Preliminary Study on Enzymatic-Based Cleaning of Cation-Exchange Membranes Used in Electrodialysis System in Red Wine Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Enzymatic Agents
2.3. Chemicals
2.4. Protocols
2.5. Analysis
2.5.1. Ion-Exchange Capacity
2.5.2. Electric Conductivity
2.5.3. Contact Angle
3. Results and Discussion
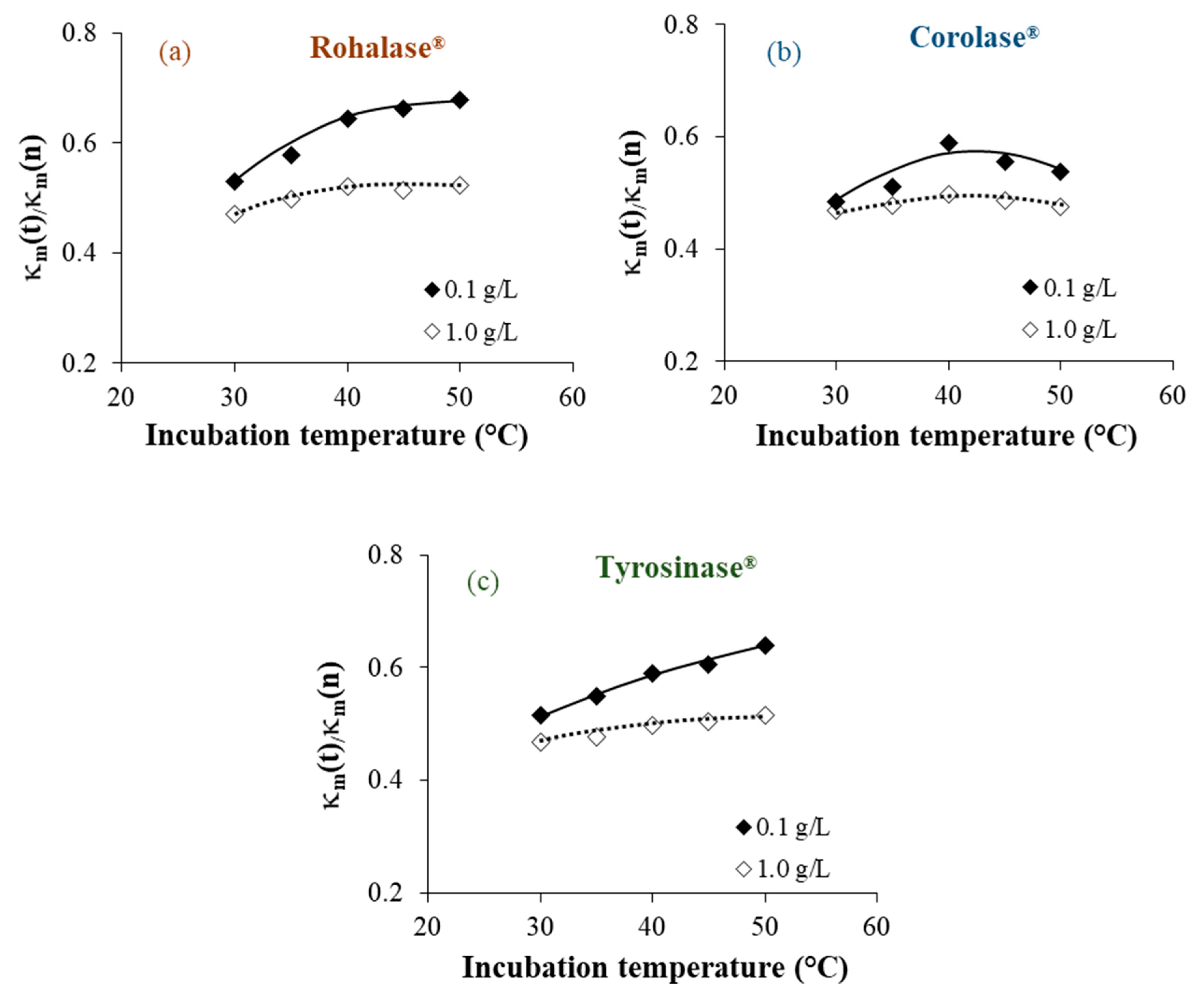
3.1. Optimizing the Operating Conditions
3.2. Membrane Cleaning Using Three Enzymatic Solutions Successively and a Mixture of Two Compatible Enzymes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langevin, M.-E.; Bazinet, L. Ion-exchange membrane fouling by peptides: A phenomenon governed by electrostatic interactions. J. Membr. Sci. 2011, 369, 359–366. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Sarapulova, V.; Nevakshenova, E.; Nebavskaya, X.; Kozmai, A.; Aleshkina, D.; Pourcelly, G.; Nikonenko, V.; Pismenskaya, N. Characterization of bulk and surface properties of anion-exchange membranes in initial stages of fouling by red wine. J. Membr. Sci. 2018, 559, 170–182. [Google Scholar] [CrossRef]
- Ghalloussi, R.; Garcia-Vasquez, W.; Chaabane, L.; Dammak, L.; Larchet, C.; Deabate, S.V.; Nevakshenova, E.; Nikonenko, V.; Grande, D. Ageing of ion-exchange membranes in electrodialysis: A structural and physicochemical investigation. J. Membr. Sci. 2013, 436, 68–78. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Chaabane, L.; Larchet, C.; Hellal, F.; Nikonenko, V.; Pismenskaya, N.D. Cleaning of cation-exchange membranes used in electrodialysis for food industry by chemical solutions. Sep. Purif. Technol. 2018, 199, 114–123. [Google Scholar] [CrossRef]
- Ghalloussi, R.; Chaabane, L.; Larchet, C.; Dammak, L.; Grande, D. Structural and physicochemical investigation of ageing of ion-exchange membranes in electrodialysis for food industry. Sep. Purif. Technol. 2014, 123, 229–234. [Google Scholar] [CrossRef]
- Šímová, H.; Kysela, V.; Černín, A. Demineralization of natural sweet whey by electrodialysis at pilot-plant scale. Desalin. Water Treat 2010, 14, 170–173. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Effects of acid–base cleaning procedure on structure and properties of anion-exchange membranes used in electrodialysis. J. Membr. Sci. 2016, 507, 12–23. [Google Scholar] [CrossRef]
- Bdiri, M.; Dammak, L.; Larchet, C.; Hellal, F.; Porozhnyy, M.; Nevakshenova, E.; Pismenskaya, N.; Nikonenko, V. Characterization and cleaning of anion-exchange membranes used in electrodialysis of polyphenol-containing food industry solutions; comparison with cation-exchange membranes. Sep. Purif. Technol. 2019, 210, 636–650. [Google Scholar] [CrossRef]
