Investigations of Alkaline and Enzymatic Membrane Cleaning of Ultrafiltration Membranes Fouled by Thermomechanical Pulping Process Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Process Water
2.2. Cleaning Agents and Protocols
- Alkaline cleaning with NaOH (NaOH + NaOH).
- Alkaline cleaning with Ultrasil 10 (U10 + U10).
- Enzymatic cleaning with the enzyme cocktail followed by NaOH (EC + NaOH).
- Enzymatic cleaning with the enzyme cocktail followed by Ultrasil 10 (EC + U10).
2.3. Membrane and Equipment
2.4. Experimental Procedures
Membrane Fouling and Cleaning
2.5. Analysis
3. Results and Discussion
3.1. Two-Step Cleaning Protocols
3.1.1. Alkaline Cleaning
3.1.2. Enzymatic Cleaning
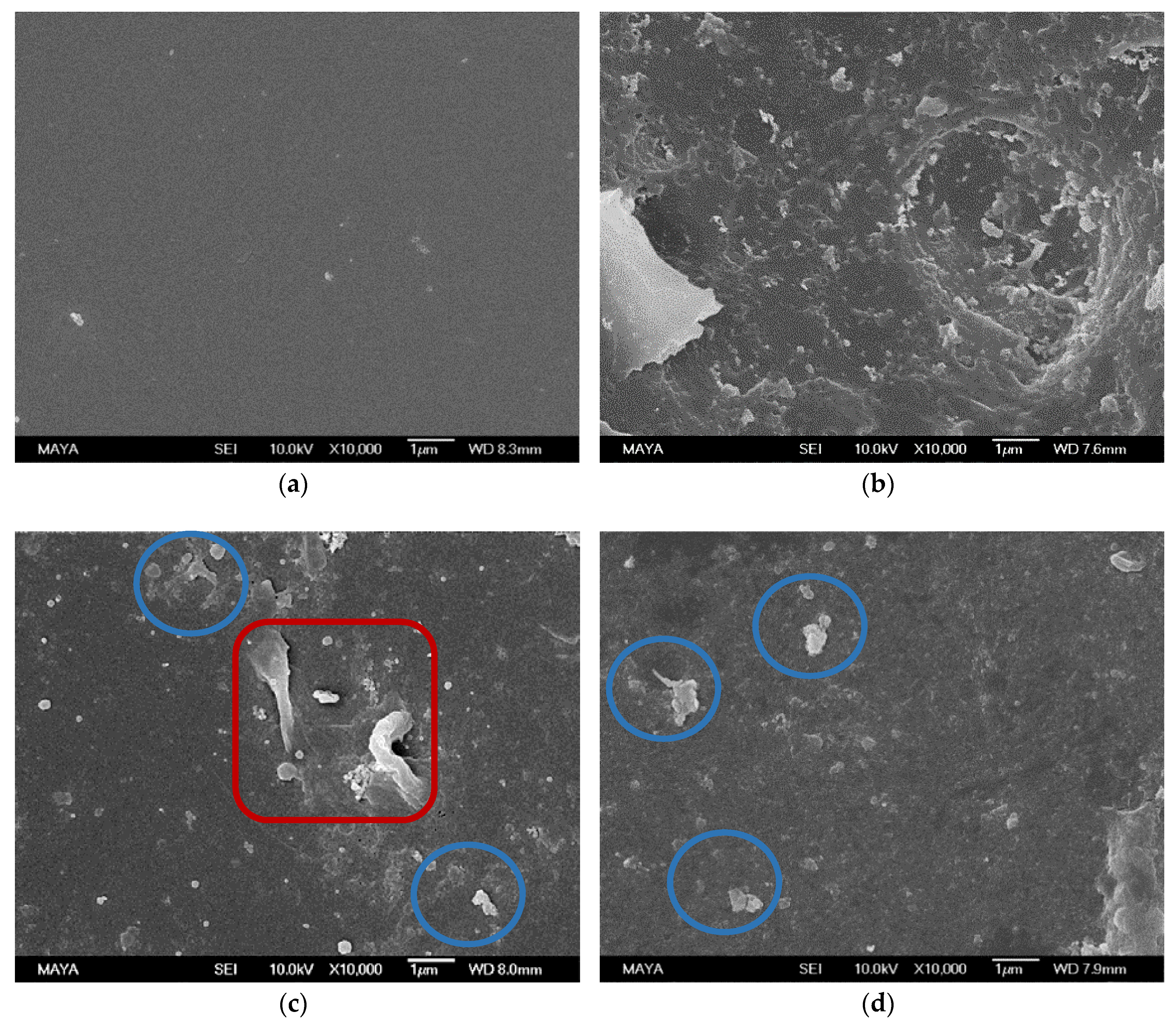
3.1.3. SEM Analysis
3.1.4. ATR-FTIR Analysis
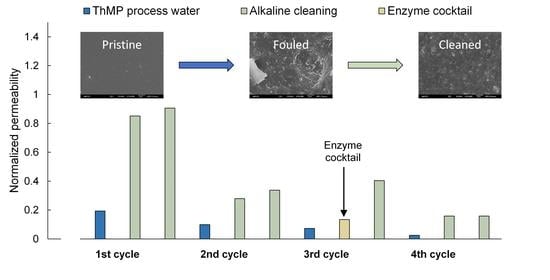
3.2. Long-Term Study
3.2.1. First and Second Cycles—Two-Step Cleaning U10 + U10
3.2.2. Third Cycle—Two-Step Cleaning EC + U10
3.2.3. Fourth Cycle—Two-Step Cleaning U10 + U10
3.2.4. SEM and Water Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bokhary, A.; Cui, L.; Lin, H.L.; Liao, B.Q. A Review of Membrane Technologies for Integrated Forest Biorefinery. J. Membr. Sci. Res. 2017, 3, 120–141. [Google Scholar]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, T.; Jönsson, A.-S. Isolation of hemicelluloses by ultrafiltration of thermomechanical pulp mill process water—Influence of operating conditions. Chem. Eng. Res. Des. 2010, 88, 1548–1554. [Google Scholar] [CrossRef]
- Thuvander, J.; Jönsson, A.-S. Extraction of galactoglucomannan from thermomechanical pulp mill process water by microfiltration and ultrafiltration—Influence of microfiltration membrane pore size on ultrafiltration performance. Chem. Eng. Res. Des. 2016, 105, 171–176. [Google Scholar] [CrossRef]
