Liquid Membranes as a Tool for Chemical Speciation of Metals in Natural Waters: Organic and Inorganic Complexes of Nickel
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Solutions
2.2. Procedures
2.3. Bulk Liquid Membrane System
2.4. Effect of Ligands on the BLM System
3. Results and Discussion
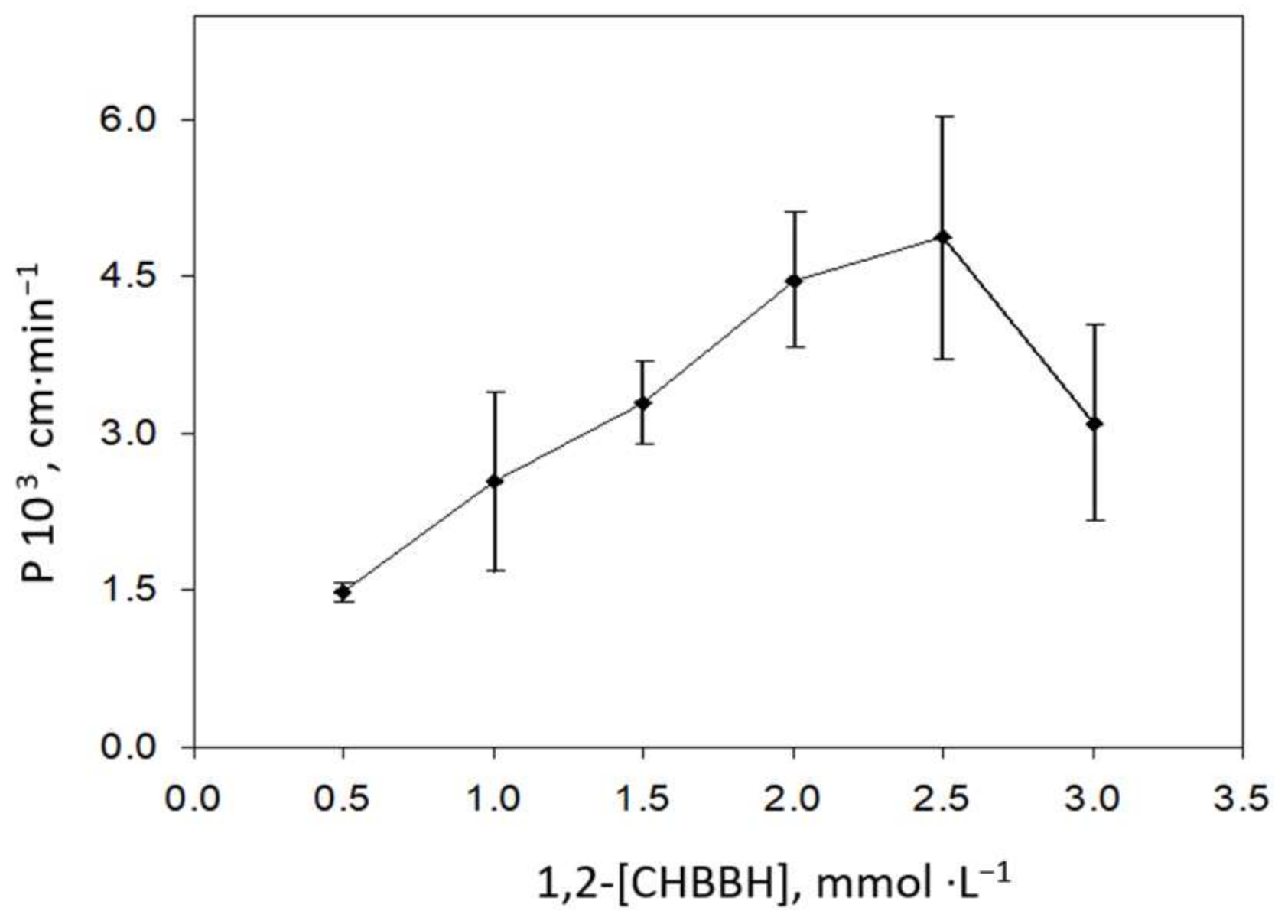
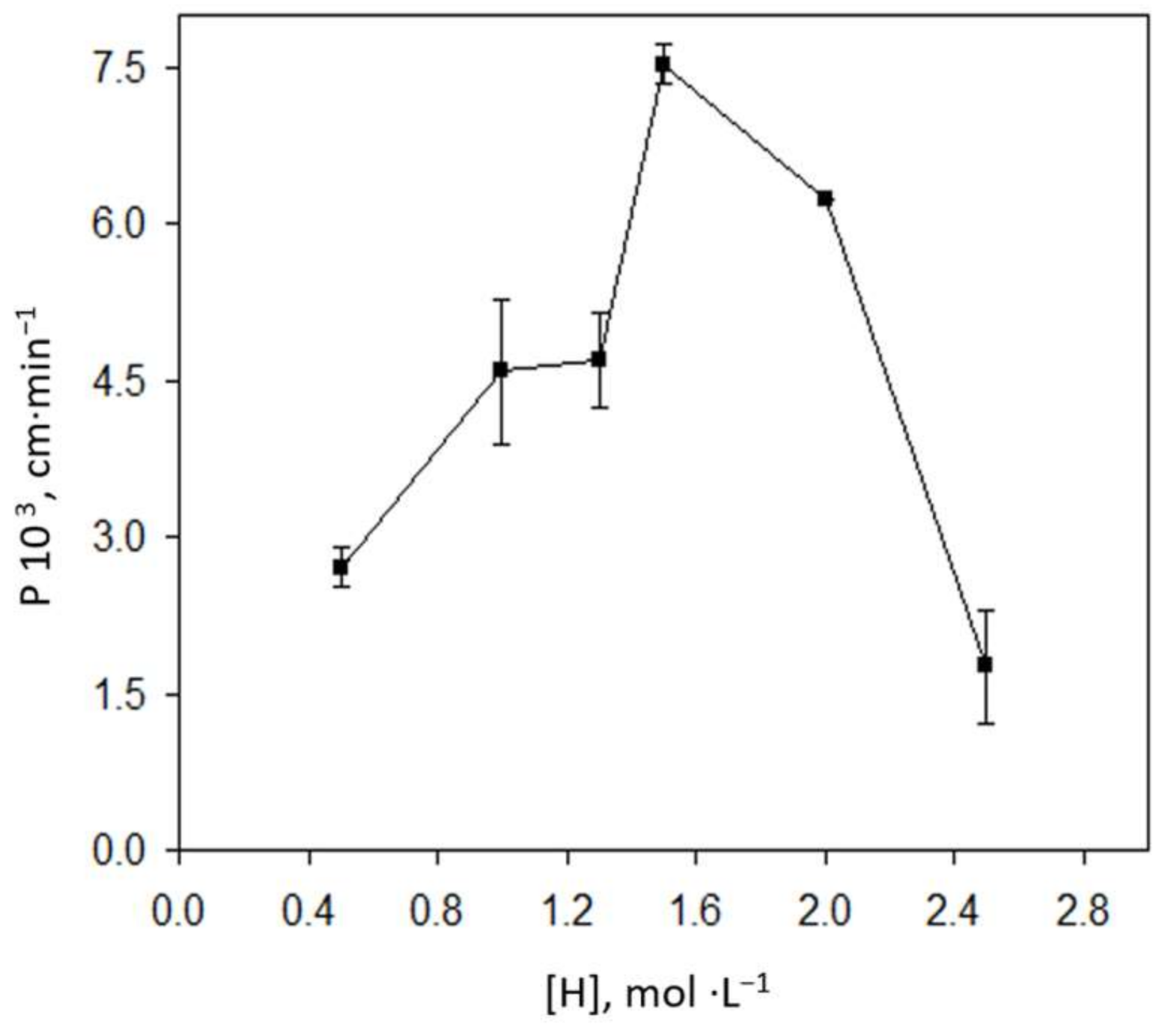
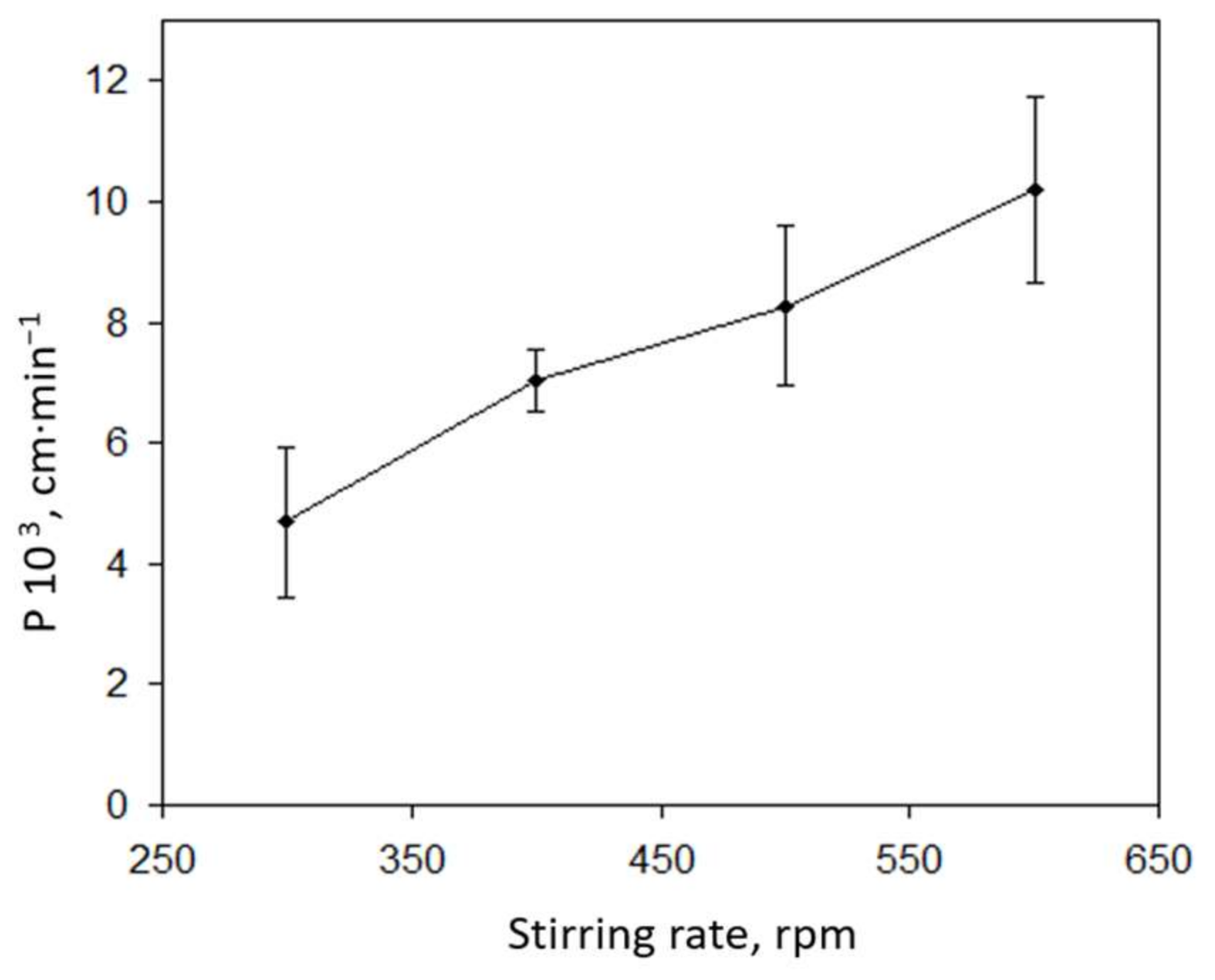
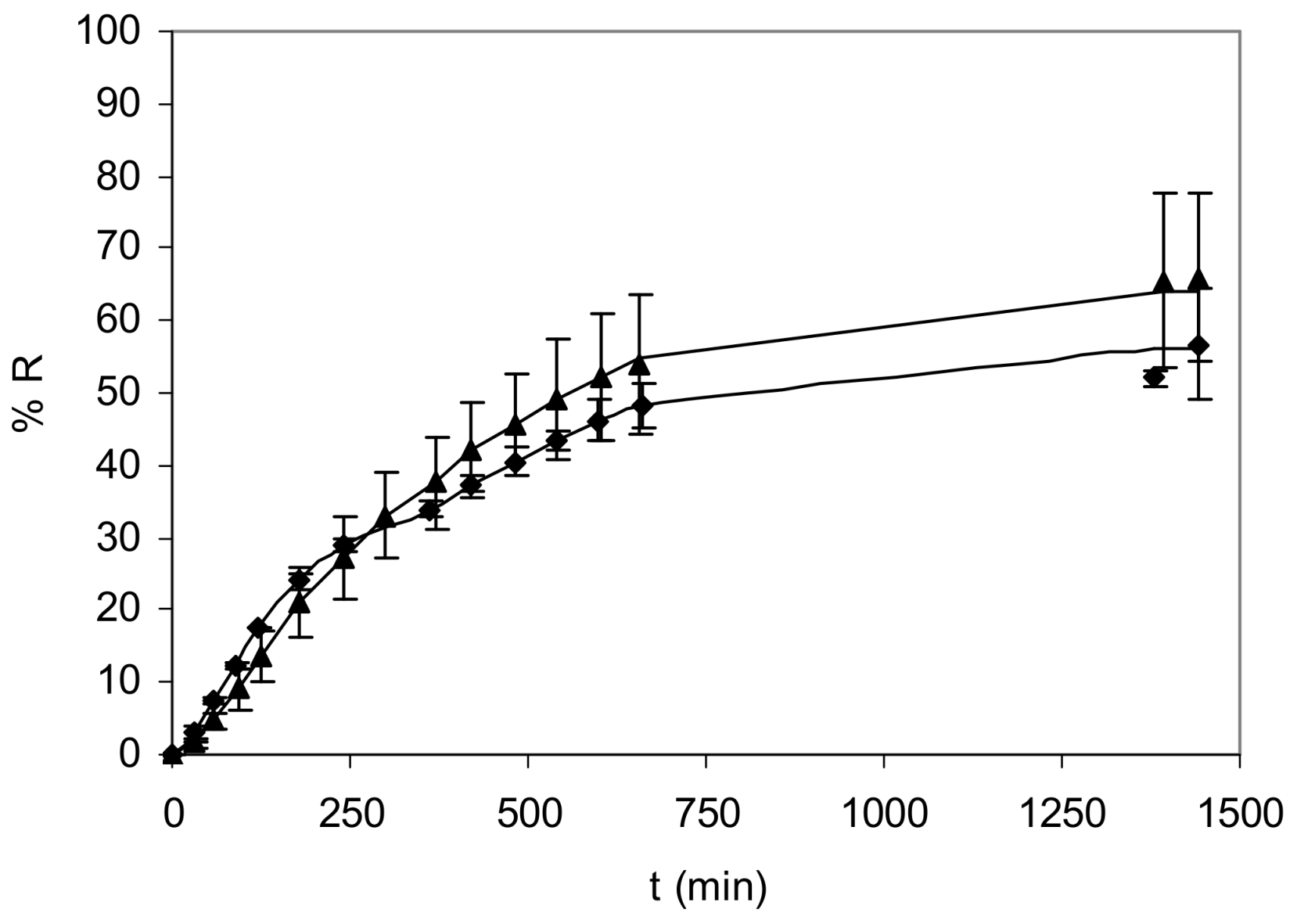
3.1. Optimization of the Bulk Liquid Membrane System
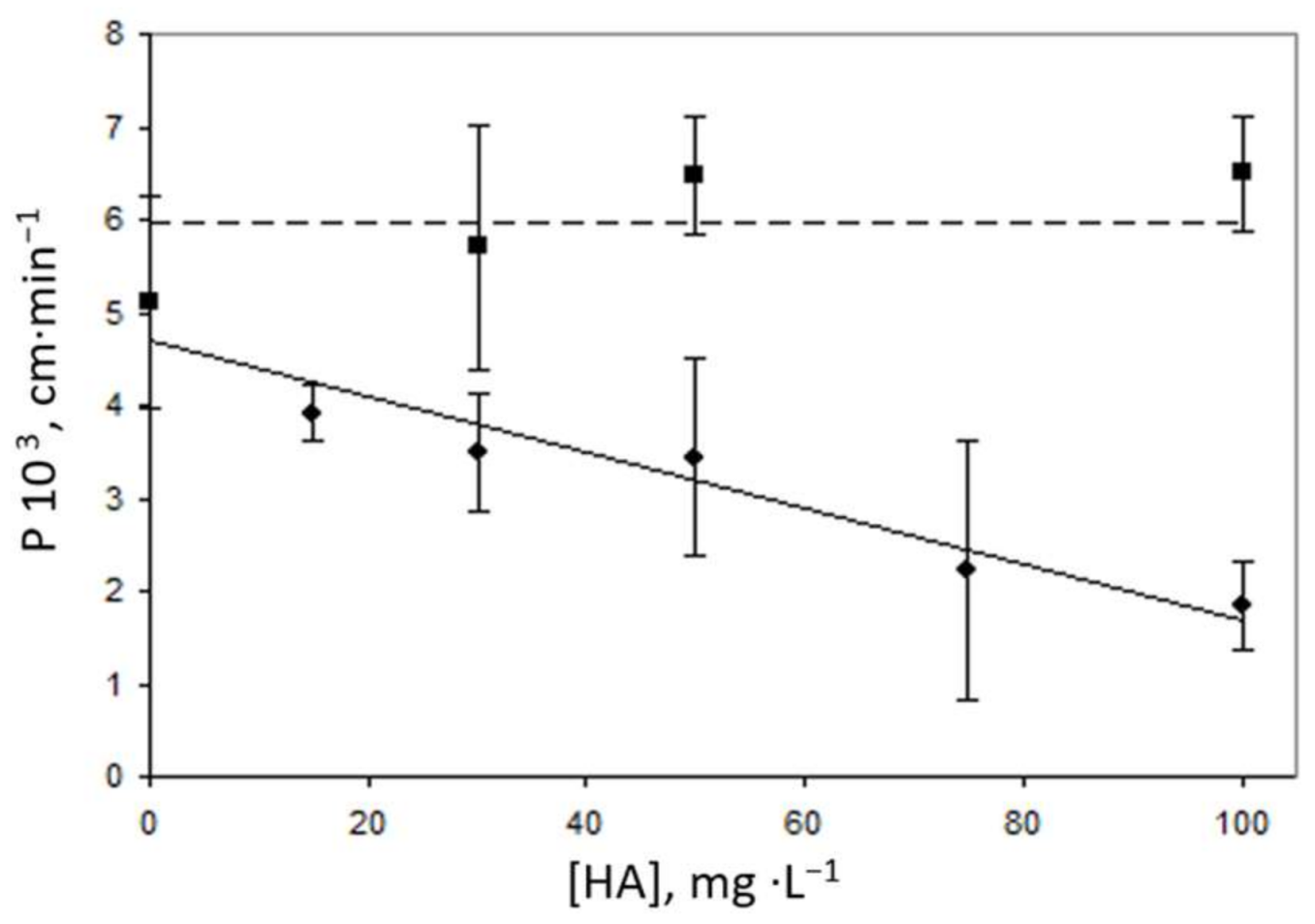
3.2. Effects of Ligands on Nickel Transport
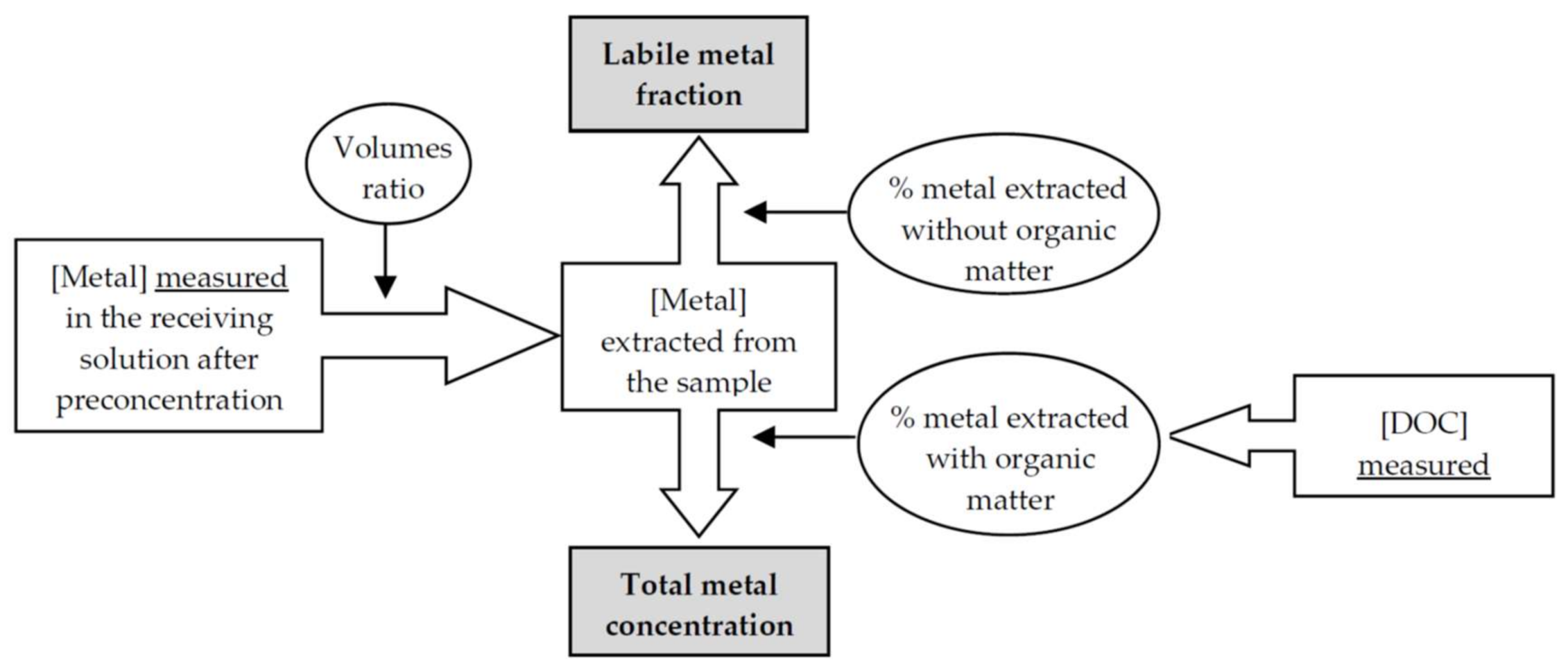
3.3. Measuring the Concentration of Nickel Species in Real Samples
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Sánchez Urias, J.E.; Sanz Medel, A. Inorganic and methylmercury speciation in environmental samples. Talanta 1998, 47, 509–524. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W. Direct in situ measurements of labile inorganic and organically bound metal species in synthetic solutions and natural waters using diffusive gradients in thin films. Anal. Chem. 2000, 72, 4447–4457. