1. Introduction
Ammonia (NH
3) emissions from livestock manure are an important source of reactive nitrogen (N) release to the environment, impacting the health of humans and livestock, and resulting in N loss from the farm production cycle negatively affecting profitability. Loss of ammonia decreases the fertilizer value of digestate. Nitrogen fertilizers are then required for crop production, contributing to additional ammonia emissions and adding an economic burden to farmers. Ammonia emissions contribute to eutrophication of surface water bodies, soil acidification, and particulate matter formation [
1]. Ammonia is a precursor to PM
2.5 (particulate matter with an aerodynamic diameter of 2.5 µm or less), which has negative human health impacts [
2]. At the same time, ammonia has a harmful impact on manure treatment processes, such as anaerobic digestion (AD). The AD process digests organic matter to produce renewable energy from the captured methane but in the process, mineralizes nitrogen to ammoniacal form. At high concentrations ammonia can inhibit the methane formation process during AD [
3]. Managing ammonia in manure can result in increased efficiencies in terms of farm productivity and improved efficiency in manure treatment through AD. Moreover, there is a potential win-win if captured ammonia is in a liquid form that could be used as fertilizer for crops, offsetting synthetic fertilizer.
Techniques and technologies to capture ammonia have been developed in attempts to reduce these negative effects in biodigesters, such as acid spray scrubbers, biofilters, acidified clays, and addition of sodium hydrogen sulphate [
4]. Polytetrafluoroethylene (ePTFE) gas permeable membranes (GPM) filled with dilute acids have been successful in capturing ammonia gas from liquid manures (LM) [
5], but have not yet been tested in dairy manure digestate. The ePTFE tubing has microscopic pores and is hydrophobic, allowing only gaseous diffusion. Mass transfer occurs across the membrane when there is a concentration difference, following Fick’s law of diffusion from high to low concentration. Ammonia gas diffuses through the GPM from the LM side into the sulphuric acid side, where it reacts to form aqueous ammonium sulphate, a salt that is also a commercial fertilizer, shown by Equation (1) and
Figure 1.
Multiple parameters influence the flux across the membrane and the capture rate of ammonia, such as, the surface area of the GPM, the flow rate of the acid throughout the GPM, the gaseous ammonia concentration in LM, and the pH of the recipient acid [
5,
6]. Increasing the flow rate of sulphuric acid throughout the GPM has shown to increase the ammonia capture concentration in the acid solution, with an approximately 30% increase from 5.6 mL·min
−1 to 36 mL·min
−1 [
5]. Mukhtar et al. [
7] tested the surface area ratio of GPM to LM and found the optimal ratio to be 1:3.25 to achieve 50% ammonia extraction in less than 20 days. Over time, the pH of the acid solution increases as ammonium salt is formed, while the pH of the LM decreases [
7]. Maintaining the recipient acid at pH of 2 (with an automated dosing pump and concentrated acid) resulted in the greatest removal of ammonia when compared to pH of 3, 4 and 5 [
5].
The concentration differential across the membrane is the driving force of diffusion. Gaseous ammonia concentration is a function of manure characteristics, especially pH, temperature, and TAN concentration, as described by the following equations. The chemical equilibrium between TAN species, ammonia (NH3) and ammonium (NH4+), in aqueous solutions is shown in Equation (2).
This equilibrium shifts towards the ammonia phase at higher pH and temperature. The concentration of unionized ammonia (NH3, right side of Equation (2) is referred to as the concentration of free ammonia nitrogen (FA-N, in mg·L−1), which can be calculated using Equation (3).
where
T is the temperature (°C), and [TAN] is the total ammonia nitrogen concentration (NH
3 and NH
4+, mg·L
−1), and pH is the potential of hydrogen [
8]. Due to the low pH on the acid side of the GPM, ammonia is only present on the LM side and the flux is always directed away from the LM and towards the acid. Ammonia gas and aqueous ammonia within a solution are in equilibrium, as demonstrated by Equation (4a), with the ratio described by Henry’s Law Constant,
KH, as follows in Equation (4b).
