Cardiotoxicity of Fluoropyrimidines: Epidemiology, Mechanisms, Diagnosis, and Management
Abstract
:1. History of 5-Fluorouracil (5-FU) Administration
2. Frequency of Fluoropyrimidine-Induced Cardiotoxicity
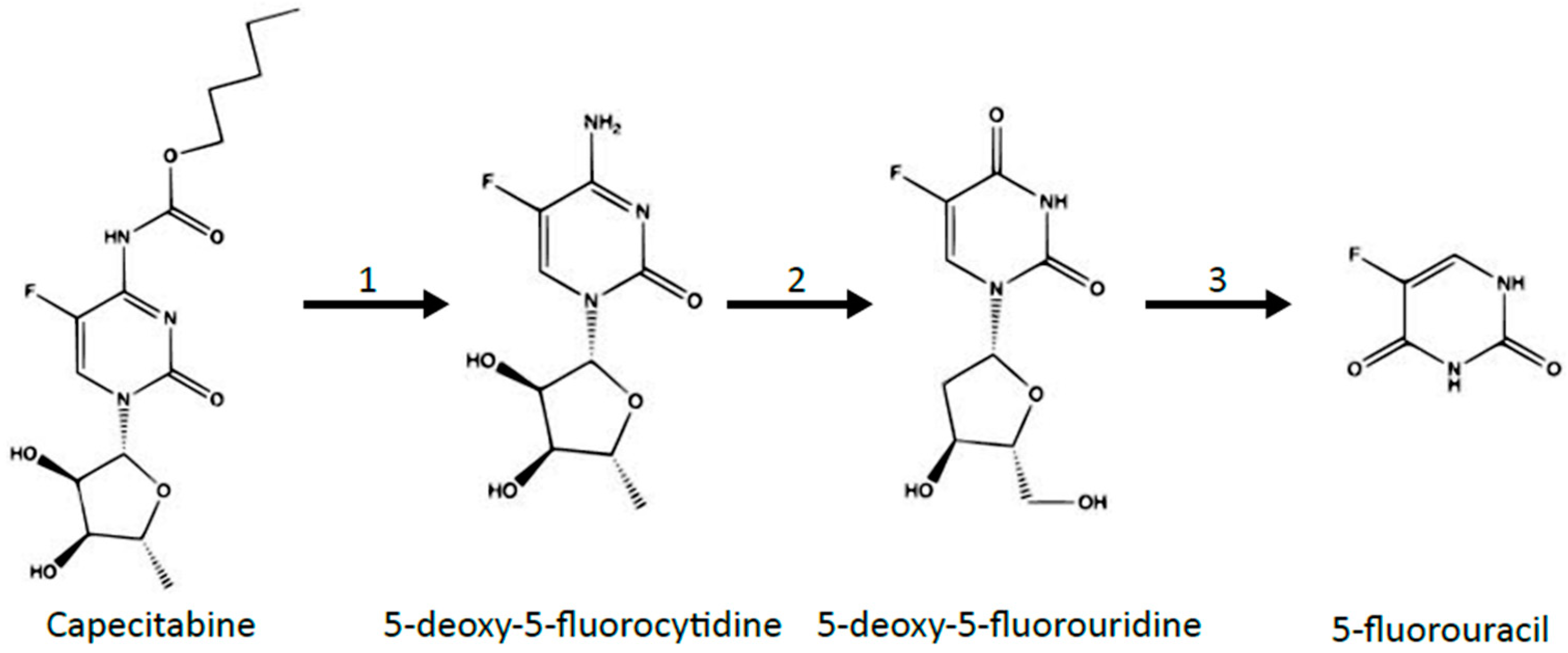
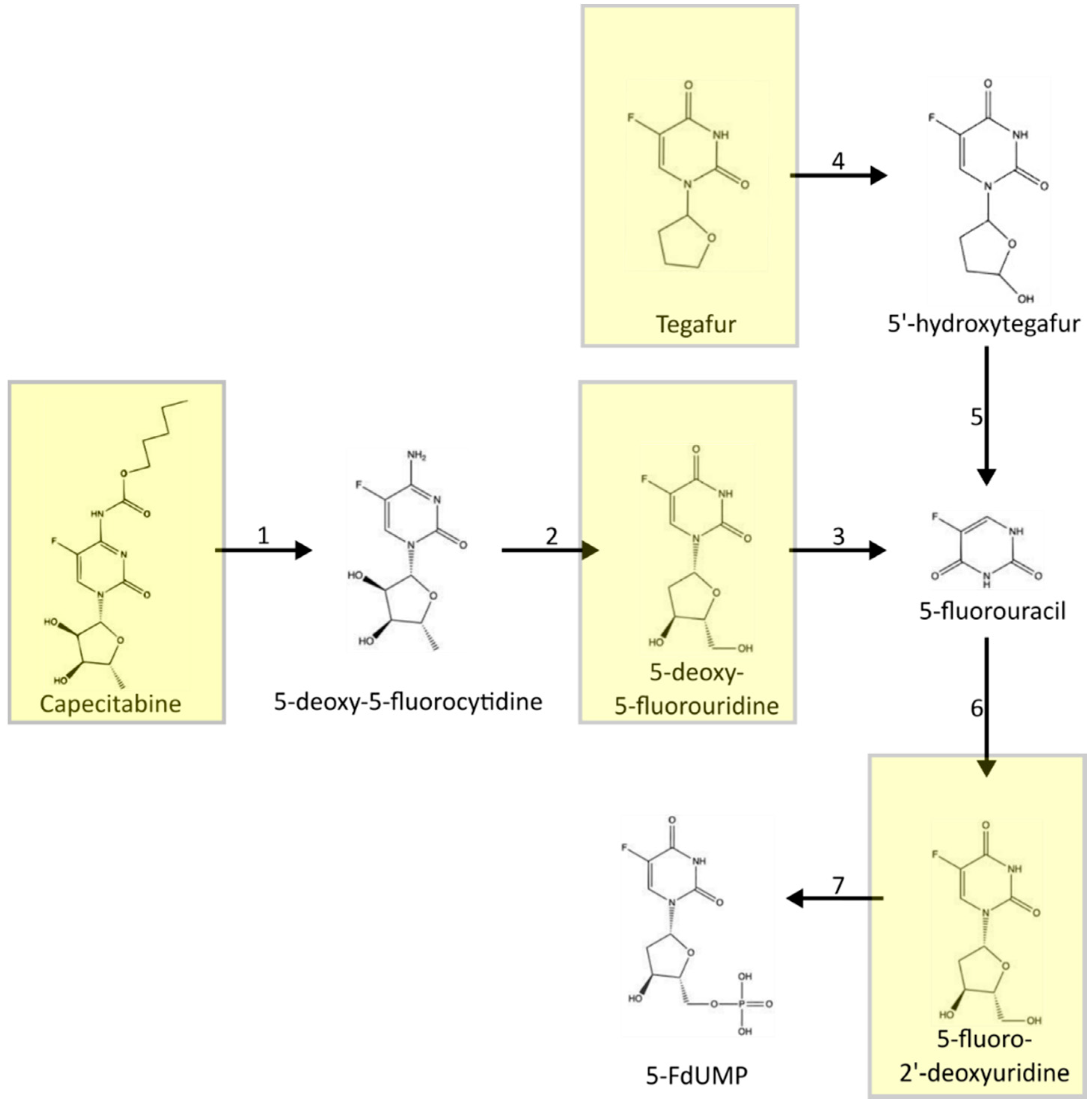
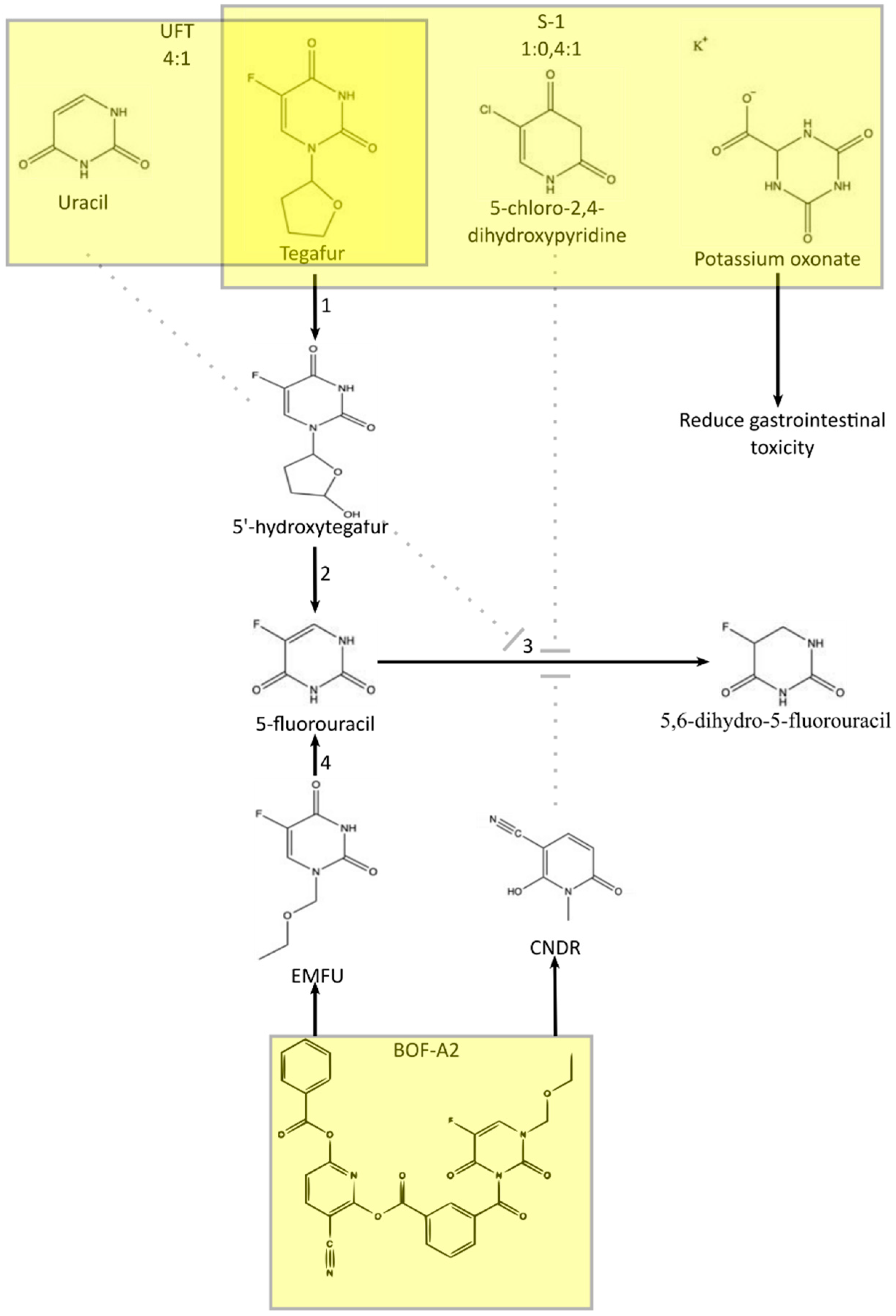
3. Metabolism of 5-FU
4. Symptoms of Fluoropyrimidine Cardiotoxicity
| Article | Frequency of Sings and Symptoms |
|---|---|
| Jensen et al. (2006) * [31] | Angina: 3.9% Arrhythmias 0.4% |
| Rezkalla et al. (1989) # [24] | ECG changes: 68% |
| De Forni et al. (1992) # [66] | Angina pectoris: 4.9% Hypotension: 0.3% |
| Tsavaris et al. (2002) # [67] | ECG changes: 4% Arrhythmias: 2.3% Chest pain: 2.1% Myocardial infarction: 1.6% Palpitation: 1.4% Conductive abnormalities: 0.9% Malaise: 0.5% Loss of consciousness: 0.5% |
| Kosmas et al. (2008) # [30] | ECG changes: 4% Chest pain: 1.7% Palpitation: 1.1% Malaise: 0.6% |
| Koca et al. (2011) # [33] | ECG changes: 30.8% Palpitation: 23% Angina: 9.6% Dyspnea 7.6% Tachycardia: 5.6% Hypotension: 3.8% Hypertension: 1.9% |
| Peng et al. (2018) # [35] | Ischemic change: 19.9% Arrhythmia: 16.8% Heart failure: 2.6% Myocardial infraction: 1.0% |
| Dyhl-Polk et al. (2020) # [37] | Acute coronary syndrome: 4% Chest pain: 0.7% |
5. Routes of 5-FU Administration
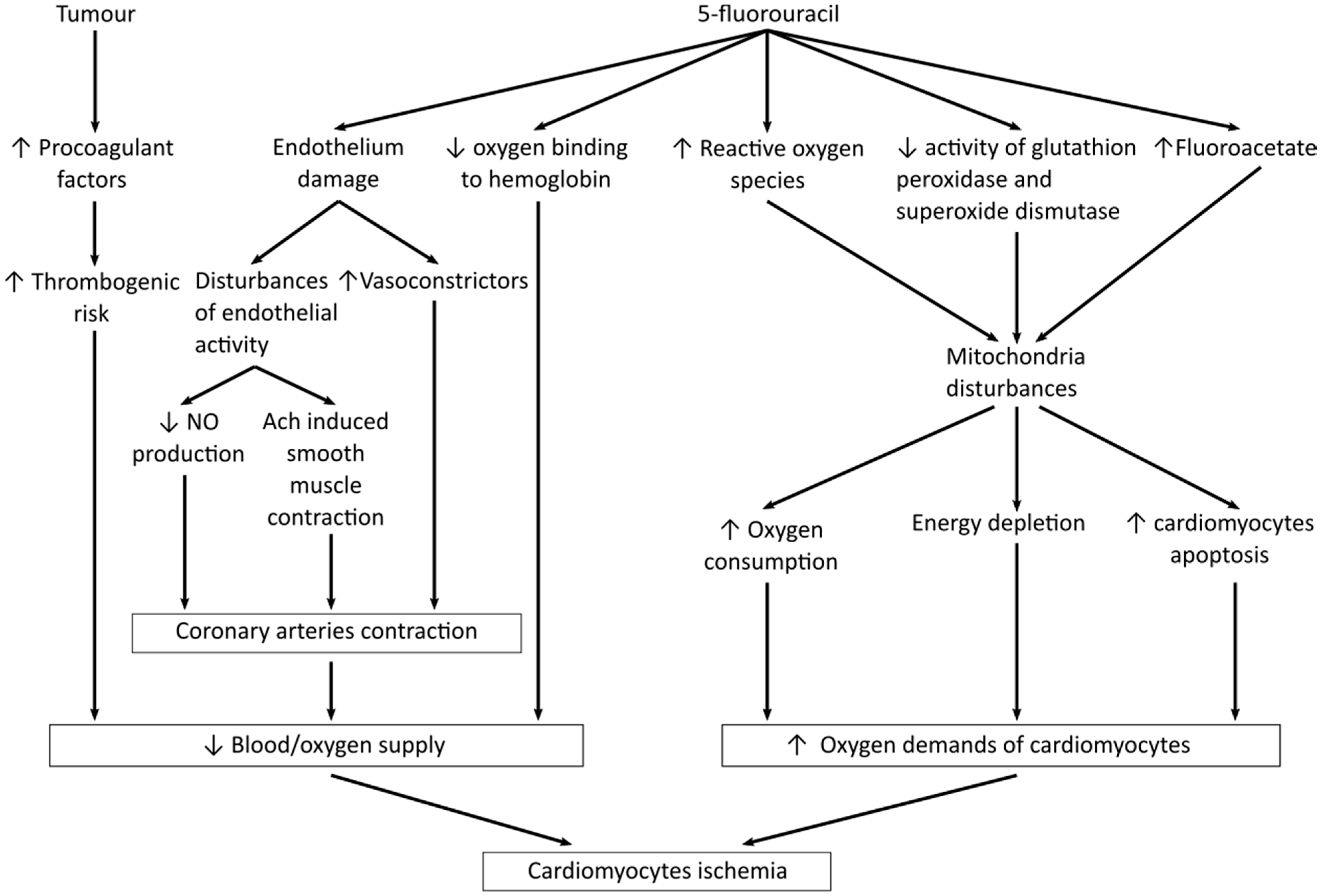
6. Mechanisms of Fluoropyrimidines Cardiotoxicity
7. Diagnostics of 5-FU Cardiotoxicity
8. Cardiotoxicity Risk Factors
9. Management of Fluoropyrimidine Cardiotoxicity
10. Reintroduction of 5-FU
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodman, L.; Wintrobe, M.; Dameshek, W.; Goodman, N.; Gilman, A.; McLennan, M. Nitrogen mustard therapy use of methyl-bis (β-chloroethyl)amine hydrochloride and tris(beta-chloroethyl)amine hydrochloride for hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA 1946, 132, 126–132. [Google Scholar] [CrossRef]
- Allison, J.D.; Tanavin, T.; Yang, Y.; Birnbaum, G.; Khalid, U. Various manifestations of 5-fluorouracil cardiotoxicity: A multicenter case series and review of literature. Cardiovas. Toxicol. 2020, 20, 437–442. [Google Scholar] [CrossRef]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated Pyrimidines, A New Class of Tumour-Inhibitory Compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef]
- Madeddu, C.; Deidda, M.; Piras, A.; Cadeddu, C.; Demurtas, L.; Puzzoni, M.; Piscopo, G.; Scartozzi, M.; Mercuro, G. Pathophysiology of cardiotoxicity induced by nonanthracycline chemotherapy. J. Cardiovasc. Med. 2016, 17, S12–S18. [Google Scholar] [CrossRef]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of chemotherapeutic agents incidence, treatment and prevention. Drug Saf. 2000, 22, 263–302. [Google Scholar] [CrossRef] [PubMed]
- Shaib, W.; Lee, V.; Saif, W. Bolus 5-fluorouracil as an alternative modality to infusion 5-fluorouracil in a patient with rectal cancer and capecitabine-induced cardiotoxicity. In Vivo 2009, 23, 821–826. [Google Scholar] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-Fluorouracil and Cardiotoxicity: A Review. Ther. Adv. Med. Oncol. 2018, 10, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, A.J. Drug Interactions and Reactions Update. Fluorouracil Cardiotoxicity. Ann. Pharmacother. 1994, 28, 374–378. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Vokes, E.E. Review 5-Fluorouracil Cardiotoxicity: A Critical Review. Ann. Oncol. 1990, 1, 409–414. [Google Scholar] [CrossRef]
- Labianca, R.; Beretta, G.; Clerici, M.; Fraschini, P.; Luporini, G. Cardiac Toxicity of 5-Fluorouracil: A Study on 1083 Patients. Tumori 1982, 68, 505–510. [Google Scholar] [CrossRef]
- Kikuchi, K.; Majima, S.; Murakami, M. Clinical survey on cardiotoxicity of tegafur (FT-207)—Compilation of a nationwide survey. Gan To Kagaku Ryoho. Cancer Chemother. 1982, 9, 1482–1488. [Google Scholar]
- Lokich, J. Infusional 5-FU: Historical evolution, rationale, and clinical experience. Oncology 1998, 12, 19–22. [Google Scholar]
- Seifert, P.; Baker, L.H.; Reed, M.L.; Vaitkevicius, V.K. Comparison of continuously infused 5-fluorouracil with bolus injection in treatment of patients with colorectal adenocarcinoma. Cancer 1975, 36, 123–128. [Google Scholar] [CrossRef]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatinas first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- De Falco, V.; Napolitano, S.; Roselló, S.; Huerta, M.; Cervantes, A.; Ciardiello, F.; Troiani, T. How we treat metastatic colorectal cancer. ESMO Open 2020, 4, e000813. [Google Scholar] [CrossRef]