- Kallioinen, M.; Huuhilo, T.; Reinikainen, S.-P.; Nuortila-Jokinen, J.; Mänttäri, M. Examination of membrane performance with multivariate methods: A case study within a pulp and paper mill filtration application. Chemometr. Intell. Lab. Syst. 2006, 84, 98–105. [Google Scholar] [CrossRef]
- Nordin, A.-K.; Jönsson, A.-S. Case study of an ultrafiltration plant treating bleach plant effluent from a pulp and paper mill. Desalination 2006, 201, 277–289. [Google Scholar] [CrossRef]
- Keyoumu, A.; Sjödahl, R.; Henriksson, G.; Ek, M.; Gellerstedt, G.; Lindström, M.E. Continuous nano- and ultra-filtration of kraft pulping black liquor with ceramic filters. Ind. Crop. Prod. 2004, 20, 143–150. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; Technomic: Lancaster, PA, USA, 1998. [Google Scholar]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef] [PubMed]
- Antón, E.; Álvarez, J.R.; Palacio, L.; Prádanos, P.; Hernández, A.; Pihlajamäki, A.; Luque, S. Ageing of polyethersulfone ultrafiltration membranes under long-term exposures to alkaline and acidic cleaning solutions. Chem. Eng. Sci. 2015, 134, 178–195. [Google Scholar] [CrossRef]
- Teella, A.; Zydney, A.L.; Zhou, H.; Olsen, C.; Robinson, C. Effects of chemical sanitization using NaOH on the properties of polysulfone and polyethersulfone ultrafiltration membranes. Biotechnol. Prog. 2015, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Aguado, M.J.; Wiley, D.E.; Fane, A.G. Enzymatic and detergent cleaning of a polysulfone ultrafiltration membrane fouled with BSA and whey. J. Membr. Sci. 1996, 117, 175–187. [Google Scholar] [CrossRef]
- Argüello, M. Enzymatic cleaning of inorganic ultrafiltration membranes used for whey protein fractionation. J. Membr. Sci. 2003, 216, 121–134. [Google Scholar] [CrossRef]