The ratio is dependent on temperature and can be calculated using Equation (5), where
T is temperature (K) [
9]:
In the context of extraction of NH
3-N from LM, a higher pH corresponds to a higher FA-N value (Equation (3)), which causes a higher concentration gradient across the membrane. With higher pH in the LM, the removal rate of ammonia increases [
6]. Temperature plays a key role in the activity and concentration of the dissolved and aqueous ammonia, and in the partitioning between ammonia gas and aqueous ammonia (Equation (5)). Higher temperature will result in an equilibrium shift towards higher concentration of gaseous ammonia; however, Henry’s Law constant decreases, and therefore, the ratio of gaseous to aqueous ammonia is lower. Temperature has a more significant impact on the equilibrium shift towards the quantity of ammonia present than on Henry’s law constant, therefore, increasing the temperature will theoretically always increase capture. Transfer rates across the membrane are regulated by the concentration of gaseous free NH
3.
Controlling ammonia within a digester improves methane production [
3]. The digestion process in an anaerobic digester is exothermic and the temperature ranges from 15 °C to 60 °C, depending on reactor type [
10]. The process can also increase the pH of manure to 8 or higher (due to bicarbonate formation during fermentation). Ammonia is excreted by livestock and also results from the digestion of proteins, urea, and nucleic acids in manure [
11]. Concentrations of mineral N in the ammoniacal form reported by Brown [
12] were 2645, 1601 and 5567 ppm for hog, dairy and poultry manures, respectively (with substantial variability associated with dry matter (DM) content). These conditions (high temperature, pH and TAN concentration) within an AD are optimal for ammonia capture. Depending on the type of reactor and the operating conditions, the TAN inhibition occurs from 4000–10,000 mg·L
−1 [
3]. Avoiding these conditions will improve reactor efficiency. Furthermore, when NH
3 concentrations are unchecked, digestate storage leads to high ammonia losses [
13], therefore, capturing ammonia also increases the environmental benefit of AD.