- Layoun, M.E.; Wickramasinghe, C.D.; Peralta, M.V.; Yang, E.H. Fluoropyrimidine-induced cardiotoxicity: Manifestations, mechanisms, and management. Curr. Oncol. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Grem, J.L. 5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development. Investig. New Drugs 2000, 18, 299–313. [Google Scholar] [CrossRef]
- Upshaw, J.N.; O’Neill, A.; Carver, J.R.; Dimond, E.P.; Denlinger, C.S.; Kircher, S.M.; Wagner, L.I.; Ky, B.; Brell, J.M. Fluoropyrimidine Cardiotoxicity: Time for a Contemporaneous Appraisal. Clin. Colorectal Cancer 2019, 18, 44–51. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef]
- Cassidy, J.; Twelves, C.; van Cutsem, E.; Hoff, P.; Bajetta, E.; Boyer, M.; Rugat, R.; Burger, U.; Garin, A.; Graeven, U.; et al. First-line oral capecitabine therapy in metastatic colorectal cancer: A favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann. Oncol. 2002, 13, 566–575. [Google Scholar] [CrossRef]
- Lestuzzi, C.; Vaccher, E.; Talamini, R.; Lleshi, A.; Meneguzzo, N.; Viel, E.; Scalone, S.; Tartuferi, L.; Buonadonna, A.; Ejiofor, L.; et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: An underestimated risk. Ann. Oncol. Off. J. Euro. Soc. Med. Oncol. 2014, 25, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Euro. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.; Kloner, R.A.; Ensley, J.; Al-Sarraf, M.; Revels, S.; Olivenstein, A.; Bhasin, S.; Kerpel-Fronious, S.; Turi, Z.G. Continuous ambulatory ECG monitoring during fluorouracil therapy: A prospective study. J. Clin. Oncol. 1989, 7, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Kanduri, J.; More, L.A.; Godishala, A.; Asnani, A. Fluoropyrimidine-associated cardiotoxicity. Cardiol. Clin. 2019, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Erckenbrecht, J.F.; Häussinger, D.; Frieling, T. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999, 57, 475–484. [Google Scholar] [CrossRef]
- Keefe, D.L.; Roistacher, N.; Pierri, M.K. Clinical Cardiotoxicity of 5-Fluorouracil. J. Clin. Pharmacol. 1993, 33, 1060–1070. [Google Scholar] [CrossRef]
- Meyer, C.C.; Calis, K.A.; Burke, L.B.; Walawander, C.A.; Grasela, T.H. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy 1997, 17, 729–736. [Google Scholar]
- Wacker, A.; Lersch, C.; Scherpinski, U.; Reindl, L.; Seyfarth, M. High incidence of angina pectoris in patients treated with 5-fluorouracil: A planned surveillance study with 102 patients. Oncology 2003, 65, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Kosmas, C.; Kallistratos, M.S.; Kopterides, P.; Syrios, J.; Skopelitis, H.; Mylonakis, N.; Karabelis, A.; Tsavaris, N. Cardiotoxicity of fluoropyrimidines in different schedules of administration: A prospective study. J. Cancer Res. Clin. Oncol. 2008, 134, 75–82. [Google Scholar] [CrossRef]
- Jensen, S.A.; Sørensen, J.B. Risk Factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother. Pharmacol. 2006, 58, 487–493. [Google Scholar] [CrossRef]
- Jensen, S.A.; Hasbak, P.; Mortensen, J.; Sørensen, J.B. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J. Clin. Oncol. 2010, 28, 5280–5286. [Google Scholar] [CrossRef]
- Koca, D.; Salman, T.; Unek, I.T.; Oztop, I.; Ellidokuz, H.; Eren, M.; Yilmaz, U. Clinical and electrocardiography changes in patients treated with capecitabine. Chemotherapy 2012, 57, 381–387. [Google Scholar] [CrossRef]
- Khan, M.A.; Masood, N.; Husain, N.; Ahmad, B.; Aziz, T.; Naccm, A. A retrospective study of cardiotoxicities induced by 5-fluouracil (5-FU) And5-FU based chemotherapy regimens in pakistani adult cancer patientsat shaukat khanum memorial cancer hospital & research center. J. Pak. Med. Assoc. 2012, 62, 430–434. [Google Scholar]
- Peng, J.; Dong, C.; Wang, C.; Li, W.; Yu, H.; Zhang, M.; Zhao, Q.; Zhu, B.; Zhang, J.; Li, W.; et al. Cardiotoxicity of 5-fluorouracil and capecitabine in chinese patients: A prospective study. Cancer Commun. 2018, 38, 22. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Bai, Y.; Gao, L.; Wu, S. Incidence of and risk factors for cardiotoxicity after fluorouracil-based chemotherapy in locally advanced or metastatic gastric cancer patients. Cancer Chemother. Pharmacol. 2019, 84, 599–607. [Google Scholar] [CrossRef]
- Dyhl-Polk, A.; Vaage-Nilsen, M.; Schou, M.; Vistisen, K.K.; Lund, C.M.; Kümler, T.; Appel, J.M.; Nielsen, D.L. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Oncol. 2020, 59, 475–483. [Google Scholar] [CrossRef]
- Liebecq, C.; Peters, A.R. The toxicity of fluoroacetate and the tricarboxylic acid cycle. Biochim. Biophys. 1949, 3, 215–230. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Malet-Martino, M.; Jolimaitre, P.; Martino, R. The prodrugs of 5-fluorouracil. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 267–310. [Google Scholar] [CrossRef] [PubMed]