- Maartens, A.; Swart, P.; Jacobs, E.P. An enzymatic approach to the cleaning of ultrafiltration membranes fouled in abattoir effluent. J. Membr. Sci. 1996, 119, 9–16. [Google Scholar] [CrossRef]
- Yu, C.-H.; Fang, L.-C.; Lateef, S.K.; Wu, C.-H.; Lin, C.-F. Enzymatic treatment for controlling irreversible membrane fouling in cross-flow humic acid-fed ultrafiltration. J. Hazard. Mater. 2010, 177, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Willför, S.; Rehn, P.; Sundberg, A.; Sundberg, K.; Holmbom, B. Recovery of water-soluble acetylgalactoglucomannans from mechanical pulp of spruce. TAPPI J. 2003, 2, 27–32. [Google Scholar]
- Persson, T.; Krawczyk, H.; Nordin, A.-K.; Jönsson, A.-S. Fractionation of process water inthermomechanical pulp mills. Bioresour. Technol. 2010, 101, 3884–3892. [Google Scholar] [CrossRef] [PubMed]
- Thuvander, J.; Zarebska, A.; Helix-Nielsen, C.; Jönsson, A.-S. Characterisation of irreversible fouling after ultrafiltration of thermomechanical pulp mill process water. J. Wood Chem. Technol. 2018, 38, 276–285. [Google Scholar] [CrossRef]
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Nyström, M. Evaluation of behavior and fouling potential of wood extractives in ultrafiltration of pulp and paper mill process water. J. Membr. Sci. 2011, 368, 150–158. [Google Scholar] [CrossRef]
- Maartens, A.; Jacobs, E.P.; Swart, P. UF of pulp and paper effluent: membrane fouling-prevention and cleaning. J. Membr. Sci. 2002, 209, 81–92. [Google Scholar] [CrossRef]
- D’Souza, N.M.; Mawson, A.J. Membrane cleaning in the dairy industry: A review. Crit. Rev. Food Sci. Nutr. 2005, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Revised January 2008. 2006. Available online: https://www.nrel.gov/docs/gen/fy08/42623.pdf (accessed on 8 October 2018).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Revised July 2011. 2008. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 8 October 2018).