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; Van leeuwen, H.P.; Lobiński, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chakrabarti, C.L. Chemical speciation of Co, Ni, Cu, and Zn in mine effluents and effects of dilution of the effluent on release of the above metals from their metal–dissolved organic carbon (DOC) complexes. Anal. Chim. Acta 2006, 571, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, L.; Smolders, E.; Degryse, F.; Janssen, C.; De Schamphelaere, K.A.C. Speciation of nickel in surface waters measured with the Donnan membrane technique. Anal. Chim. Acta 2006, 578, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurman, E.M. Organic Geochemistry of Natural Waters; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1985; ISBN 90-247-3143-7. [Google Scholar]
- Van Loon, G.W.; Duffy, S.J. Environmental Chemistry: A Global Perspective, 3rd ed.; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-108924-4. [Google Scholar]
- Irving, H.M.N.H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Doig, K.; Liber, K. Nickel speciation in the presence of different sources and fractions of dissolved organic matter. Ecotoxicol. Environ. Saf. 2007, 66, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Abasse, G.; Ouddane, B.; Fischer, J.C. Determination of total and labile fraction of metals in seawater using solid phase extraction and inductively coupled plasma atomic emission spectrometry (ICP-AES). J. Anal. At. Spectrom. 2002, 17, 1354–1358. [Google Scholar] [CrossRef]
