Surface-Engineered Inorganic Nanoporous Membranes for Vapor and Pervaporative Separations of Water–Ethanol Mixtures †
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Membranes
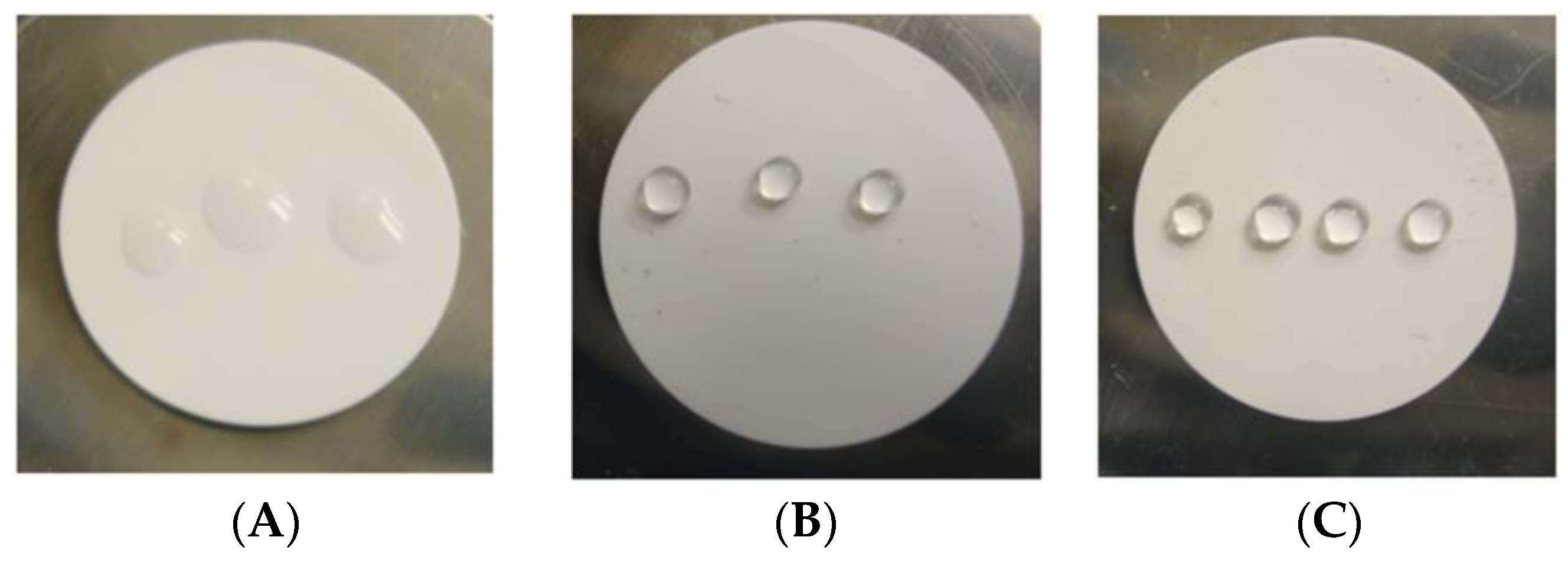
2.2. Characterization of Membrane Materials
2.3. Membrane Separation Study on Dehydration of Ethanol–Water Mixed Vapors
2.4. Membrane Separation Study on Liquid Ethanol–Water Mixtures by Ethanol Pervaporation
3. Results and Discussion
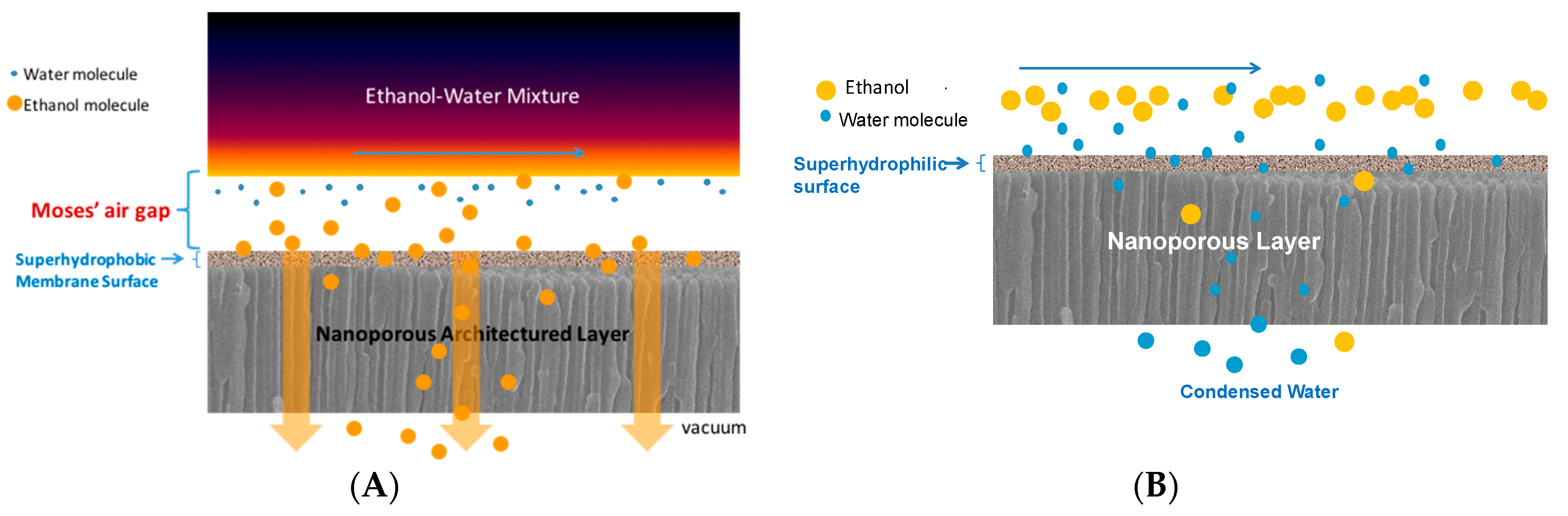
3.1. Vapor Phase Dehydration of Ethanol–water Mixtures with ~6 nm HiPAS Membranes
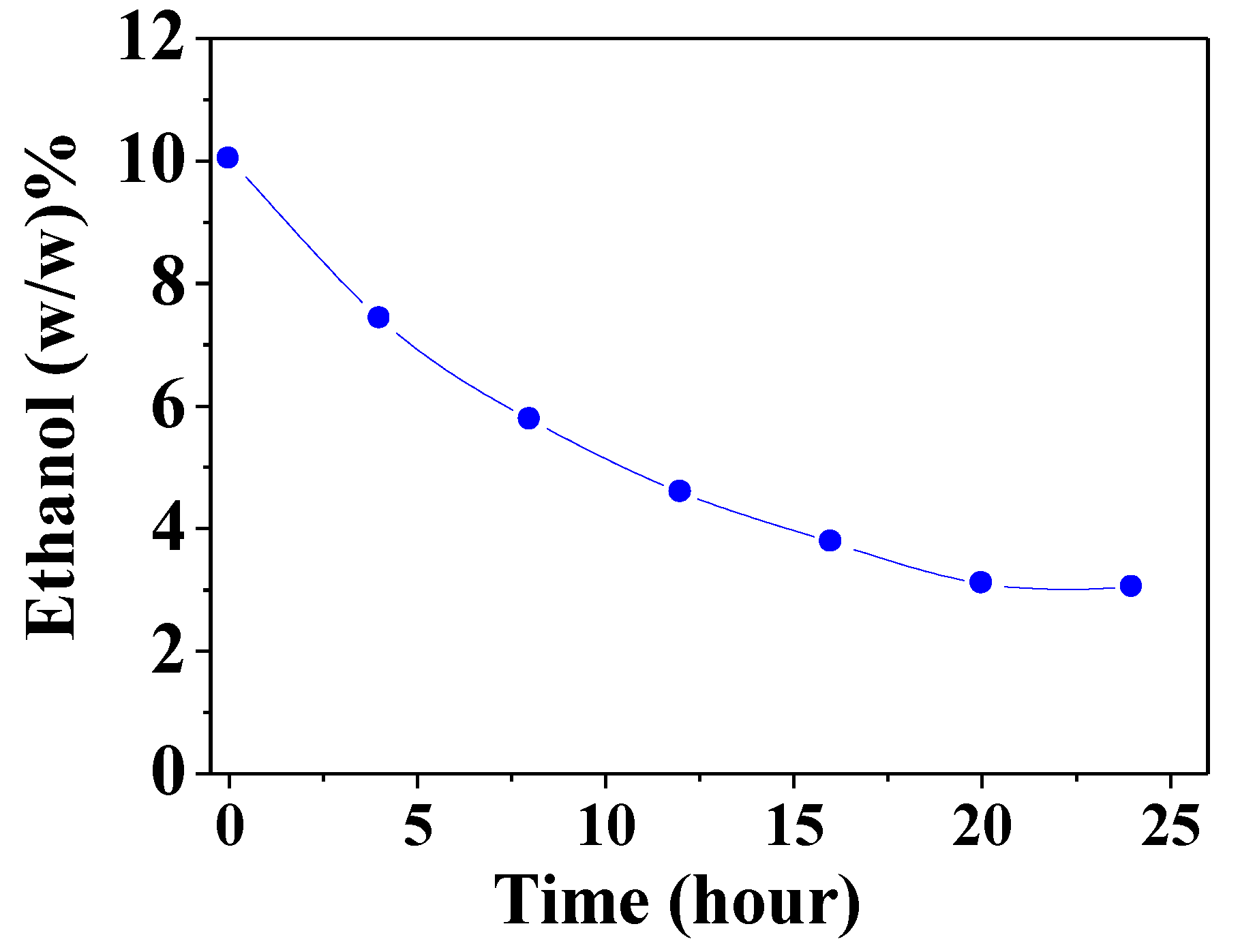
3.2. Liquid Separation by Pervaporation of Ethanol–Water Mixture
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HiPAS | High-performance architectured surface selective |
| SS | Stainless steel |
| SI | Superhydrophilic |
| SO | Superhydrophobic |
| PDTMS | 1H,1H,2H, 2H-perfluoro decyl trimethoxy silane |
| MBMS | Molecular beam mass spectrometer |
References
- Bioenergy Technologies Office, US Department of Energy. Multi-Year Program Plan (MYPP). 2015. Available online: https://www.energy.gov/eere/buildings/downloads/multi-year-program-plan (accessed on 11 October 2018).
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons. NREL/TP-5100-60223; 2013. Available online: https://www.nrel.gov/docs/fy14osti/60223.pdf (accessed on 11 October 2018).
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons. NREL/TP-5100-62498; 2015. Available online: https://www.nrel.gov/docs/fy15osti/62498.pdf (accessed on 11 October 2018).
- Sukhabaatar, B.; Li, Q.; Wan, C.X.; Yu, F.; Hassan, E.-B.; Steele, P. Inhibitors removal from bio-oil aqueous fraction for increased ethanol production. Bioresour. Technol. 2014, 161, 379. [Google Scholar] [CrossRef] [PubMed]
- Uragami, T.; Katayama, T.; Miyata, T.; Tamura, H.; Shiraiwa, T.; Higuchi, A. Dehydration of an ethanol/water azeotrope by novel organic-inorganic hybrid membranes based on quaternized chitosan and tetraethoxysilane. Biomacromolecules 2004, 5, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Vane, L.M.; Alvarez, F.R. Membrane-assisted vapor stripping: Energy efficient hybrid distillation–vapor permeation process for alcohol–water separation. J. Chem. Technol. Biotechnol. 