The objectives of this research were to test ePTFE GPM to (1) determine the optimal capture rate conditions considering total ammonium nitrogen (TAN) concentration, temperature, and pH, and (2) to mimic the conditions within a dairy manure anaerobic digester and in so doing, to evaluate whether it could be feasible to adopt within the AD reactor. If successful, GPMs could be implemented into the anaerobic digester cycle, whether within or prior, to reduce the ammonia inhibition of methanogenesis. Research has shown success in the use of GPM for ammonia recovery of anaerobically digested swine manure, but there is a lack of research using dairy manure digestate [
14]. It is hypothesized that with higher pH, temperature, and initial TAN concentration, there will be more capture due to more gaseous ammonia present, and that the conditions within an AD will increase ammonia capture compared to raw manure.
3. Materials and Methods
3.1. Gas-Permeable Membrane
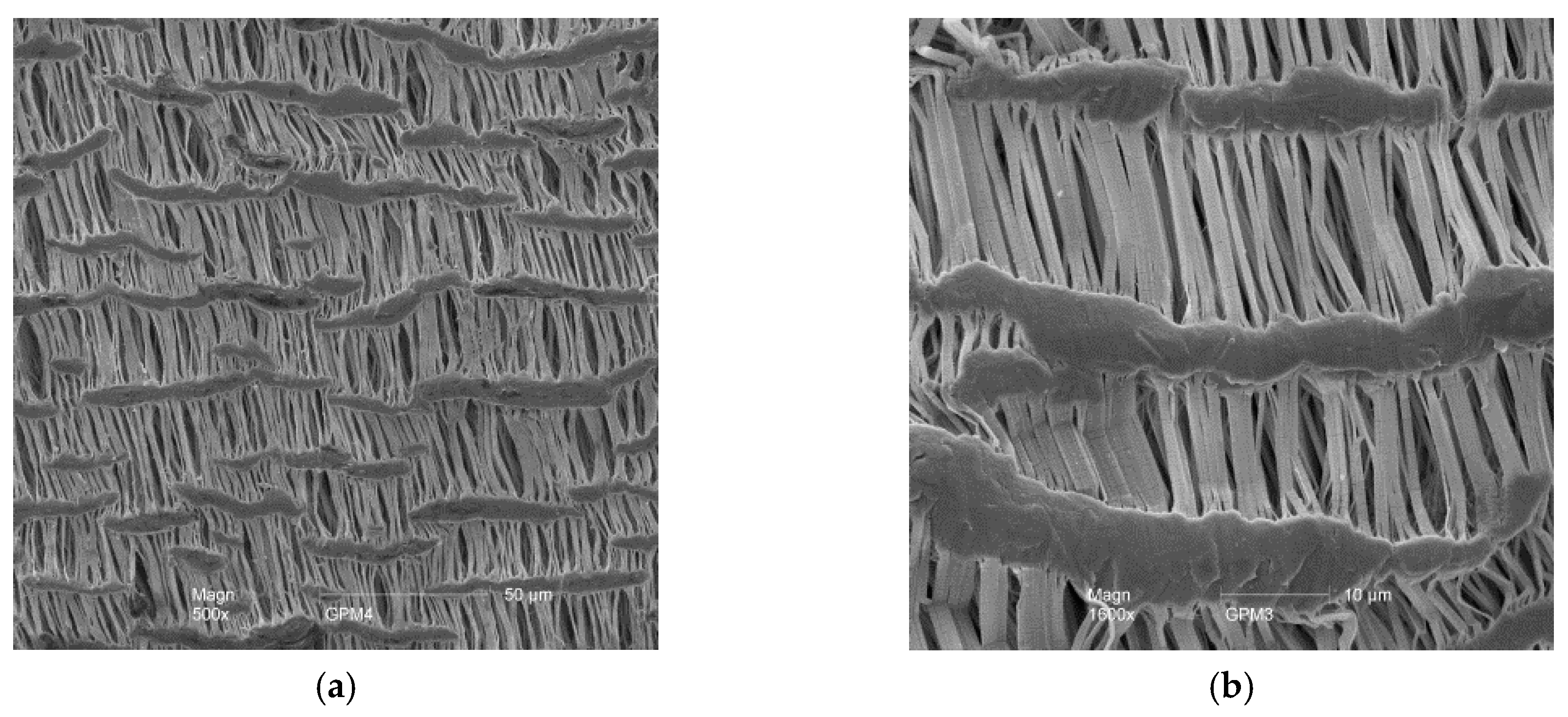
The gas permeable tubular membranes used were made from expanded polytetrafluoroethylene (ePTFE) (FL1001, Phillips Scientific, Mahwah, NJ, USA) (
Table 2). Pore characteristics of the ePTFE were examined with an environmental scanning electron microscope (XL-30 ESEM, Philips Scientific, Mahwah, NJ, USA) (
Figure 5).
3.2. System Configurations
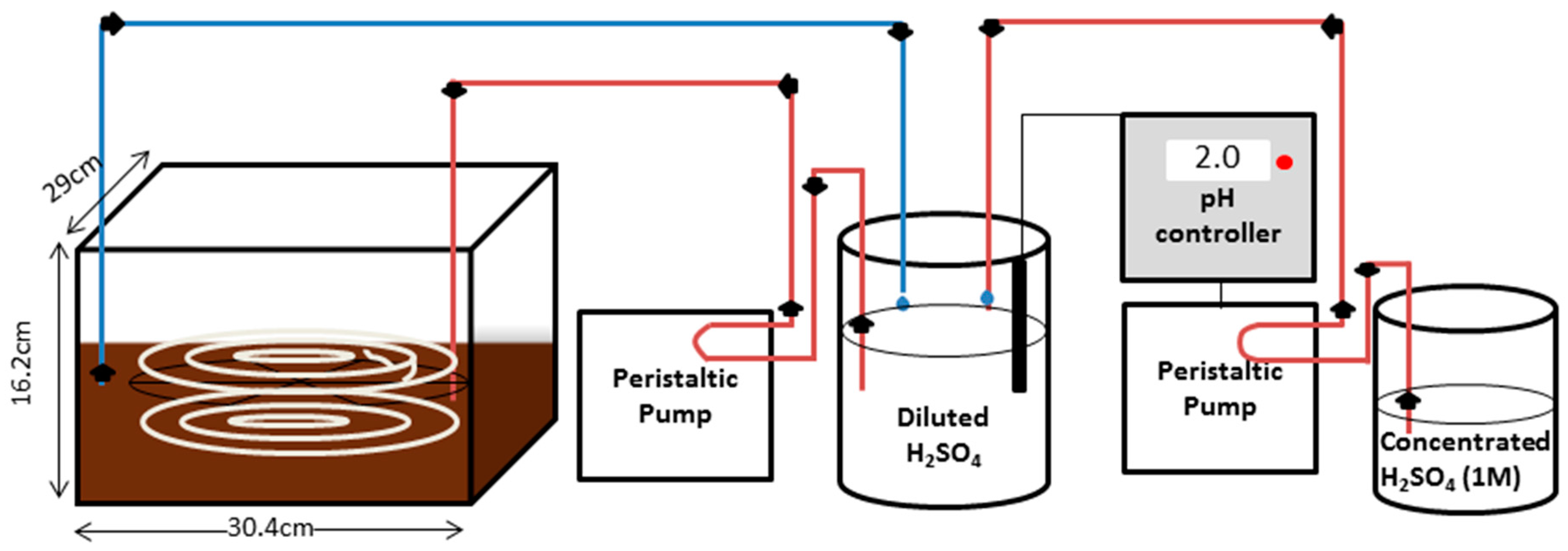
The overall GPM system consisted of two identical 14 L chambers (30.4 cm width, 29 cm length and 16.2 cm height) made of 1/4 Plexiglas which were filled with either 8 L of ammonium chloride solution, 8 L digested dairy manure, or 8 L raw dairy manure (
Figure 6). Aqueous ammonium chloride was used for practical laboratory testing [
15]. The GPM was completely immersed in the liquid in a web-like configuration to optimize the contact area within the chamber. The web configuration was made using a stainless steel circular base with four crossed arms, dividing the circle into eight sections of equal size. The circular base had three levels of duplicate holes on each arm, one with a diameter of 26 cm, one with a diameter of 17.3 cm and one with a diameter of 8.7 cm, all from the same centre point. Cable clips were clipped into the holes, one on the top side of the pair and one on the bottom side. The GPM tubing was then strung through these clips in a spiral configuration. The length of the tubing was held constant at 360 cm for each trial, resulting in a GPM surface area of 905 cm