- Martino, M.; Turner, A.; Nimmo, M. Distribution, speciation and particle-water interactions of nickel in the Mersey Estuary, UK. Mar. Chem. 2004, 88, 161–177. [Google Scholar] [CrossRef]
- Pesavento, M.; Alberti, G.; Biesuz, R. Analytical methods for determination of free metal ion concentration, labile species fraction and metal complexation capacity of environmental waters: A review. Anal. Chim. Acta 2009, 631, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Companys, E.; Galceran, J.; Pinheiro, J.P.; Puy, J.; Salaün, P. A review on electrochemical methods for trace metal speciation in environmental media. Curr. Opin. Electrochem. 2017, 3, 144–162. [Google Scholar] [CrossRef]
- Mota, A.M.; Pinheiro, J.P.; Simões Gonçalves, M.L. Electrochemical methods for speciation of trace elements in marine waters. Dynamic aspects. J. Phys. Chem. A 2012, 116, 6433–6442. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, D.; Caprara, S. Voltammetric tools for trace element speciation in fresh waters: Methodologies, outcomes and future perspectives. Environ. Chem. 2015, 12, 683–705. [Google Scholar] [CrossRef]
- Tipping, E.; Lofts, S.; Stockdale, A. Metal speciation from stream to open ocean: Modelling v. measurement. Environ. Chem. 2016, 13, 464–477. [Google Scholar] [CrossRef]