2008, 83, 1275–1287. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Li, J.; Wang, J.; Lu, J.; Yin, D.; Zhang, Y. High Performance ZSM-5 Membranes on Coarse Macroporous a-Al2O3 Supports for Dehydration of Alcohols. AIChE J. 2016, 62, 2813–2824. [Google Scholar] [CrossRef]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Jiang, J.; Wang, X.; Zhang, C.; Gu, X. High-performance NaA zeolite membranes supported on four-channel ceramic hollow fibers for ethanol dehydration. RSC Adv. 2015, 5, 95866–95871. [Google Scholar] [CrossRef]
- Zhou, H.; Korelsky, D.; Leppajavi, T.; Grahn, M.; Tanskanen, J.; Hedlund, J. Ultrathin zeolite X membranes for pervaporation dehydration of ethanol. J. Membr. Sci. 2012, 399–400, 106–111. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, W.; Yang, J.; Chu, N.; Lu, J.; Yin, D.; Wang, J. Synthesis of silicalite-1 membrane with two silicon source by secondary growth method and its pervaporation performance. Sep. Purif. Technol. 2011, 76, 308–315. [Google Scholar] [CrossRef]
- Wee, S.L.; Tye, C.T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Moussa, M.; Souchon, I.; Athes, V. Pervaporative Dehydration of Binary Ethanol/Water and Ternary Ethanol/Water/Methanol Mixtures Using a Methylated Silica Membrane: A Mechanistic Approach. Sep. Sci. Technol. 2015, 50, 2708–2716. [Google Scholar] [CrossRef]
- Araki, S.; Kiyohara, Y.; Imasaka, S.; Tanaka, S.; Miyake, Y. Preparation and pervaporation properties of silica–zirconia membranes. Desalination 2011, 266, 46–50. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Tsuru, T. Pervaporation of water/ethanol mixtures through microporous silica membranes. Sep. Purif. Technol. 2009, 66, 479–485. [Google Scholar] [CrossRef]
- Agirre, I.; Arias, P.L.; Castricum, H.L.; Creatore, M.; Elshof, J.E.; Paradis, G.G.; Ngamou, P.H.T.; van Veen, H.M.; Vente, J.F. Hybrid organosilica membranes and processes: Status and outlook. Sep. Purif. Technol. 2014, 121, 2–12. [Google Scholar] [CrossRef]