- Golias, C.; Dimitriadis, G.; Dimitriadis, D.; Graidis, C.; Dimitrelos, I.; Tsiakou, A.; Bitsis, T.; Charalabopoulos, K. Acute presentation of vasospastic angina induced by oral capecitabine: A case report. J. Med. Case Rep. 2014, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Shirasaka, T. Development history and concept of an oral anticancer agent S-1 (TS-1®): Its clinical usefulness and future vistas. Jpn. J. Clin. Oncol. 2009, 39, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Muhale, F.A.; Wetmore, B.A.; Thomas, R.S.; McLeod, H.L. Systems pharmacology assessment of the 5-fluorouracil pathway. Pharmacogenomics 2011, 12, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Rooseboom, M.; Commandeur, J.N.M.; Vermeulen, N.P.E. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004, 56, 53–102. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-FU metabolism in cancer and orally-administrable 5-FU drugs. Cancers 2010, 14, 1717–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, M.G.; Farrell, M.P.; Schmitz, J.C. Thymidylate synthase: A critical target for cancer chemotherapy. Clin. Colorectal Cancer 2002, 1, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; McLeod, H.L. Can dihydropyrimidine dehydrogenase impact 5-fluorouracil-based treatment? Euro. J. Cancer 2000, 36, 37–42. [Google Scholar] [CrossRef]
- Lischke, J.; Lang, C.; Sawodny, O.; Feuer, R. Impairment of Energy Metabolism in Cardiomyocytes Caused by 5-FU Catabolites Can Be Compensated by Administration of Amino Acids. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 1 November 1998. [Google Scholar] [CrossRef]
- Fraile, R.J.; Baker, L.H.; Buroker, T.R.; Horwitz, J.; Vaitkevicius, V.K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980, 40, 2223–2228. [Google Scholar] [PubMed]
- Tanaka, F.; Fukuse, T.; Wada, H.; Fukushima, M. The history, mechanism and clinical use of oral 5-fluorouracil derivative chemotherapeutic agents. Curr. Pharm. Biotechnol. 2000, 1, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Mannino, S. 5-fluorouracil-induced coronary vasospasm. Am. Heart J. 1987, 114, 433–436. [Google Scholar] [CrossRef]
- Karakulak, U.N.U.N.; Aladaǧ, E.; Maharjan, N.; Övünç, K. Capecitabine-induced coronary artery vasospasm in a patient who previously experienced a similar episode with fluorouracil therapy. Turk. Kardiyol. Dern. Ars. 2016, 44, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, S.S.; Salim, K.P.; Bano, Z.A. Symptomatic cardiotoxicity with high-dose 5-fluorouracil infusion: A prospective study. Oncology 1993, 50, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Płońska-Gościniak, E.; Różewicz, M.; Kasprzak, J.; Wojtarowicz, A.; Mizia-Stec, K.; Hryniewiecki, T.; Pysz, P.; Kułach, A.; Bodys, A.; Sulżyc, V.; et al. Tissue doppler echocardiography detects subclinical left ventricular dysfunction in patients undergoing chemotherapy for colon cancer: Insights from ONCOECHO multicentre study. Kardiol. Pol. 2017, 75, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, U.; Oztop, I.; Ciloglu, A.; Okan, T.; Tekin, U.; Yaren, A.; Somali, I.; Alacacioglu, A.; Kirimli, O. 5-fluorouracil increases the number and complexity of premature complexes in the heart: A prospective study using ambulatory ECG monitoring. Int. J. Clin. Pract. 2007, 61, 795–801. [Google Scholar] [CrossRef]
- Amraotkar, A.R.; Pachika, A.; Grubb, K.J.; DeFilippis, A.P. Rapid extracorporeal membrane oxygenation overcomes fulminant myocarditis induced by 5-fluorouracil. Tex. Heart Inst. J. 2016, 43, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Çalik, A.N.; Çeliker, E.; Velibey, Y.; Çaǧdaş, M.; Güzelburç, Ö. Initial dose effect of 5-fluorouracil: Rapidly improving severe, acute toxic myopericarditis. Am. J. Emerg. Med. 2012, 30, 257.e1–257.e3. [Google Scholar] [CrossRef] [PubMed]
- Sundaravel, S.; Alrifai, A.; Kabach, M.; Ghumman, W. FOLFOX induced takotsubo cardiomyopathy treated with impella assist device. Case Rep. Cardiol. 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Joy, G.; Eissa, H.; Al Karoudi, R.; White, S.K. Fluorouracil-induced takotsubo cardiomyopathy causing cardiogenic shock: A case report of clinical and acute cardiac magnetic resonance imaging features. Euro. Heart J. Case Rep. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Gianni, M.; Dentali, F.; Lonn, E. 5 flourouracil-induced apical ballooning syndrome: A case report. Blood Coagul. Fibrinolysis 2009, 20, 306–308. [Google Scholar] [CrossRef]