- López-López, J.A.; Mendiguchía, C.; Pinto, J.J.; Moreno, C. Liquid membranes for quantification and speciation of trace metals in natural waters. TrAC-Trend Anal. Chem. 2010, 29, 645–653. [Google Scholar] [CrossRef]
- Diaconu, I.; Ruse, E.; Aboul-Enein, H.Y.; Bunaciu, A.A. Analytical Applications of Transport through Bulk Liquid Membranes. Crit. Rev. Anal. Chem. 2016, 46, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Benomar, M.; Mendiguchía, C.; García-Vargas, M.; Moreno, C. A liquid membrane-based green method for the separation and determination of lead in saline waters. Spectrosc. Lett. 2011, 44, 83–87. [Google Scholar] [CrossRef]
- Irigoyen, L.; Moreno, C.; Mendiguchía, C.; García-Vargas, M. Application of liquid membranes to sample preconcentration for the spectrometric determination of cadmium in seawater. J. Membr. Sci. 2006, 274, 169–172. [Google Scholar] [CrossRef]
- López-López, J.A.; García-Vargas, M.; Moreno, C. A new contamination-free method for the determination of traces of anthropogenic silver in freshwaters. Int. J. Environ. Anal. Chem. 2012, 92, 636–643. [Google Scholar] [CrossRef]
- Mendiguchía, C.; García-Vargas, M.; Moreno, C. Screening of dissolved heavy metals (Cu, Zn, Mn, Al, Cd, Ni, Pb) in seawater by a liquid-membrane–ICP–MS approach. Anal. Bioanal. Chem. 2008, 391, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Aouarram, A.; Galindo-Riaño, M.D.; García-Vargas, M.; Stitou, M.; El Yousfi, F. A permeation liquid membrane system for determination of nickel in seawater. Talanta 2007, 71, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.J.; García-Vargas, M.; Moreno, C. A bulk liquid membrane–flow injection (BLM–FI) coupled system for the preconcentration and determination of vanadium in saline waters. Talanta 2013, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Fahmideh-Rad, E.; Ronagi, E.G.; Zavar, M.H.A.; Chamsaz, M. Spectrometric determination of Pb2+ cation after selective bulk liquid membrane transport using benzo-18-crown-6 as carrier. Der Pharma Chem. 2010, 2, 8–18. [Google Scholar]
- López-López, J.A.; García-Vargas, M.; Moreno, C. A new analytical method for selective pre-concentration of free silver in estuarine waters using liquid membranes. Talanta 2013, 108, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Bayen, S.; Worm, I.; Parthasarathy, N.; Wilkinson, K.; Buffle, J. Cadmium bioavailability and speciation using the permeation liquid membrane. Anal. Chim. Acta 2004, 575, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gunkel-Grillon, P.; Buffle, J. Speciation of Cu(II) with a flow-through permeation liquid membrane: Discrimination between free copper, labile and inert Cu(II) complexes, under natural waters conditions. Analyst 2008, 133, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Ndungu, K.; Hurst, M.P.; Bruland, K.W. Comparison of copper speciation in estuarine water measured using analytical voltammetry and supported liquid membrane techniques. Environ. Sci. Technol. 2005, 39, 3166–3175. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, N.; Pelletier, M.; Buffle, J. Permeation liquid membrane for trace metal speciation in natural waters. Transport of liposoluble Cu(II) complexes. J. Chromatogr. A 2004, 1025, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Jönsson, J.Å. Determination of free copper concentration in natural waters based on a supported liquid membrane extraction system. Anal. Bioanal. Chem. 2005, 38, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Vergel, C.; Mendiguchía, C.; García-Vargas, M.; Moreno, C. Quantification of free and bound fractions of nickel in natural waters by solvent extraction with 1,2-cyclohexanedione bis-benzoyl-hydrazone. Solvent Extr. Ion Exch. 2010, 28, 625–635. [Google Scholar] [CrossRef]
- García-Vargas, M.; Trevilla, S.; Milla, M. Synthesis and characterization of 1,2-cyclohexanedione bis-benzoylhydrazone and its application to the determination of Ti in minerals and rocks. Talanta 1986, 33, 209–214. [Google Scholar] [CrossRef]