- Castricum, H.L.; Paradis, G.G.; Mittelmeijer-Hazeleger, M.C.; Bras, W.; Eeckhaut, G.; Vente, J.F.; Rothernberg, G.; Elshof, J.E. Tuning the nanopore structure and separation behavior of hybrid organosilica membranes. Microporous Mesoporous Mater. 2014, 185, 224–234. [Google Scholar] [CrossRef]
- Castricum, H.L.; Sah, A.; Kreiter, R.; Blank, D.H.A.; Vente, J.F.; Elshof, J.E. Hybrid ceramic nanosieves: Stabilizing nanopores with organic links. Chem. Commun. 2008, 9, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Bakhtiari, O.; Mosleh, S.; Khosravi, T.; Mohammadi, T. Synthesis and Characterization of Polyimide Mixed Matrix Membranes. Sep. Sci. Technol. 2011, 46, 2138–2147. [Google Scholar] [CrossRef]
- Shao, J.; Ge, Q.Q.; Shan, L.J.; Wang, Z.B.; Yan, Y.S. Influences of seeds on the properties of zeolite NaA membranes on alumina hollow fibers. Ind. Eng. Chem. Res. 2011, 50, 9718–9726. [Google Scholar] [CrossRef]
- Vane, L.M.; Namboodiri, V.V.; Bowen, T.C. Hydrophobic zeolite–silicone rubber mixed matrix membranes for ethanol–water separation: Effect of zeolite and silicone component selection on pervaporation performance. J. Membr. Sci. 2008, 308, 230–241. [Google Scholar] [CrossRef]
- Tanaka, S.; Yasuda, T.; Katayama, Y.; Miyake, Y. Pervaporation dehydration performance of microporous carbon membranes prepared from resorcinol/formaldehyde polymer. J. Membr. Sci. 2011, 379, 52–59. [Google Scholar] [CrossRef]
- Vane, L.M. Pervaporation and Vapor Permeation Tutorial: Membrane Processes for the Selective Separation of Liquid and Vapor Mixtures. Sep. Sci. Technol. 2013, 48, 429–437. [Google Scholar] [CrossRef]
- Vane, L.M. Separation technologies for the recovery and dehydration of alcohols from fermentation broths Biofuels. Bioprod. Bioref. 2008, 2, 553–588. [Google Scholar] [CrossRef]
- Chai, L.; Li, H.; Zheng, X.; Wang, J.; Yang, J.; Lu, J.; Yin, D.; Zhang, Y. Pervaporation separation of ethanol–water mixtures through B-ZSM-11 zeolite membranes on macroporous supports. J. Membr. Sci. 2015, 491, 168–175. [Google Scholar] [CrossRef]