- Moriyama, S.; Yokoyama, T.; Irie, K.; Ito, M.; Tsuchihashi, K.; Fukata, M.; Kusaba, H.; Maruyama, T.; Akashi, K. Atrial fibrillation observed in a patient with esophageal cancer treated with fluorouracil. J. Cardiol. Cases 2019, 20, 183–186. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.; Mahmood, A.; Dogar, M.; Guo, J.; Nwamaghinna, F.; Salciccioli, L.; Mcfarlane, S.I. Simultaneous cardiotoxicity and neurotoxicity associated with 5-fluorouracil containing chemotherapy: A case report and literature review. Am. J. Med. Case Rep. 2020, 8, 73–75. [Google Scholar] [CrossRef]
- Polk, A.; Shahmarvand, N.; Vistisen, K.; Vaage-Nilsen, M.; Ole Larsen, F.; Schou, M.; Lisbeth Nielsen, D. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: A retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open 2016, 6, e012798. [Google Scholar] [CrossRef] [Green Version]
- Saunders, S.; Anwar, M. Capecitabine-induced myopericarditis-a case report and review of literature. J. Oncol. Pharm. Prac. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2019, 25, 1006–1010. [Google Scholar] [CrossRef]
- Qasem, A.; bin Abdulhak, A.A.; Aly, A.; Moormeier, J. Capecitabine-induced takotsubo cardiomyopathy: A case report and literature review. Am. J. Ther. 2014, 23, 1–5. [Google Scholar] [CrossRef]
- de Forni, M.; Malet-Martino, M.C.; Jaillais, P.; Shubinski, R.E.; Bachaud, J.M.; Lemaire, L.; Canal, P.; Chevreau, C.; Carrié, D.; Soulié, P. Cardiotoxicity of high-dose continuous infusion fluorouracil: A prospective clinical study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1992, 10, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Tsavaris, N.; Kosmas, C.; Vadiaka, M.; Zinelis, A.; Beldecos, D.; Sakelariou, D.; Koufos, C.; Stamatelos, G. Cardiotoxicity following different doses and schedules of 5-fluorouracil administration for malignancy—A survey of 427 patients. Med. Sci. Monit. 2002, 8, 51–57. [Google Scholar]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef]
- Saif, M.W.; Shah, M.M.; Shah, A.R. Fluoropyrimidine-associated cardiotoxicity: Revisited. Exp. Opin. Drug Safety 2009, 8, 191–202. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Colin, P.; Louvet, C.; Gomelin, E.; Bouche, O.; Achille, E.; Colbert, N.; Boaziz, C.; Piedbois, P.; Tubiana-Mathieu, N.; et al. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: Results of a randomized trial. J. Clin. Oncol. 2003, 21, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Arkenau, H.T.; Rettig, K.; Porschen, R. Adjuvant chemotherapy in curative resected colon carcinoma: 5-fluorouracil/leucovorin versus high-dose 5-fluorouracil 24-h infusion/leucovorin versus high-dose 5-fluorouracil 24-h infusion. Int. J. Colorectal Dis. 2005, 20, 258–261. [Google Scholar] [CrossRef] [PubMed]
- De Gramont, A.; Bosset, J.-F.; Milan, C.; Rougier, P.; Bouch, O.; Etienne, P.-L.; Morvan, F.; Louvet, C.; Guillot, T.; Frangois, E.; et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: A French intergroup study. J. Clin. Oncol. 1997, 15, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Meydan, N.; Kundak, I.; Yavuzsen, T.; Oztop, I.; Barutca, S.; Yilmaz, U.; Alakavuklar, M.N. Cardiotoxicity of de gramont’s regimen: Incidence, clinical characteristics and long-term follow-up. Jpn. J. Clin. Oncol. 2005, 35, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holubec, L., Jr.; Topolcan, O.; Finek, J.; Salvet, J.; Svoboda, T.; Svobodova, S.; Mrazkova, P.; Ludvikowa, M. Dynamic monitoring of cardio-specific markers and markers of thyroid gland function in cancer patients-A pilot study. Anticancr Res. 2007, 27, 1883–1886. [Google Scholar]
- Schaaf, L.J.; Dobbs, B.R.; Edwards, I.R.; Pettier, D.G. European journal of clinical pharmacology nonlinear pharmacokinetic characteristics of 5-fluorouracil (5-FU) in colorectal cancer patients. Eur. J. Clin. Pharmacol. 1987, 32, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mcdermott, B.J.; van den Berg, H.W.; Murphy, R.F. Ancer hemotherapy and harmacology nonlinear pharmacokinetics for the elimination of 5-fluorouracil after intravenous administration in cancer patients. Cancer Chemother. Pharmacol. 1982, 9, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Sitar, D.; Shaw, D.; Thirwell, M.; Ruedy, J. Disposition of 5-fluorouracil after intravenous bolus dosesof a commercial formulation to cancer patients1. Cancer Res. 1977, 37, 3961–3964. [Google Scholar]
- Heggie, G.D.; Sommadossi, J.-P.; Cross, D.S.; Huster, W.J.; Diasio2, R.B. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile1. Cancer Res. 1987, 47, 2203–2206. [Google Scholar] [PubMed]
- Lerner-Tung, M.B.; Chang, A.Y.; Ong, L.S.; Kreiser, D. Pharmacokinetics of intrapericardial administration of 5-fluorouracil. Cancer Chemother. Pharmacol. 1997, 40, 318–320. [Google Scholar] [CrossRef]
- Twelves, C.; Gollins, S.; Grieve, R.; Samuel, L. A randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Ann. Oncol. 2006, 17, 239–245. [Google Scholar] [CrossRef]