2.
Tubing throughout the rest of the system was made of Tygon (OD 7 mm, ID 4 mm). Sulphuric acid (0.001 M, initial pH 2) was circulated at a flow rate of 18.2 mL·min−1 from a 1 L closed beaker (#2070350000, Fisher Scientific, Ottawa, ON, Canada) through the immersed GPM tubing and back to the 1 L beaker by means of a Gilson Minipuls 2 multichannel peristaltic pump. The closed beaker contained 800 mL of acid trapping solution, after the tubing was filled (1 L total being circulated). Industrial flat tip pH electrodes (#HI 1006-1007, Hanna Instruments, Laval, QC, Canada) were submerged in each of the 1 L beakers of diluted sulphuric acid. The pH electrodes were connected to controllers (BL 931700, Hanna Instruments, Laval, QC, Canada) that activated peristaltic pumps (Fisher Scientific, Ottawa, ON, Canada) to add concentrated 1M sulphuric acid from a 500 mL beaker when the pH within the 1 L beaker exceeded 3. The pH of the acid trapping solution within both 1 L beakers were logged every half hour using two data loggers (DataSuite MicroLite II LITE5032P-4/20-A, Fourier Technologies, Orlando Park, FL, USA).
Temperature and pH of the ammonium chloride solutions, digested manure, or raw manure, were measured and logged once every half hour by an IntelliCAL pH electrode with a built-in temperature sensor (PHC28101, Hach Company, Loveland, CO, USA) connected to a portable meter (HQ40d53000000, Hach Company, Loveland, CO, USA). The ammonium chloride solutions were brought back to the target pH daily by adding 1 M sodium hydroxide. The pH of manure and digestate was not altered.
Ammonia concentrations were tested daily using the HACH Meter attached with a HACH IntelliCAL ammonia ISE electrode (ISENH318103, Hach, Loveland, CO, USA). The TAN concentrations within the liquid manure were only measured at the start and finish of the trial. The TAN concentrations of the sulphuric acid trapping solution were tested daily in all experiments using the Hach electrode and confirmed by analysing a 15 mL subsample using Flow Injection Analysis [
17]. Daily subsamples were refrigerated at 5 °C until analysis. The TAN concentrations in the ammonium chloride trials were brought back to the target concentration daily, sampling before and after concentration adjustment, to ensure similar conditions throughout the trial.
