Reverse Triggered Breath during Pressure Support Ventilation and Neurally Adjusted Ventilatory Assist at Increasing Propofol Infusion
Abstract
:1. Introduction
2. Materials and Methods
3. Study Protocol
4. Data Acquisition and Analysis
- -
- Triggered breath: a mechanical (i.e., ventilator) insufflation triggered by a neural effort (i.e., a contraction of the diaphragm), defined by EAdi greater than 1 μV.
- -
- Ineffective effort: a neural effort, as defined above, not followed by a ventilator pressurization.
- -
- Auto-triggered breath: a mechanical insufflation without a negative deflection in Paw (i.e., not triggered by the patient) and with no neural effort.
- -
- Double-triggered breath: two ventilator insufflations separated by a very short expiratory time (i.e., <30% of the mean inspiratory time) triggered by one patient’s effort.
- -
- Reverse triggered breath (RTB): presence of a ventilator insufflation without a negative deflection in Paw followed by the initiation of a neural effort (i.e., increase in EAdi) greater than 1 μV [10]. BS phenomena were counted whenever they occurred.
5. Statistical Analysis
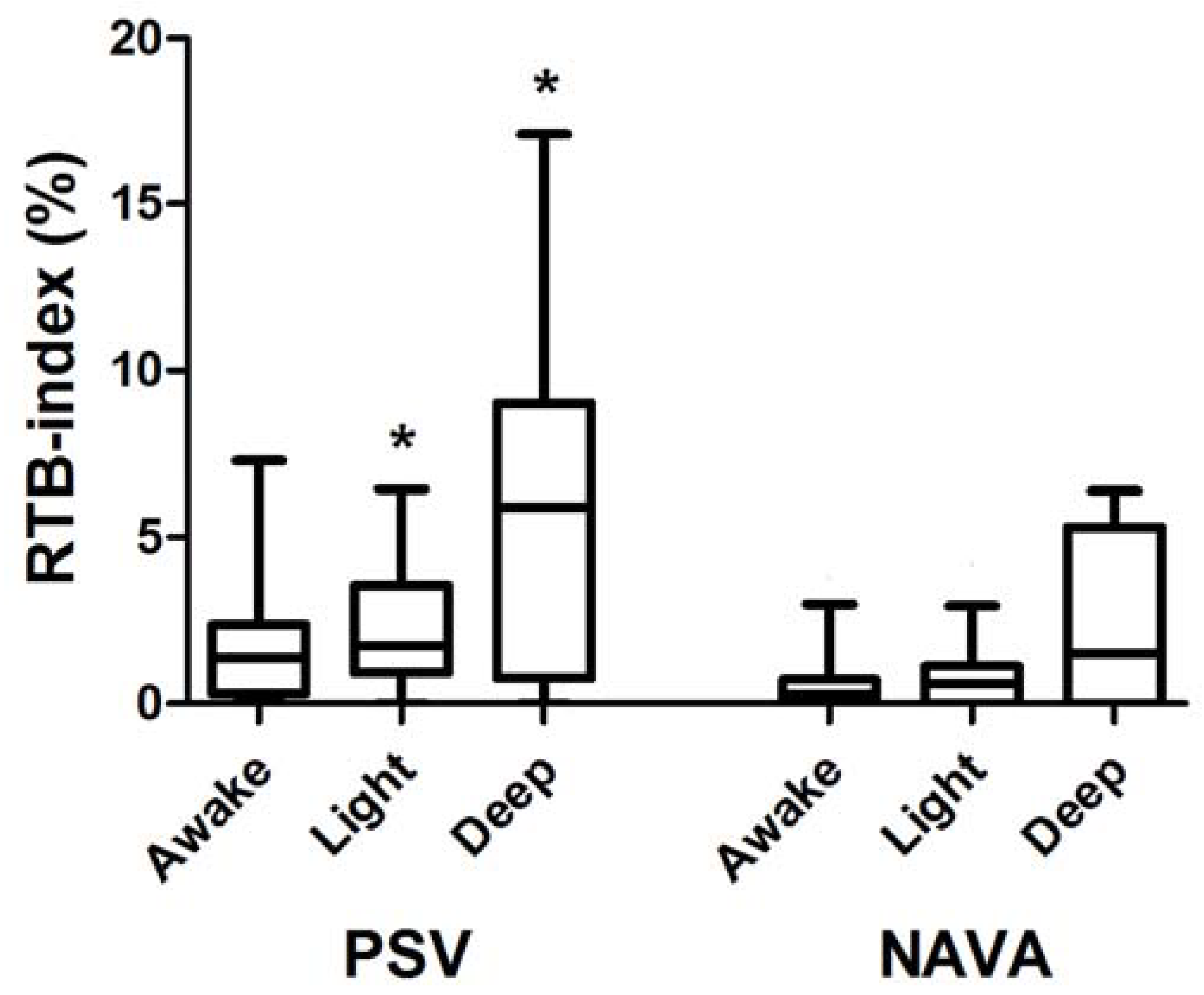
6. Results
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garofalo, E.; Bruni, A.; Pelaia, C.; Liparota, L.; Lombardo, N.; Longhini, F.; Navalesi, P. Recognizing, quantifying and managing patient-ventilator asynchrony in invasive and noninvasive ventilation. Expert Rev. Respir. Med. 2018, 12, 557–567. [Google Scholar] [CrossRef]
- Bruni, A.; Garofalo, E.; Pelaia, C.; Messina, A.; Cammarota, G.; Murabito, P.; Corrado, S.; Vetrugno, L.; Longhini, F.; Navalesi, P. Patient-ventilator asynchrony in adult critically ill patients. Minerva Anestesiol. 2019, 85, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Akoumianaki, E.; Lyazidi, A.; Rey, N.; Matamis, D.; Perez-Martinez, N.; Giraud, R.; Mancebo, J.; Brochard, L.; Marie Richard, J.C. Mechanical ventilation-induced reverse-triggered breaths: A frequently unrecognized form of neuromechanical coupling. Chest 2013, 143, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Vaschetto, R.; Cammarota, G.; Colombo, D.; Longhini, F.; Grossi, F.; Giovanniello, A.; Della Corte, F.; Navalesi, P. Effects of propofol on patient-ventilator synchrony and interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit. Care Med. 2014, 42, 74–82. [Google Scholar] [CrossRef]