- Ben-Yakov, M.; Mattu, A.; Brady, W.J.; Dubbs, S.B. Prinzmetal angina (coronary vasospasm) associated with 5-fluorouracil chemotherapy. Am. J. Emerg. Med. 2017, 35, 1038.e3–1038.e5. [Google Scholar] [CrossRef]
- Depetris, I.; Marino, D.; Bonzano, A.; Cagnazzo, C.; Filippi, R.; Aglietta, M.; Leone, F. Fluoropyrimidine-induced cardiotoxicity. Crit. Rev. Oncol. Hematol. 2018, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Polk, A.; Vistisen, K.; Vaage-Nilsen, M.; Nielsen, D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 2014, 15, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.H.; Ghosh, A.K. Coronary artery vasospasm induced by 5-fluorouracil: Proposed mechanisms, existing management options and future directions. Interv. Cardiol. Rev. 2019, 14, 89. [Google Scholar] [CrossRef] [Green Version]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sessa, F.; Noonan, D.M.; et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ros production in endothelial cells and cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef]
- Lamberti, M.; Porto, S.; Zappavigna, S.; Addeo, E.; Marra, M.; Miraglia, N.; Sannolo, N.; Vanacore, D.; Stiuso, P.; Caraglia, M. A Mechanistic study on the cardiotoxicity of 5-fluorouracil in vitro and clinical and occupational perspectives. Toxicol. Lett. 2014, 227, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Thyss, A.; Gaspard, M.H.; Marsault, R.; Milano, G.; Frelin, C.; Schneider, M. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann. Oncol. 1992, 3, 88. [Google Scholar] [CrossRef]
- Seker, M.; Isen, H.C.; Çevirme, N.; Aydln, S.; Bilici, A.; Bulut, H.; Yasin, A.I.; Coban, E.; Demir, T.; Aliyev, A.; et al. Role of urotensin-2 in 5-fluorouracil-related arterial vasoconstriction in cancer patients. Oncol. Res. Treat. 2018, 41, 545–549. [Google Scholar] [CrossRef]
- Kinhult, S.; Albertsson, M.; Eskilsson, J.; Cwikiel, M. Antithrombotic treatment in protection against thrombogenic effects of 5-fluorouracil on vascular endothelium: A scanning microscopy evaluation. Scanning 2001, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Falanga, A.; Piccioli, A. Cancer and venous thromboembolism. Lancet Oncol. 2005, 6, 401–410. [Google Scholar] [CrossRef]
- Jensen, S.A.; Sørensen, J.B. 5-fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer Chemother. Pharmacol. 2012, 69, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A.; Treasure, C.B.; Nabel, E.G.; McLenachan, J.M.; Fish, R.D.; Yeung, A.C.; Vekshtein, V.I.; Selwyn, A.P.; Ganz, P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 1990, 81, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Hasdai, D.; Gibbons, R.J.; Holmes, D.R.; Higano, S.T.; Lerman, A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects background coronary endothelial dysfunction may occur in patients with minimally. Circulation 1997, 96, 3390–3395. [Google Scholar] [CrossRef] [Green Version]
- Mosseri, M.; Fingert, H.J.; Varticovski, L.; Chokshi, S.; Isner2, J.M. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle1. Cancer Res. 1993, 53, 30–33. [Google Scholar]
- Salepci, T.; Seker, M.; Uyarel, H.; Gumus, M.; Bilici, A.; Ustaalioğlu, B.B.O.; Öztürk, A.; Sonmez, B.; Orcun, A.; Ozates, M.; et al. 5-fluorouracil induces arterial vasoconstrictions but does not increase angiotensin II levels. Med. Oncol. 2010, 27, 416–420. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Q.; Zhou, L.; Liu, K.; Jiao, K. Complex regulation of mitochondrial function during cardiac development. J. Am. Heart Assoc. 2019, 8, e012731. [Google Scholar] [CrossRef]
- Durak, I.; Karaayvaz, M.; Kavutcu, M.; Cimen, M.Y.; Kaçmaz, M.; Büyükkoçak, S.; Oztürk, H.S. reduced antioxidant defense capacity in myocardial tissue from guinea pigs treated with 5-fluorouracil. J. Toxicol. Environ. Health. Part A 2000, 59, 585–589. [Google Scholar] [CrossRef]
- Arellanol, M.; Malet-Martino’, M.; Martino1, R.; Gires2, P. The Anti-Cancer Drug 5-Fluorouracil Is Metabolized by the Isolated Perfused Rat Liver and in Rats into Highly Toxic Fluoroacetate. Br. J. Cancer 1998, 77, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, H.; Patel, A. Biochemical basis for fluorouracil neurotoxicity the role of krebs cycle inhibition by fluoroacetate. Arch. Neurol. 1970, 23, 155–160. [Google Scholar] [CrossRef]
- Muneoka, K.; Shirai, Y.; Yokoyama, N.; Wakai, T.; Hatakeyama, K. 5-fluorouracil cardiotoxicity induced by α-fluoro-β-alanine. Int. J. Clin. Oncol. 2005, 10, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Spasojević, I.; Maksimović, V.; Zakrzewska, J.; Bacić, G. Effects of 5-fluorouracil on erythrocytes in relation to its cardiotoxicity: Membrane structure and functioning. J. Chem. Inf. Modeling 2005, 45, 1680–1685. [Google Scholar] [CrossRef]