3.3. Experimental Approach
Eighteen trials were conducted to test the performance of the GPM system and to characterize the effects of changing the three parameters that affect the concentration of aqueous ammonia: temperature, concentration, and pH. Each trial lasted seven days. To represent the characteristics of different manures and digested manures, ammonium chloride solutions were prepared with specific levels of pH and TAN. Ammonium chloride was selected as a “synthetic manure” because it enabled consistency across trials which would not be possible with manure, and it facilitated manipulation of the desired characteristics which would be more difficult to alter using liquid manure. After trends and relationships among variables were observed using ammonium chloride, the system was switched over to using liquid dairy manure and digestate obtained from two commercial dairy farms, each with a biodigester receiving raw manure and co-substrates from other industries.
In the initial experiments, two parameters were held constant while the third was changed to analyse the influence of each separately. The “base conditions” were a pH of 8.3, an initial TAN concentration of 1800 mg N-NH
3 L
−1, and room temperature (21–24 °C). Previous studies have found that pH of the liquid manure (or ammonium chloride) decreased as ammonia was captured and the TAN concentration decreased [
7]. Within a continuously mixed AD, manure would consistently be added and removed at steady state, therefore maintaining near constant concentration and pH. To mimic this, the pH and concentration of ammonium chloride were brought back up to the target each day at a consistent time, using the HACH pH and ammonia probes to determine the amount of NaOH and NH
4Cl to be added to achieve base conditions.
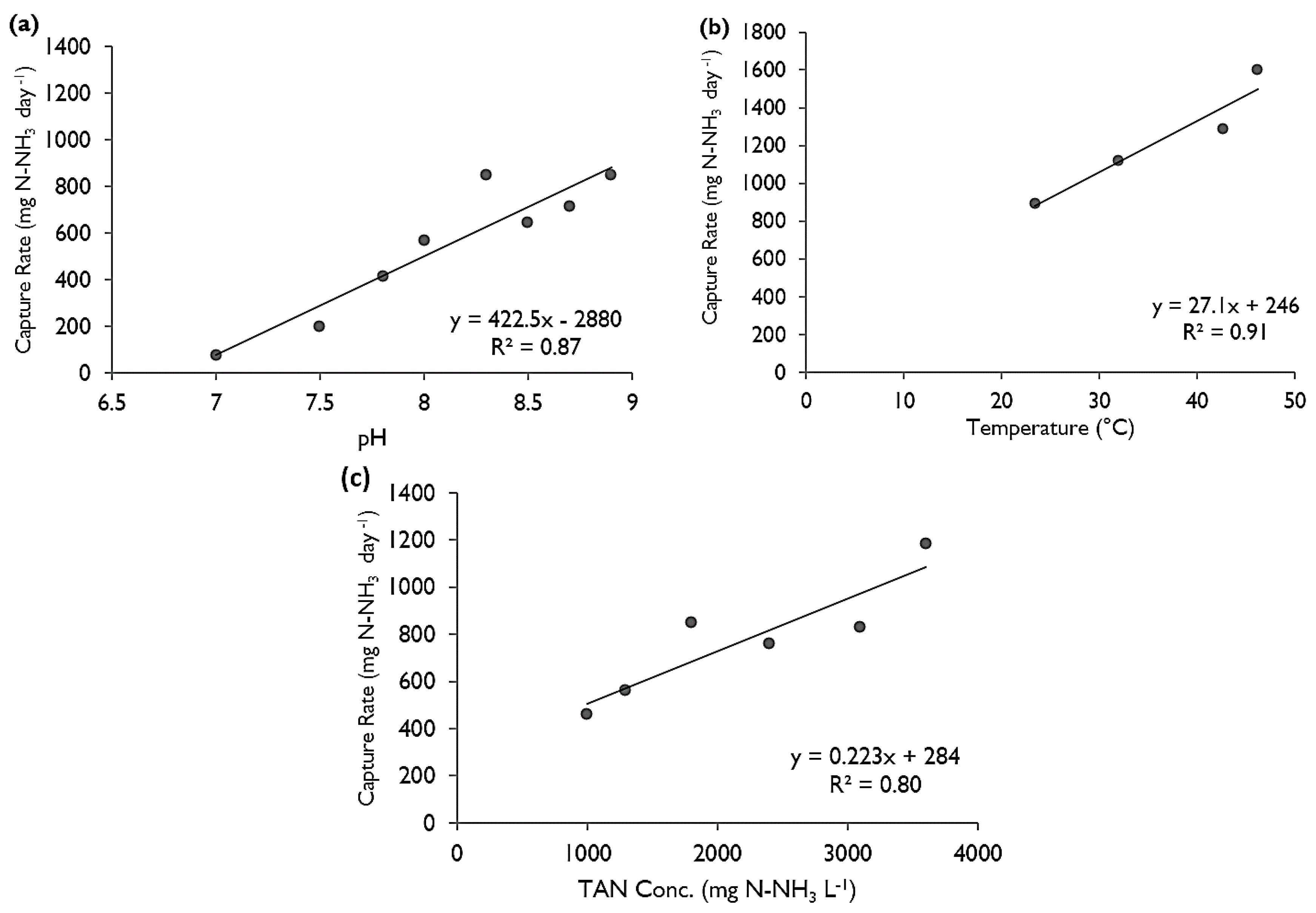
The first parameter that was changed was pH. A trial was conducted representing “normal” manure conditions (i.e., pH 8, room temperature (21–24 °C), and 1800 mg N-NH3 L−1). While keeping temperature and concentration constant, multiple trials were performed to test pH values of 7, through 8.9.