- Yu, L.; Korelskiy, D.; Grahn, M.; Hedlund, J. Very high flux MFI membranes for alcohol recovery via pervaporation at high temperature and pressure. Sep. Purif. Technol. 2015, 153, 138–145. [Google Scholar] [CrossRef]
- Elyassi, B.; Jeon, M.Y.; Tsapatsis, M.; Narasimharao, K.; Basahel, S.N.; Al-Thabaiti, S. Ethanol/Water Mixture Pervaporation Performance of b-Oriented Silicalite-1 Membranes Made by Gel-Free Secondary Growth. AIChE J. 2016, 62, 556–563. [Google Scholar] [CrossRef]
- Korelskiy, D.; Leppajarvi, T.; Zhou, H.; Grahn, M.; Tanskanen, J.; Hedlund, J. High flux MFI membranes for pervaporation. J. Membr. Sci. 2013, 427, 381–389. [Google Scholar] [CrossRef]
- Shan, L.; Shao, J.; Wang, Z.; Yan, Y. Preparation of zeolite MFI membranes on alumina hollow fibers with high flux for pervaporation. J. Membr. Sci. 2011, 378, 319–329. [Google Scholar] [CrossRef]
- Rozicka, A.; Niemisto, J.; Keiski, R.L.; Kujawski, W. Apparent and intrinsic properties of commercial PDMS based membranes in pervaporative removal of acetone, butanol and ethanol from binary aqueous mixtures. J. Membr. Sci. 2014, 453, 108–118. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Fang, M.; Wang, T.; Yu, L.; Li, J. ZIF-7/PDMS mixed matrix membranes for pervaporation recovery of butanol from aqueous solution. Sep. Purif. Technol. 2016, 163, 39–47. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Zhan, X.; Fang, M.; Wang, T.; Li, J. Preparation and characterization of ZSM-5/PDMS hybrid pervaporation membranes: Laboratory results and pilot-scale performance. Sep. Purif. Technol. 2015, 150, 257–267. [Google Scholar] [CrossRef]
- Wang, N.; Liu, J.; Li, J.; Gao, J.; Li, S.; Li, J. Tuning properties of silicalite-1 for enhanced ethanol/water pervaporation separation in its PDMS hybrid membrane. Microporous Mesoporous Mater. 2015, 201, 35–42. [Google Scholar] [CrossRef]
- Naik, P.V.; Kerkhofs, S.; Martens, J.A.; Vankelecom, I.F.J. PDMS mixed matrix membranes containing hollow silicalite sphere for ethanol/water separation by pervaporation. J. Membr. Sci. 2016, 502, 48–56. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Separation of ethanol from water by pervaporation using mixed matrix copolymer membranes. Sep. Purif. Technol. 2015, 146, 176–186. [Google Scholar] [CrossRef]