- Spasojević, I.; Jelić, S.; Zakrzewska, J.; Bačić, G. Decreased oxygen transfer capacity of erythrocytes as a cause of 5-fluorouracil related ischemia. Molecules 2009, 14, 53–67. [Google Scholar] [CrossRef]
- Ceyhan, C.; Meydan, N.; Barutca, S.; Tekten, T.; Onbasılı, A.O.; Ozturkà, B.; Unal, S.; Bayrak, I. Influence of high-dose leucovorin and 5-fluorouracil chemotherapy regimen on p wave duration and dispersion. J. Clin. Pharm. Ther. 2004, 29, 267–271. [Google Scholar] [CrossRef]
- Vasić, M.; Lončar-Turukalo, T.; Tasić, T.; Matić, M.; Glumac, S.; Bajić, D.; Popović, B.; Japundžić-Žigon, N. Cardiovascular variability and β-ars gene expression at two stages of doxorubicin–induced cardiomyopathy. Toxicol. Appl. Pharmacol. 2019, 362, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, C.; Meydan, N.; Barutca, S.; Tekten, T.; Onbasili, A.O.; Ozturk, B.; Unal, S. Ultrasound tissue characterization by integrated backscatter for analyzing fluorouracil induced myocardial damage. Echocardiography 2005, 22, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Barutca, S.; Ceyhan, C.; Meydan, N.; Ozturk, B.; Tekten, T.; Onbasili, A.; Kadikoylu, G.; Bolaman, Z. A New perspective on cardiotoxicity of 5-fluorouracil: A novel research tool “cardiac ultrasonic integrated backscatter analysis” indicates transient, subclinical myocardial dysfunction due to high-dose leucovorin and infusional 5-fluorouracil regimen. Chemotherapy 2004, 50, 113–118. [Google Scholar] [CrossRef]
- Turan, T.; Agac, M.T.; Aykan, A.Ç.; Kul, S.; Akyüz, A.R.; Gökdeniz, T.; Gül, İ.; Cengiz, E.; Boyacı, F.; Erkan, H.; et al. Usefulness of heart-type fatty acid-binding protein and myocardial performance index for early detection of 5-fluorouracil cardiotoxicity. Angiology 2017, 68, 52–58. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Robben, N.C.; Pippas, A.W.; Moore, J.O. The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer 1993, 71, 493–509. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. 5-fluorouracil-related cardiotoxicity; findings from five randomized studies of 5-fluorouracil-based regimens in metastatic colorectal cancer. Clin. Colorectal Cancer 2019, 18, 58–63. [Google Scholar] [CrossRef]
- Kohne’, C.-H.; Thuss-Patience’, P.; Friedrich2, M.; Daniel’, P.T.; Kretzschmarl, A.; Benterl, T.; Bauer1, B.; Dietz2, R.; Dorken1, B. Raltitrexed (tomudex): An alternative drug for patients with colorectal cancer and 5-fluorouracil associated cardiotoxicity. Br. J. Cancer 1998, 6, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Cocconi, G.; Cunningham, D.; van Cutsem, E.; Francois, E.; Gustavsson, B.; van Hazel, G.; Kerr, D.; Possinger, K.; Hietschold, S.M. Open, randomized, multicenter trial of raltitrexed versus fluorouracil plus high-dose leucovorin in patients with advanced colorectal cancer. J. Clin. Oncol. 1998, 16, 2943–2952. [Google Scholar] [CrossRef]
- Jackman, A.L.; Taylor, G.A.; Gibson, W.; Kimbell, R.; Brown, M.; Calvert, A.H.; Judson, I.R.; Hughes, L.R. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of li 210 tumor cell growth in vitro and in vivo: A new agent for clinical study1. Cancer Res. 1991, 51, 5579–5586. [Google Scholar]
- Kelly, C.; Bhuva, N.; Harrison, M.; Buckley, A.; Saunders, M. Use of raltitrexed as an alternative to 5-fluorouracil and capecitabine in cancer patients with cardiac history. Euro. J. Cancer 2013, 49, 2303–2310. [Google Scholar] [CrossRef]
- Hooning, M.J.; Botma, A.; Aleman, B.M.P.; Baaijens, M.H.A.; Bartelink, H.; Klijn, J.G.M.; Taylor, C.W.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Nat. Cancer Instit. 2007, 99, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Milano, G.; Etienne, M.C.; Pierrefite, V.; Barberi-Heyob, M.; Deporte-Fety, R.; Renée, N. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br. J. Cancer 1999, 4, 627–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.W.; Saif, M.W.; El-Rayes, B.F.; Fakih, M.G.; Cartwright, T.H.; Posey, J.A.; King, T.R.; von Borstel, R.W.; Bamat, M.K. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017, 123, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.A.; Milano, G.; Thyss, A.; Etienne, M.-C.; Renã©e, N.; Schneider, M.; Demani, F. Correlation between dihydropyrimidine dehydrogenase activity in peripheral mononuclear cells and systemic clearance of fluorouracil in cancer patients1. Cancer Res. 1992, 52, 2899–2902. [Google Scholar]