The second parameter changed was temperature. The temperature was increased and maintained with custom-made, programmable heating pads below each chamber that evenly distributed heat over the base of the chamber and a thermistor within the chamber measured the temperature and adjusted the heat supplied. Data from the HACH pH and temperature probe were recorded every half hour, to ensure the temperature was maintained at the target. The pH and concentration for each of the temperature tests were held constant, at 8.3 and 1800 mg N-NH3 L−1 respectively. Temperatures tested were room temperature (22–24 °C), 32, 42.5 and 46.2 °C.
The final parameter tested was concentration. The concentration was increased by simply starting with a stronger ammonium chloride solution. The concentrations tested, while pH and temperature remained constant were 1000, 1400, 1800, 2400, 3200 and 3500 mg N-NH3 L−1.
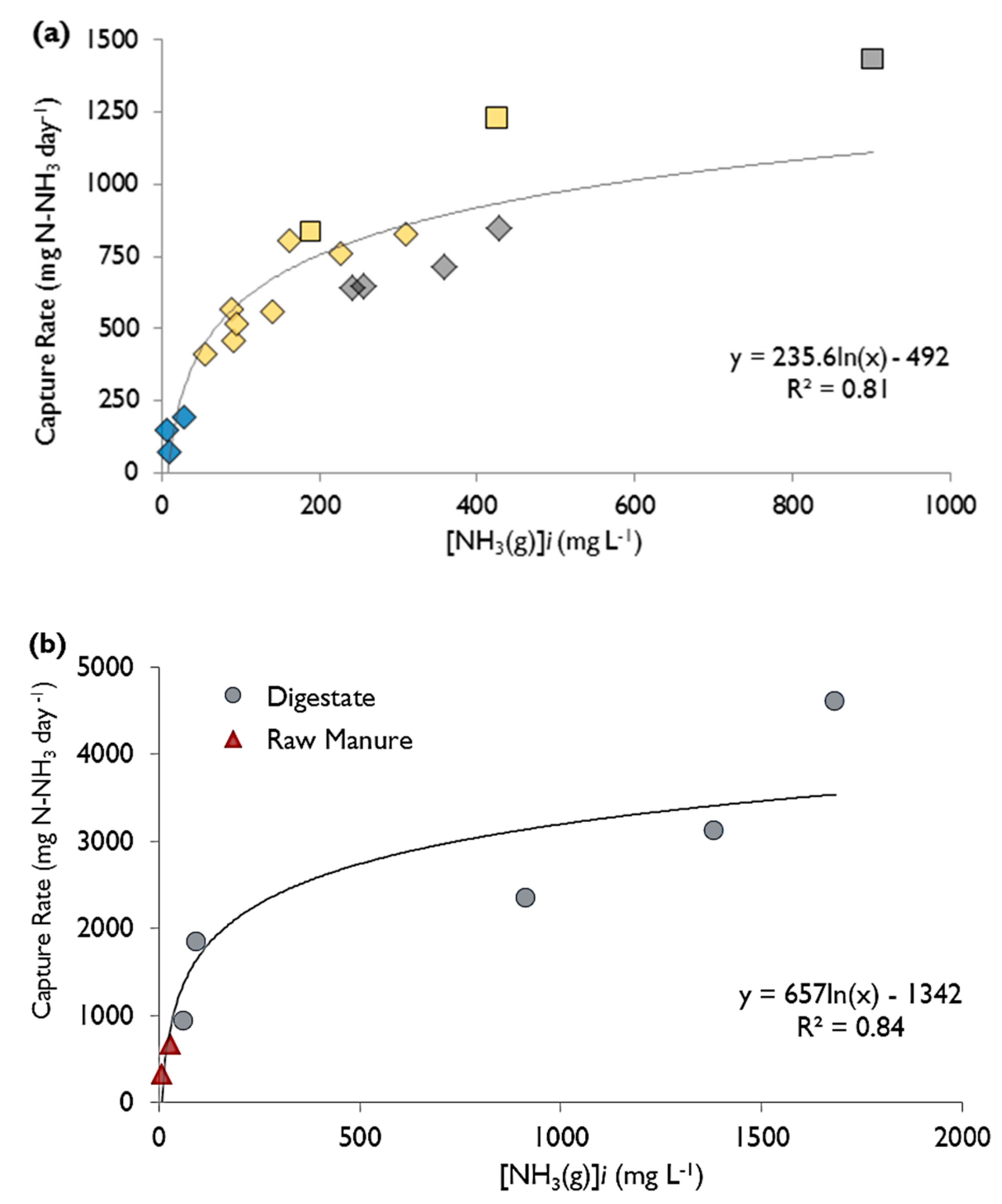
After the above trials using ammonium chloride were conducted, the system was switched from ammonium chloride to liquid manure and digestate to determine whether the findings and trends were transferrable. The procedure changed such that the concentration and pH were left to decrease over the trial (not being adjusted daily) and the ammonia concentration was only tested at the start and end of the trial, while the concentration of ammonia captured was still measured daily. Raw manure was collected twice from the same farm in Eastern Ontario—in one case the characteristics were 1430 mg N-NH3 L−1, pH 7.0 and 8.8% dry matter (DM), and the other case had characteristics 3280 mg N-NH3 L−1, pH 7.2 and 9.2% DM. Digestate was