- Wang, N.; Shi, G.; Gao, J.; Li, J.; Wang, L.; Guo, H.; Zhang, G.; Ji, S. MCM-41@ZIF-8/PDMS hybrid membranes with micro- and nanoscaled hierarchical structure for alcohol permselective pervaporation. Sep. Purif. Technol. 2015, 153, 146–155. [Google Scholar] [CrossRef]
- Liu, G.; Hou, D.; Wei, W.; Xiangli, F.; Jin, W. Pervaporation Separation of Butanol-Water Mixtures Using Polydimethylsiloxane/Ceramic Composite Membrane. Sep. Sci. Eng. 2011, 19, 40–44. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, R.; Jin, W. Novel organic–inorganic pervaporation membrane with a superhydrophobic surface for the separation of ethanol from an aqueous solution. Sep. Purif. Technol. 2014, 127, 61–69. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, B.; Huang, A. Polydopamine-based superhydrophobic membranes for biofuel recovery. J. Mater. Chem. A 2013, 1, 11970–11974. [Google Scholar] [CrossRef]
- Hu, M.Z.; Engtrakul, C.; Bischoff, B.L.; Jang, G.G.; Theiss, T.J.; Davis, M.F. Superhydrophobic and Superhydrophilic Surface-Enhanced Separation Performance of Porous Inorganic Membranes for Biomass-to-Biofuel Conversion Applications. Sep. Sci. Technol. 2016, 3, 528–543. [Google Scholar] [CrossRef]
- Engtrakul, C.; Hu, M.Z.; Bischoff, B.L.; Jang, G.G. Surface-Enhanced Separation of Water from Hydrocarbons: Potential Dewatering Membranes for the Catalytic Fast Pyrolysis of Pine Biomass. Energy Fuels 2016, 30, 8343–8348. [Google Scholar] [CrossRef]
- Simpson, J.T.; D’Urso, B.R. Superhydrophobic Diatomaceous Earth. U.S. Patent 8,216,674 B2, 10 July 2012. [Google Scholar]
- Hu, M.Z.; Simpson, J.T.; Aytug, T.; Paranthaman, M.P.; Sturgeon, M.R. Super-Surface Selective Nanomembranes Providing Simultaneous High Permeation Flux and High Selectivity. U.S. Patent 9,308,501 B2, 12 April 2016. [Google Scholar]
- ASTM F316-03—Standard Test Methods for Pore Size Characteristics of Membrane Filters by Bubble Point and Mean Flow Pore Test; ASTM International (ASTM): West Conshohocken, PA, USA, 2011.