- Horie, N.; Aiba, H.; Oguro, K.; Hojo, H.; Takeishi, K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5-terminal regulatory region of the humangene for thymidylate synthase. Cell Struct. Funct. 1995, 20, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullarkat, S.T.; Stoehlmacher, J.; Ghaderi, V.; Xiong, Y.-P.; Ingles, S.A.; Sherrod, A.; Warren, R.; Tsao-Wei, D.; Groshen, S.; Lenz, H.-J. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogen. J. 2001, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Shahrokni, A.; Rajebi, M.R.; Harold, L.; Saif, M.W. Cardiotoxicity of 5-fluorouracil and capecitabine in a pancreatic cancer patient with a novel mutation in the dihydropyrimidine dehydrogenase gene. JOP J. Pancreas 2009, 10, 215–220. [Google Scholar]
- Ichikawa, W.; Takahashi, T.; Suto, K.; Sasaki, Y.; Hirayama, R. Orotate phosphoribosyltransferase gene polymorphism predicts toxicity in patients treated with bolus 5-fluorouracil regimen. Clin. Cancer Res. 2006, 12, 3928–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, G.J.; van Groeningen, C.J.; van der Wilt, C.L.; Meijer, S.; Smid, K.; Laurensse, E.; Pinedo, H.M. Time course of inhibition of thymidylate synthase in patients treated with fluorouracil and leucovorin. Semin. Oncol. 1992, 19, 26–35. [Google Scholar] [PubMed]
- Jakobsen, A.; Nielsen, J.N.; Gyldenkerne, N.; Lindeberg, J. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in normal tissue as predictors of fluorouracil sensitivity. J. Clin. Oncol. 2005, 23, 1365–1369. [Google Scholar] [CrossRef]
- Saif, M.W.; Smith, M.; Maloney, A. The first case of severe takotsubo cardiomyopathy associated with 5-fluorouracil in a patient with abnormalities of both Dihydropyrimidine Dehydrogenase (DPYD) and Thymidylate Synthase (TYMS) Genes. Cureus 2016, 8, e783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raber, I.; Warack, S.; Kanduri, J.; Pribish, A.; Godishala, A.; Abovich, A.; Orbite, A.; Dommaraju, S.; Frazer, M.; Peters, M.L.; et al. Fluoropyrimidine-associated cardiotoxicity: A retrospective case-control study. Oncologist 2020, 25, e606–e609. [Google Scholar] [CrossRef] [Green Version]
- Vargo, C.A.; Blazer, M.; Reardon, J.; Gulati, M.; Bekaii-Saab, T. Successful completion of adjuvant chemotherapy in a patient with colon cancer experiencing 5-fluorouracil–induced cardiac vasospasm. Clin. Colorectal Cancer 2016, 15, e61–e63. [Google Scholar] [CrossRef]
- Lecomte, T.; Ferraz, J.-M.; Zinzindohoué, F.; Loriot, M.-A.; Tregouet, D.-A.; Landi, B.; Berger, A.; Cugnenc, P.-H.; Jian, R.; Beaune, P.; et al. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin. Cancer Res. 2004, 10, 5880–5888. [Google Scholar] [CrossRef] [Green Version]
- Caudle, K.E.; Thorn, C.F.; Klein, T.E.; Swen, J.J.; McLeod, H.L.; Diasio, R.B.; Schwab, M. Clinical pharmacogenetics implementation consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin. Pharmacol. Ther. 2013, 94, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Thalambedu, N.; Khan, Y. Fluorouracil (5-FU)-Induced Cardiomyopathy. Cureus 2019, 11, e5162. [Google Scholar] [CrossRef] [Green Version]
- Bovelli, D.; Plataniotis, G.; Roila, F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann. Oncol. 2010, 21, v277–v282. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Suter, T.; Plataniotis, G.; de Azambuja, E.; Sandri, M.T.; Criscitiello, C.; Goldhirsch, A.; Cipolla, C.; Roila, F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: Esmo clinical practice guidelines. Ann. Oncol. 2012, 23, vii155–vii166. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; Bridges, C.R.; Califf, R.M.; Casey, D.E.; Chavey, W.E.; Fesmire, F.M.; Hochman, J.S.; Levin, T.N.; et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-st-elevation myocardial infarction: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 61, 179–347. [Google Scholar] [CrossRef] [Green Version]
- Perrino, C.; Schiattarella, G.G.; Magliulo, F.; Ilardi, F.; Carotenuto, G.; Gargiulo, G.; Serino, F.; Ferrone, M.; Scudiero, F.; Carbone, A.; et al. Cardiac side effects of chemotherapy: State of art and strategies for a correct management. Curr. Vasc. Pharmacol. 2014, 12, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Steger, F.; Hautmann, M.G.; Kölbl, O. 5-FU-induced cardiac toxicity--an underestimated problem in radiooncology? Radiat. Oncol. 2012, 7, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.-F.; Li, X.-K.; Lin, Y.; Wu, F.; Liang, L.-M.; Fu, X.-B. Protective effects of non-mitogenic human acidic fibroblast growth factor on hydrogen peroxide-induced damage to cardiomyocytes in vitro. World J. Gastroenterol. 2005, 11, 5492–5497. [Google Scholar] [CrossRef]