- Li, Q.; Bi, Q.-Y.; Lin, H.-H.; Bian, L.-X.; Wang, X.-L. A novel ultrafiltration (UF) membrane with controllable selectivity for protein separation. J. Membr. Sci. 2013, 427, 155–167. [Google Scholar] [CrossRef]
- Kim, J.T.; Shin, G.H. Adsorption behavior of β-lactoglobulin onto polyethersulfone membrane surface. J. Adhes. Sci. Technol. 2015, 29, 2245–2255. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Hoek, E.M.V.; Bui, N.; Lind, M.L. Nanostructured Membranes for Engineered Osmosis Applications. International Patent Application No. PCT/US2010/056426, 11 November 2010. [Google Scholar]
- Kumar, R.; Isloor, A.M.; Ismail, A.F.; Rashid, S.A.; Matsuura, T. Polysulfone–Chitosan blend ultrafiltration membranes: preparation, characterization, permeation and antifouling properties. RSC Adv. 2013, 3, 7855–7861. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Wang, J.; Wang, S. A novel method of surface modification to polysulfone ultrafiltration membrane by preadsorption of citric acid or sodium bisulfite. Membr. Water Treat. 2012, 3, 35–49. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Glucose and fructose hydrates in aqueous solution by IR spectroscopy. J. Phys. Chem. A 2007, 111, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.; Brites Alves, A.M.; de Lurdes Serrano, M.; de Sousa, A.P.M. Isolation and purification of concentrated and non-concentrated hemicellulose alkaline extracts. Sep. Purif. Technol. 2017, 173, 233–239. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Insights into polysaccharide fouling of ultrafiltration membranes. Desalination 2006, 200, 181–182. [Google Scholar] [CrossRef]





| Total solids (g/L) | 5.4 |
| Ash (g/L) | 1.85 |
| Hemicelluloses (g/L) | 2.60 |
| Arabinan (g/L) | 0.12 |
| Galactan (g/L) | 0.35 |
| Glucan (g/L) | 0.64 |
| Mannan (g/L) | 1.49 |
| Total lignin (g/L) | 0.96 |
| Turbidity (NTU 3) | 307 |
| Cleaning Agent | Duration | Stirring | Pressure | Concentration |
|---|---|---|---|---|
| NaOH | 1 h, then VR 1 50% | 500 rpm | Ambient, then 2 bar | 0.2% (w/v) |
| Ultrasil 10 (20% NaOH) | 1 h, then VR 50% | 500 rpm | Ambient, then 2 bar | 1.0% (w/v) |
| Enzyme cocktail: | ||||
| B-glucanase, cellulose, cutinase, lipase, mannanase, xylanase | 3 h | 500 rpm | Ambient | 0.1% (v/v) each |
| Wavenumber (cm−1) | Assignment | References |
|---|---|---|
| 635–875 | C–H bending, rocking of PSU 1 | [28] |
| 1014, 1080 | Skeletal aliphatic C–C bonds/aromatic C–H bonds of PSU and polysaccharides | [28,26] |
| 1107, 1169 | C–C bonds /aromatic hydrogen bending/rocking of PSU | [26] |
| 1157 | C–SO2–C symmetric stretching of PSU | [26,27,28] |
| 1240 | C–O–C symmetric stretching of PSU | [26,27,28] |
| 1295 | S=O stretching of PSU | [26,27,28] |
| 1323 | C–SO2–C asymmetric stretching of PSU | [28] |
| 1503, 1580, 1586 | C=C stretching of PSU in the aromatic rings | [26,28] |
| 1690–1750 | C=O stretching of extractives | [19,28] |
| 2967 | Aromatic C–H stretching of PSU and polysaccharides | [27] |
| 3000–3400 | O–H stretching of polysaccharides | [28] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, G.; Schagerlöf, H.; Morkeberg Krogh, K.B.; Jönsson, A.-S.; Lipnizki, F. Investigations of Alkaline and Enzymatic Membrane Cleaning of Ultrafiltration Membranes Fouled by Thermomechanical Pulping Process Water. Membranes 2018, 8, 91. https://doi.org/10.3390/membranes8040091
Rudolph G, Schagerlöf H, Morkeberg Krogh KB, Jönsson A-S, Lipnizki F. Investigations of Alkaline and Enzymatic Membrane Cleaning of Ultrafiltration Membranes Fouled by Thermomechanical Pulping Process Water. Membranes. 2018; 8(4):91. https://doi.org/10.3390/membranes8040091
Chicago/Turabian StyleRudolph, Gregor, Herje Schagerlöf, Kristian B. Morkeberg Krogh, Ann-Sofi Jönsson, and Frank Lipnizki. 2018. "Investigations of Alkaline and Enzymatic Membrane Cleaning of Ultrafiltration Membranes Fouled by Thermomechanical Pulping Process Water" Membranes 8, no. 4: 91. https://doi.org/10.3390/membranes8040091