption in a microfluidic Y-junction. Soft Matter 2016, 12, 9025–9029. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.A.; van Vliet, T. Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Adv. Colloid Interface Sci. 2001, 437–471. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. food Sci. food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Muijlwijk, K.; Hinderink, E.; Ershov, D.; Berton-Carabin, C.; Schroën, K. Interfacial tension measured at high expansion rates and within milliseconds using microfluidics. J. Colloid Interface Sci. 2016, 470, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.J.M.; Boon, A.; Agterof, W.G.M. Influence of dynamic interfacial properties on droplet breakup in simple shear flow. AIChE J. 1994, 40, 1929–1939. [Google Scholar] [CrossRef]
- Schröder, V.; Behrend, O.; Schubert, H. Effect of Dynamic Interfacial Tension on the Emulsification Process Using Microporous, Ceramic Membranes. J. Colloid Interface Sci. 1998, 202, 334–340. [Google Scholar] [CrossRef]
- Eggleton, C.D.; Tsai, T.M.; Stebe, K.J. Tip streaming from a drop in the presence of surfactants. Phys. Rev. Lett. 2001, 87, 48302. [Google Scholar] [CrossRef] [PubMed]
- Steegmans, M.L.J.; Warmerdam, A.; Schroen, K.G.P.H.; Boom, R.M. Dynamic interfacial tension measurements with microfluidic Y-junctions. Langmuir 2009, 25, 9751–9758. [Google Scholar] [CrossRef] [PubMed]
- Scherze, I.; Marzilger, K.; Muschiolik, G. Emulsification using micro porous glass (MPG): Surface behaviour of milk proteins. Colloids Surf. Biointerfaces 1999, 12, 213–221. [Google Scholar] [CrossRef]
- Berendsen, R.; Güell, C.; Henry, O.; Ferrando, M. Premix membrane emulsification to produce oil-in-water emulsions stabilized with various interfacial structures of whey protein and carboxymethyl cellulose. Food Hydrocoll. 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Rossier-Miranda, F.J.; Schroën, K.; Boom, R. Mechanical characterization and pH response of fibril-reinforced microcapsules prepared by layer-by-layer adsorption. Langmuir 2010, 26, 19106–19113. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yin, L.; Kobayashi, I.; Nakajima, M. Preparation characteristics of monodispersed oil-in-water emulsions with large particles stabilized by proteins in straight-through microchannel emulsification. Food Hydrocoll. 2005, 19, 745–751. [Google Scholar] [CrossRef]
- Trentin, A.; de Lamo, S.; Güell, C.; López, F.; Ferrando, M. Protein-stabilized emulsions containing beta-carotene produced by premix membrane emulsification. J. Food Eng. 2011, 106, 267–274. [Google Scholar] [CrossRef]
- Maan, A.A.; Schroen, K.; Boom, R. Spontaneous droplet formation techniques for monodisperse emulsions preparation-Perspectives for food applications. J. Food Eng. 2011, 107, 334–346. [Google Scholar] [CrossRef]
- ISO 9101:1987. Surface Active Agents-Determination of Interfacial Tension-Drop Volume Method; International Organization for Standardization: Geneva, Switzerland, 1987. [Google Scholar]
- Rühs, P.; Scheuble, N.; Windhab, E.; Fischer, P. Protein adsorption and interfacial rheology interfering in dilatational experiment. Eur. Phys. J. Spec. Top. 2013, 222, 47–60. [Google Scholar] [CrossRef]


| Oil Phase | Continuous Phase | Concentration (w/w % of Cont. Phase) | Interfacial Tension (mN/m) |
|---|---|---|---|
| Hexadecane | Tween 20 | 0.10 | 4.2 |
| 0.50 | 3.7 | ||
| 1.25 | 3.4 | ||
| 2.00 | 3.1 | ||
| BSA | 0.25 | 14.6 | |
| 0.50 | 13.6 | ||
| 1.00 | 13.6 | ||
| Whey protein | 0.25 | 12.9 | |
| 0.50 | 12.5 | ||
| 1.00 | 10.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güell, C.; Ferrando, M.; Trentin, A.; Schroën, K. Apparent Interfacial Tension Effects in Protein Stabilized Emulsions Prepared with Microstructured Systems. Membranes 2017, 7, 19. https://doi.org/10.3390/membranes7020019
Güell C, Ferrando M, Trentin A, Schroën K. Apparent Interfacial Tension Effects in Protein Stabilized Emulsions Prepared with Microstructured Systems. Membranes. 2017; 7(2):19. https://doi.org/10.3390/membranes7020019
Chicago/Turabian StyleGüell, Carme, Montserrat Ferrando, Alexandre Trentin, and Karin Schroën. 2017. "Apparent Interfacial Tension Effects in Protein Stabilized Emulsions Prepared with Microstructured Systems" Membranes 7, no. 2: 19. https://doi.org/10.3390/membranes7020019