- Tipping, E. WHAMC—A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput. Geosci. 1994, 20, 973–1023. [Google Scholar] [CrossRef]
- Mendiguchía, C.; Moreno, C.; García-Vargas, M. Separation of heavy metals in seawater by liquid membranes: Preconcentration of copper. Sep. Sci. Technol. 2002, 37, 2337–2351. [Google Scholar] [CrossRef]
- Pinto, J.J. Nuevas Alternativas Para Simplificación y Mejora de Lametodologia de Analísis de Metales Pesados en Muestras Ambientales. Ph.D. Thesis, University of Cadiz, Cadiz, Spain, 2010. [Google Scholar]
- Vergel, C. Desarrollo de Procesos Químicos de Separación por Membranas Para la Caracterización de Sistemas Marinos Afectados por Contaminación Metálica. Ph.D. Thesis, University of Cádiz, Cádiz, Spain, 2011. [Google Scholar]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
| Humic Acid, mg·L−1 | NiHA | Ni(OH)2 | Ni2+ | NiCl+ | NiOH+ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mol·L−1 | % | mol·L−1 | % | mol·L−1 | % | mol·L−1 | % | mol·L−1 | % | |
| 0 | 0 | 0 | 2.9 × 10−9 | 0 | 1.3 × 10−5 | 74 | 4.3 × 10−6 | 26 | 6.8 × 10−8 | 0 |
| 15 | 8.1 × 10−7 | 5 | 2.7 × 10−9 | 0 | 1.2 × 10−5 | 70 | 4.0 × 10−6 | 24 | 6.3 × 10−8 | 0 |
| 30 | 1.5 × 10−6 | 9 | 2.5 × 10−9 | 0 | 1.1 × 10−5 | 67 | 3.8 × 10−6 | 23 | 5.9 × 10−8 | 0 |