- Doi, S.; Yasukawa, M.; Kakihana, Y.; Higa, M. Alkali attack on anion exchange membranes with PVC backing and binder: Effect on performance and correlation between them. J. Membr. Sci. 2019, 573, 85–96. [Google Scholar] [CrossRef]
- Doi, S.; Kinoshita, M.; Yasukawa, M.; Higa, M. Alkali Attack on Anion Exchange Membranes with PVC Backing and Binder: II Prediction of Electrical and Mechanical Performances from Simple Optical Analyses. Membranes 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed.; W.H. Freeman: New York, NY, USA, 1985; ISBN 978-0-7167-1614-3. [Google Scholar]
- Petrus, H.B.; Li, H.; Chen, V.; Norazman, N. Enzymatic cleaning of ultrafiltration membranes fouled by protein mixture solutions. J. Membr. Sci. 2008, 325, 783–792. [Google Scholar] [CrossRef]
- te Poele, S.; van der Graaf, J. Enzymatic cleaning in ultrafiltration of wastewater treatment plant effluent. Desalination 2005, 179, 73–81. [Google Scholar] [CrossRef]
- Muñoz-Aguado, M.J.; Wiley, D.; Fane, A.G. Enzymatic detergent cleaning of polysul-phone membrane fouled with BSA and whey. J. Membr. Sci. 1996, 117, 175–187. [Google Scholar] [CrossRef]
- Rudolph, G.; Schagerlöf, H.; Morkeberg Krogh, K.B.; Jönsson, A.-S.; Lipnizki, F. Investigations of Alkaline and Enzymatic Membrane Cleaning of Ultrafiltration Membranes Fouled by Thermomechanical Pulping Process Water. Membranes 2018, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.R.; Baten, M.; Pollet, A.; Courtin, C.; Wouters, J.; Verbiest, T.; Vankelecom, I.F.J. A novel in-situ Enzymatic Cleaning Method for Reducing Membrane Fouling in Membrane Bioreactors (MBRs). Indones. J. Sci. Technol. 2016, 1, 1–22. [Google Scholar] [CrossRef]
- Gonçalves, F.; Fernandes, C.; Cameira dos Santos, P.; de Pinho, M.N. Wine tartaric stabilization by electrodialysis and its assessment by the saturation temperature. J. Food Eng. 2003, 59, 229–235. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Pismenskaya, N.; Grande, D. Evolution of anion-exchange membrane properties in a full scale electrodialysis stack. J. Membr. Sci. 2013, 446, 255–265. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- AB Enzymes, Rohalase Rohament, Products Brochure. 2011. Available online: https://www.abenzymes.com/media/2375/abenzymes_brochure_grainprocessingproducts_jul2018.pdf (accessed on 18 September 2018).
- AB Enzymes, Corolase 7089, Products Brochure. 2009. Available online: http://usc.ge/files/produqtebi/ludi/eng/4.%20Corolase_7089.pdf (accessed on 18 September 2018).
- Sigma Aldrich, Tyrosinase, Products Catalog. Available online: https://www.sigmaaldrich.com/catalog/search?term=Tyrosine&interface=All&N=0&mode=partialmax&lang=en®ion=SX&focus=product&F=PR&ST=RS&N3=mode%20matchpartialmax&N5=All (accessed on 18 September 2018).