collected from two commercial dairy farms within Ontario, one of which was the same location the raw manure was collected. The digestate was collected on two separate occasions from the first farm (same location as the RM), with the conditions being 1860 mg N-NH3 L−1 with pH 7.65 and 5.0% DM, and 1800 mg N-NH3 L−1 with pH 7.55 and 5.5% DM, respectively. Sufficient digestate was collected from the second farm on one occasion for multiple trials, with the conditions being 6130 mg N-NH3 L−1 with pH 8.5 and 5.2% DM. All manure/digestate was stored at 5°C when not in use. The temperature of the RM and digestate was first run at room temperature for all samples. The digestate was also tested at a temperature of 38 ± 1 °C for samples from both locations.
3.4. Data Analysis
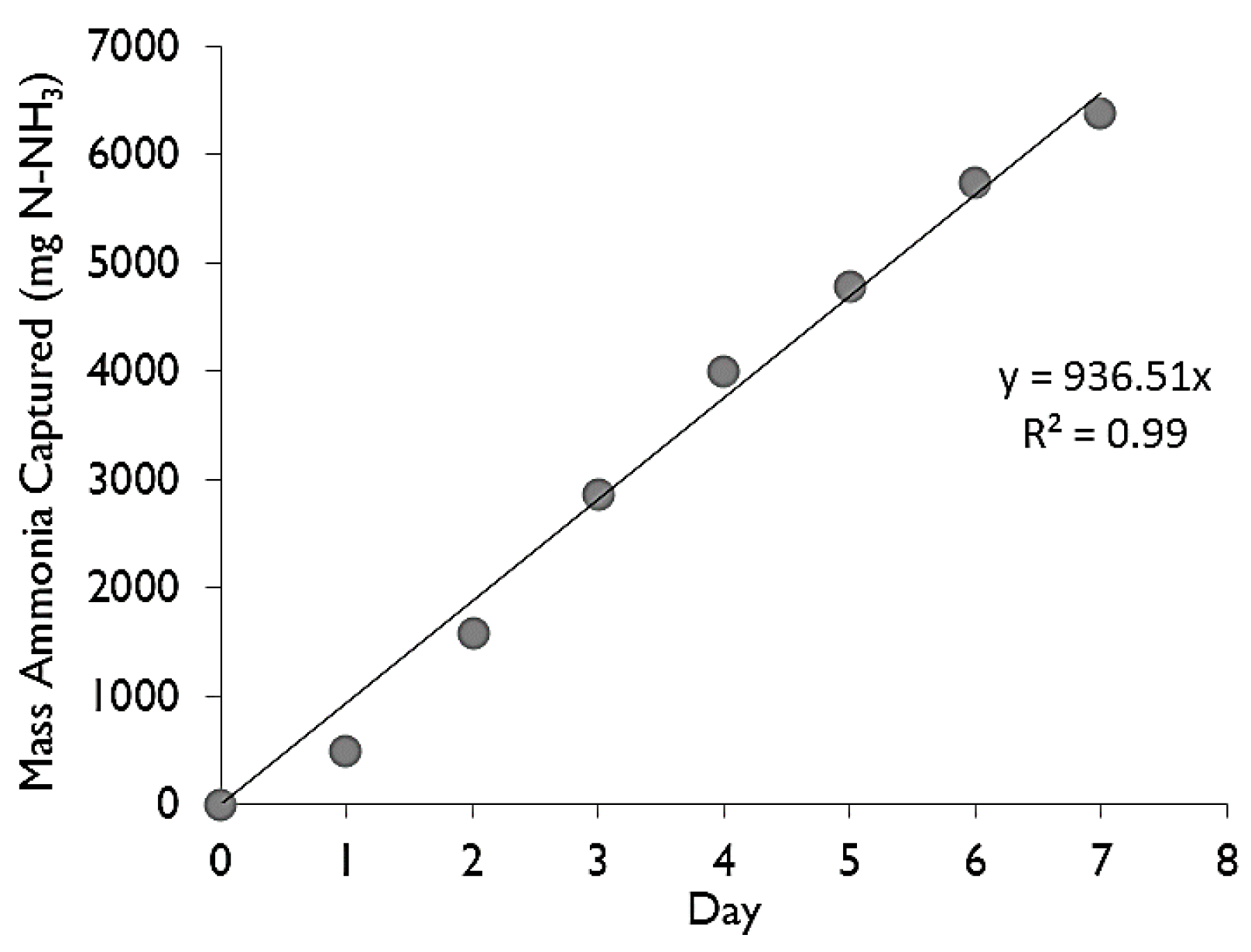
The concentration of the recipient sulfuric acid solution was tested daily at approximately the same time of day. The concentration was converted into mass by multiplying by the volume of the recipient solution. The mass captured versus time (in days) was then plotted for each trial and a linear regression was performed to find the rate at which ammonia was being trapped, hereon termed as the “capture rate” (in mg·day−1). When two of the three parameters were held constant across trials, the third could be compared simply by the change in the trapping rate.
To compare trials of different pH, temperature and concentrations, the concentration of ammonia gas corresponding to the liquid manure/ammonium chloride solution was calculated using Equation (6), which was derived knowing that Henry’s Law constant (KH) is a ratio between NH3(s) and NH3(aq) (Equation (4b)) and that free Ammonia (FA) is the summation of NH3(s) and NH3(aq).