- Costa, R.; Navalesi, P.; Cammarota, G.; Longhini, F.; Spinazzola, G.; Cipriani, F.; Ferrone, G.; Festa, O.; Antonelli, M.; Conti, G. Remifentanil effects on respiratory drive and timing during pressure support ventilation and neurally adjusted ventilatory assist. Respir. Physiol. Neurobiol. 2017, 244, 10–16. [Google Scholar] [CrossRef]
- Conti, G.; Ranieri, V.M.; Costa, R.; Garratt, C.; Wighton, A.; Spinazzola, G.; Urbino, R.; Mascia, L.; Ferrone, G.; Pohjanjousi, P.; et al. Effects of dexmedetomidine and propofol on patient-ventilator interaction in difficult-to-wean, mechanically ventilated patients: A prospective, open-label, randomised, multicentre study. Crit. Care 2016, 20, 206. [Google Scholar] [CrossRef] [Green Version]
- Murias, G.; Villagra, A.; Blanch, L. Patient-ventilator dyssynchrony during assisted invasive mechanical ventilation. Minerva Anestesiol. 2013, 79, 434–444. [Google Scholar]
- Imanaka, H.; Nishimura, M.; Takeuchi, M.; Kimball, W.R.; Yahagi, N.; Kumon, K. Autotriggering caused by cardiogenic oscillation during flow-triggered mechanical ventilation. Crit. Care Med. 2000, 28, 402–407. [Google Scholar] [CrossRef]
- Vignaux, L.; Vargas, F.; Roeseler, J.; Tassaux, D.; Thille, A.W.; Kossowsky, M.P.; Brochard, L.; Jolliet, P. Patient-ventilator asynchrony during non-invasive ventilation for acute respiratory failure: A multicenter study. Intensive Care Med. 2009, 35, 840–846. [Google Scholar] [CrossRef] [Green Version]
- Mellado Artigas, R.; Damiani, L.F.; Piraino, T.; Pham, T.; Chen, L.; Rauseo, M.; Telias, I.; Soliman, I.; Junhasavasdikul, D.; Santis, C.; et al. Reverse triggering dyssynchrony 24 h after initiation of mechanical ventilation. Anesthesiology 2021, 134, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Navalesi, P.; Longhini, F. Neurally adjusted ventilatory assist. Curr. Opin Crit. Care 2015, 21, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.; Telias, I.; Piraino, T.; Yoshida, T.; Brochard, L.J. Asynchrony consequences and management. Crit. Care Clin. 2018, 34, 325–341. [Google Scholar] [CrossRef]
- Simon, P.M.; Zurob, A.S.; Wies, W.M.; Leiter, J.C.; Hubmayr, R.D. Entrainment of respiration in humans by periodic lung inflations. Effect of state and co(2). Am. J. Respir. Crit. Care Med. 1999, 160, 950–960. [Google Scholar] [CrossRef]
- Gea, J.; Zhu, E.; Galdiz, J.B.; Comtois, N.; Salazkin, I.; Fiz, J.A.; Grassino, A. Functional consequences of eccentric contractions of the diaphragm. Arch. Bronconeumol. 2009, 45, 68–74. [Google Scholar] [CrossRef]