- Faccio, G.; Kruus, K.; Saloheimo, M.; Thöny-Meyer, L. Bacterial tyrosinases and their applications. Process Biochem. 2012, 47, 1749–1760. [Google Scholar] [CrossRef]
- Macheix, J.-J.; Fleuriet, A.; Jay-Allemand, C. Les composés phénoliques des végétaux: un exemple de métabolites secondaires d’importance économique; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 2005; ISBN 978-2-88074-625-4. [Google Scholar]
- French Standard NF X 45-200; AFNOR: La Plaine Saint-Denis, France, 1995.
- Ulbricht, M.; Ansorge, W.; Danielzik, I.; König, M.; Schuster, O. Fouling in microfiltration of wine: The influence of the membrane polymer on adsorption of polyphenols and polysaccharides. Sep. Purif. Technol. 2009, 68, 335–342. [Google Scholar] [CrossRef]
- Loubser, P. The Role of Polysaccharides in Vinification and their Contribution to Aspects such as Body, Better Mouthfeel and Stability. Wineland Magazine, 1 November 2001. [Google Scholar]
- Knee, M. Polysaccharides and glycoproteins of apple fruit cell walls. Phytochemistry 1973, 12, 637–653. [Google Scholar] [CrossRef]
- Fairhead, M.; Thöny-Meyer, L. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. New Biotechnol. 2012, 29, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Le Roes-Hill, M.; Palmer, Z.; Rohland, J.; Kirby, B.M.; Burton, S.G. Partial purification and characterisation of two actinomycete tyrosinases and their application in cross-linking reactions. J. Mol. Catal. B Enzym. 2015, 122, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine—effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Bell, E.A.; Charlwood, B.V.; Harborne, J.B. (Eds.) Plant Phenolics. In Secondary Plant Products; Encyclopedia of Plant Physiology; Springer: Berlin/Heidelberg, Germany, 1980; Volume 8, ISBN 978-3-642-67362-7. [Google Scholar]
- Macheix, J.-J.; Fleuriet, A. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-0-8493-4968-3. [Google Scholar]
- Sarapulova, V.; Nevakshenova, E.; Pismenskaya, N.; Dammak, L.; Nikonenko, V. Unusual concentration dependence of ion-exchange membrane conductivity in ampholyte-containing solutions: Effect of ampholyte nature. J. Membr. Sci. 2015, 479, 28–38. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compounds. In Handbook of Enology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; ISBN 978-0-470-01039-6. [Google Scholar]
- Ibeas, V.; Correia, A.C.; Jordão, A.M. Wine tartrate stabilization by different levels of cation exchange resin treatments: impact on chemical composition, phenolic profile and organoleptic properties of red wines. Food Res. Int. 2015, 69, 364–372. [Google Scholar] [CrossRef]



| Characteristics | CMX-Sb(n) | CMX-Sb(u) |
|---|---|---|
| IEC (mmol g−1) | 2.47 ± 0.13 | 1.40 ± 0.06 |
| Tm (µm) | 177 ± 3 | 270 ± 4 |
| κm (mS cm−1) | 13.4 ± 0.6 | 6.2 ± 0.4 |
| θ (°) | 47 ± 1.3 | 49.3 ± 0.9 |
| WC (%) | 26.1 ± 0.9 | 40.0 ± 2.1 |
| f2 | 0.11 ± 0.01 | 0.04 ± 0.01 |
| E (MPa) | 555 ± 11 | 264 ± 17 |
| Rp (MPa) | 29 ± 3 | 11 ± 1 |
| Cleaning Operations | Total Duration (h) | % κm (mS cm−1) | % IEC (mmol g−1) | % θ (°) |
|---|---|---|---|---|
| Rohalase® BXL | 20 | +45 | +23 | −16 |
| Corolase® 7089 | 20 | +26 | +14 | −13 |
| Tyrosinase® | 20 | +37 | +18 | −12 |
| Successive cleanings | 30 (10 h/enzyme) | +50 | +33 | −15 |
| Enzymes cocktail | 10 | +25 | +18 | −13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bdiri, M.; Bensghaier, A.; Chaabane, L.; Kozmai, A.; Baklouti, L.; Larchet, C. Preliminary Study on Enzymatic-Based Cleaning of Cation-Exchange Membranes Used in Electrodialysis System in Red Wine Production. Membranes 2019, 9, 114. https://doi.org/10.3390/membranes9090114
Bdiri M, Bensghaier A, Chaabane L, Kozmai A, Baklouti L, Larchet C. Preliminary Study on Enzymatic-Based Cleaning of Cation-Exchange Membranes Used in Electrodialysis System in Red Wine Production. Membranes. 2019; 9(9):114. https://doi.org/10.3390/membranes9090114
Chicago/Turabian StyleBdiri, Myriam, Asma Bensghaier, Lobna Chaabane, Anton Kozmai, Lassaad Baklouti, and Christian Larchet. 2019. "Preliminary Study on Enzymatic-Based Cleaning of Cation-Exchange Membranes Used in Electrodialysis System in Red Wine Production" Membranes 9, no. 9: 114. https://doi.org/10.3390/membranes9090114