Equation (6) takes into consideration the three variables that affect the concentration and hence the mass transfer across the GPM: temperature, pH and TAN concentration. The ammonia gas concentration for each trial was then calculated and used in regression analysis with the trapping rate. The concentration of ammonia gas can be found for both the LM/ammonium chloride side of the GPM or for the sulphuric acid recipient side. The capture rate (mg·day
−1) was calculated from a linear regression fitted to the trend from the mass captured vs. time (an example can be seen in
Figure 1). Flux (
J) was determined from the mass captured per day divided by the surface area of the GPM tubing, as shown in Equation (7).
where CR is the capture rate (mg·day
−1) of ammonia gas in the trapping solution (sulfuric acid), and
A is the area (m
2) of the gas permeable membrane.
The flux across the membrane can also be described following the mass transfer model expressed in Equation (8) [
5,
9]:
where
J is the mass flux per area (g·m
−2·s
−1),
Km is the mass transfer coefficient, and
C1 and
C2 are the concentrations of the ammonia gas (i.e., [NH
3(g)] from Equation (6)) in the donor solution (LM or ammonium chloride) and the trapping solution (sulfuric acid), respectively. The difference between
C1 and
C2, the concentration gradient, is the driving force of the flux according to Fick’s Law. The mass transfer coefficient,
Km, is dependent on characteristics of the gas permeable membrane, such as porosity and pore size, as well as on the flowrate of dilute acid within the membrane [
6,
16].