- Kinhult, S.; Albertsson, M.; Eskilsson, J.; Cwikiel, M. Effects of Probucol on Endothelial Damage by 5-Fluorouracil. Acta Oncol. 2003, 42, 304–308. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, J.; Zhao, L.; Yang, L.; Hu, B.; Fu, Q. Effect of beta2-adrenergic agonist clenbuterol on ischemia/reperfusion injury in isolated rat hearts and cardiomyocyte apoptosis induced by hydrogen peroxide. Acta Pharmacol. Sin. 2008, 29, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.-J.; Lu, Y.; Wang, H.-W.; Zhang, H.; Wang, S.-R.; Xu, W.-Y.; Fu, H.-L.; Yao, X.-Y.; Yang, F.; Yuan, H.-B. Activation of endocannabinoid receptor 2 as a mechanism of propofol pretreatment-induced cardioprotection against ischemia-reperfusion injury in rats. Oxidative Med. Cell. Longev. 2017, 2017, 2186383. [Google Scholar] [CrossRef] [Green Version]
- Altieri, P.; Murialdo, R.; Barisione, C.; Lazzarini, E.; Garibaldi, S.; Fabbi, P.; Ruggeri, C.; Borile, S.; Carbone, F.; Armirotti, A.; et al. 5-fluorouracil causes endothelial cell senescence: Potential protective role of glucagon-like peptide 1. Br. J. Pharmacol. 2017, 174, 3713–3726. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-Y.; Lu, X. Coenzyme complex decreased cardiotoxicity when combined with chemotherapy in treating elderly patients with gastrointestinal cancer. As. Pac. J. Cancer Prev. APJCP 2015, 16, 4045–4049. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Ke, C.Y.; Subeq, Y.M.; Yang, W.T.; Huang, S.G.; Shiao, A.S.; Lee, R.P. Protective effect of calcitriol on organ damage induced by 5-fluorouracil treatment. Nutr. Cancer 2020, 73, 1687–1696. [Google Scholar] [CrossRef]
- Bi, W.; Bi, Y.; Li, P.; Hou, S.; Yan, X.; Hensley, C.; Bammert, C.E.; Zhang, Y.; Gibson, K.M.; Ju, J.; et al. Indole alkaloid derivative b, a novel bifunctional agent that mitigates 5-fluorouracil-induced cardiotoxicity. ACS Omega 2018, 3, 15850–15864. [Google Scholar] [CrossRef]
- Rateesh, S.; Shekar, K.; Naidoo, R.; Mittal, D.; Bhaskar, B. Use of extracorporeal membrane oxygenation for mechanical circulatory support in a patient with 5-fluorouracil induced acute heart failure. Circ. Heart Fail. 2015, 8, 381–383. [Google Scholar] [CrossRef] [Green Version]
- Lestuzzi, C.; Crivellari, D.; Rigo, F.; Viel, E.; Meneguzzo, N. Capecitabine cardiac toxicity presenting as effort angina: A case report. J. Cardiovasc. Med. 2010, 11, 700–703. [Google Scholar] [CrossRef]
- Sorrentino, M.F.; Kim, J.; Foderaro, A.E.; Truesdell, A.G. 5-fluorouracil induced cardiotoxicity: Review of the literature. Cardiol. J. 2012, 19, 453–458. [Google Scholar] [CrossRef]
- Saneeymehri, S.S.; Markey, K.R.; Mahipal, A. Paradoxical effect of capecitabine in 5-fluorouracil-induced cardiotoxicity: A case vignette and literature review. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2016, 22, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Bathina, J.D.; Yusuf, S.W. 5-Fluorouracil-induced coronary vasospasm. J. Cardiovasc. Med. 2010, 11, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.; Malfertheiner, P.; Venerito, M. Safe Administration of S-1 after 5-fluorouracil-induced cardiotoxicity in a patient with colorectal cancer. BMJ Case Rep. 2017, 2017, bcr-2016. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, F.; Barni, S.; Bertocchi, P.; Zaniboni, A. TAS-102, the first “cardio-gentle” fluoropyrimidine in the colorectal cancer landscape? BMC Cancer 2016, 16, 1–4. [Google Scholar] [CrossRef] [Green Version]


| Article | Frequency of Cardiotoxicity |
|---|---|
| Meyer et al. (1997) # [28] | 5-FU-related: 1.9% |
| Wacker et al. (2003) # [29] | 5-FU-related: 19% |
| Kosmas et al. (2006) # [30] | Fluoropyrimidines-related: 4.0% |
| Jensen et al. (2006) * [31] | 5-FU-related: 5.9% Capecitabine-related: 2.8% |
| Jensen et al. (2010) # [32] | 5-FU-related: 8.5% |
| Koca et al. (2011) # [33] | Capecitabine-related: 34.6% |
| Khan et al. (2012) * [34] | 5-FU-related: 19.9% |
| Lestuzzi et al. (2014) # [21] | 5-FU-related: 5.9% |
| Peng et al. (2018) # [35] | 5-FU-related: 25% Capecitabine-related: 33.8% |
| Jin et al. (2019) * [36] | 5-FU-related: 29.5% |
| Dyhl-Polk et al. (2020) * [37] | Fluoropyrimidines-related: 5.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczyk, M.; Król, M.; Midro, A.; Kurnik-Łucka, M.; Poniatowski, A.; Gil, K. Cardiotoxicity of Fluoropyrimidines: Epidemiology, Mechanisms, Diagnosis, and Management. J. Clin. Med. 2021, 10, 4426. https://doi.org/10.3390/jcm10194426
Jurczyk M, Król M, Midro A, Kurnik-Łucka M, Poniatowski A, Gil K. Cardiotoxicity of Fluoropyrimidines: Epidemiology, Mechanisms, Diagnosis, and Management. Journal of Clinical Medicine. 2021; 10(19):4426. https://doi.org/10.3390/jcm10194426
Chicago/Turabian StyleJurczyk, Michał, Magdalena Król, Aleksandra Midro, Magdalena Kurnik-Łucka, Adrian Poniatowski, and Krzysztof Gil. 2021. "Cardiotoxicity of Fluoropyrimidines: Epidemiology, Mechanisms, Diagnosis, and Management" Journal of Clinical Medicine 10, no. 19: 4426. https://doi.org/10.3390/jcm10194426