- Zhang, P.; Lv, F.Y. A review of the recent advances in superhydrophobic surfaces and the emerging energy-related applications. Energy 2015, 82, 1068–1087. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef] [PubMed]
- Celia, E.; Carmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Inter. Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Uhlhornm, R.J.R.; Burggraaf, A.J. Inorganic Membranes Synthesis, Characteristics and Applications. In Gas Separations with Inorganic Membranes; Bhave, R.R., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1991; pp. 155–176. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W. Adsorption. In Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Kujawski, W. Pervaporative removal of organics from water using hydrophobic membranes: Binary mixtures. Sep. Sci. Technol. 2000, 35, 89–108. [Google Scholar] [CrossRef]
- Lipnizki, F.; Hausmanns, S. Hydrophobic pervaporation of binary and ternary solutions: Evaluation of fluxes, selectivities, and coupling effects. Sep. Sci. Technol. 2004, 39, 2235–2259. [Google Scholar] [CrossRef]
- Lipnizki, F.; Olsson, J.; Wu, O.; Weis, A.; Tragardh, G.; Field, R.W. Hydrophobic pervaporation: Influence of the support layer of composite membranes on the mass transfer. Sep. Sci. Technol. 2002, 37, 1747–1770. [Google Scholar] [CrossRef]
- Hickey, P.J.; Juricic, F.P.; Skater, C.S. The effect of process parameters on the pervaporation of alcohols through organophilic membranes. Sep. Sci. Technol. 1992, 27, 843–861. [Google Scholar] [CrossRef]





| Membrane Separation Performance Evaluation Tests | Membrane Types | Membrane Substrates | Surface Coatings | Membrane ID | |
|---|---|---|---|---|---|
| Vapor Phase Separation | Separations of ethanol–water vapors by membranes (~6 nm membrane pores) | Bare alumina-coated support (hydrophilic) | 4.3-µm porous SS434 tube | 6-nm porous alumina + no surface ligand modification | #0 Baseline tube |
| Superhydrophilic | 4.3-µm porous SS434 tube | 5.79-nm porous alumina + hydroxylated silica aerogel nanoparticle coating | #8 SI-tube | ||
| Superhydrophobic | 4.3-µm porous SS434 tube | 6.4-nm porous alumina + diatomaceous earth (DE) coating +1H,1H,2H, 2H-perfluoro decyl trimethoxy silane (PDTMS) modification | #12 SO-tube | ||
| Liquid Phase Solution Pervaporation | Pervaporative extraction of ethanol from ethanol–water mixtures (~60 nm membrane pores) | Bare alumina disc (Hydrophilic surface) | Alumina ceramic disc with ~60-nm pores | DE coating + PDTMS modification → superhydrophobic surface | Baseline Disc |
| Superhydrophobic | Alumina ceramic disc with ~60-nm pores | DE coating + PDTMS modification → superhydrophobic surface | SO Disc | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.Z.; Engtrakul, C.; Bischoff, B.L.; Lu, M.; Alemseghed, M. Surface-Engineered Inorganic Nanoporous Membranes for Vapor and Pervaporative Separations of Water–Ethanol Mixtures. Membranes 2018, 8, 95. https://doi.org/10.3390/membranes8040095
Hu MZ, Engtrakul C, Bischoff BL, Lu M, Alemseghed M. Surface-Engineered Inorganic Nanoporous Membranes for Vapor and Pervaporative Separations of Water–Ethanol Mixtures. Membranes. 2018; 8(4):95. https://doi.org/10.3390/membranes8040095
Chicago/Turabian StyleHu, Michael Z., Chaiwat Engtrakul, Brian L. Bischoff, Mi Lu, and Mussie Alemseghed. 2018. "Surface-Engineered Inorganic Nanoporous Membranes for Vapor and Pervaporative Separations of Water–Ethanol Mixtures" Membranes 8, no. 4: 95. https://doi.org/10.3390/membranes8040095