| 50 | 2.4 × 10−6 | 15 | 2.3 × 10−9 | 0 | 9.9 × 10−6 | 63 | 3.4 × 10−6 | 22 | 5.4 × 10−8 | 0 |
| 75 | 3.3 × 10−6 | 21 | 2.1 × 10−9 | 0 | 9.0 × 10−6 | 58 | 3.1 × 10−6 | 20 | 4.8 × 10−8 | 0 |
| 100 | 4.0 × 10−6 | 27 | 1.7 × 10−9 | 0 | 8.1 × 10−6 | 54 | 2.8 × 10−6 | 19 | 4.4 × 10−8 | 0 |
| Humic Acid, mg·L−1 | DOC, mg·L−1 | Recovery Efficiency, % | Standard Deviation |
|---|---|---|---|
| 0 | 0 | 50.5 | 3.2 |
| 15 | 3.65 | 48.0 | 2.6 |
| 50 | 12.20 | 47.2 | 4.4 |
| 75 | 18.58 | 33.7 | 7.5 |
| 100 | 25.95 | 37.5 | 6.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergel, C.; Mendiguchía, C.; Moreno, C. Liquid Membranes as a Tool for Chemical Speciation of Metals in Natural Waters: Organic and Inorganic Complexes of Nickel. Membranes 2018, 8, 19. https://doi.org/10.3390/membranes8020019
Vergel C, Mendiguchía C, Moreno C. Liquid Membranes as a Tool for Chemical Speciation of Metals in Natural Waters: Organic and Inorganic Complexes of Nickel. Membranes. 2018; 8(2):19. https://doi.org/10.3390/membranes8020019
Chicago/Turabian StyleVergel, Cristina, Carolina Mendiguchía, and Carlos Moreno. 2018. "Liquid Membranes as a Tool for Chemical Speciation of Metals in Natural Waters: Organic and Inorganic Complexes of Nickel" Membranes 8, no. 2: 19. https://doi.org/10.3390/membranes8020019