- Beitler, J.R.; Sands, S.A.; Loring, S.H.; Owens, R.L.; Malhotra, A.; Spragg, R.G.; Matthay, M.A.; Thompson, B.T.; Talmor, D. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ards: The breathe criteria. Intensive Care Med. 2016, 42, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- Colombo, D.; Cammarota, G.; Alemani, M.; Carenzo, L.; Barra, F.L.; Vaschetto, R.; Slutsky, A.S.; Della Corte, F.; Navalesi, P. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit. Care Med. 2011, 39, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Longhini, F.; Colombo, D.; Pisani, L.; Idone, F.; Chun, P.; Doorduin, J.; Ling, L.; Alemani, M.; Bruni, A.; Zhaochen, J.; et al. Efficacy of ventilator waveform observation for detection of patient-ventilator asynchrony during niv: A multicentre study. ERJ Open Res. 2017, 3, 00075-2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, P.O.; Tiribelli, N.; Gogniat, E.; Plotnikow, G.A.; Fredes, S.; Fernandez Ceballos, I.; Pratto, R.A.; Madorno, M.; Ilutovich, S.; San Roman, E.; et al. Automatic detection of reverse-triggering related asynchronies during mechanical ventilation in ards patients using flow and pressure signals. J. Clin. Monit. Comput. 2020, 34, 1239–1246. [Google Scholar] [CrossRef]
- Cammarota, G.; Simonte, R.; Longhini, F.; Spadaro, S.; Vetrugno, L.; De Robertis, E. Advanced point-of-care bedside monitoring for acute respiratory failure. Anesthesiology 2023, 138, 317–334. [Google Scholar] [CrossRef]
- Turbil, E.; Guerin, C.; Schwebel, C.; Terzi, N. Reverse triggering: Sometimes it is not only the diaphragm. Am. J. Respir. Crit. Care Med. 2020, 201, e24–e25. [Google Scholar] [CrossRef]
- Cecchini, J.; Schmidt, M.; Demoule, A.; Similowski, T. Increased diaphragmatic contribution to inspiratory effort during neurally adjusted ventilatory assistance versus pressure support: An electromyographic study. Anesthesiology 2014, 121, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
| PSV | NAVA | |||||
|---|---|---|---|---|---|---|
| Awake | Light | Deep | Awake | Light | Deep | |
| Mechanical insufflations (n) | 500 [400; 570] | 457 [341; 530] | 414 [252; 625] | 536 [410; 594] | 527 [398; 662] | 552 [352; 696] |
| Neural efforts (n) | 495 [398; 619] | 442 [375; 523] | 463 [251; 748] | 536 [410; 594] | 527 [398; 662] | 552 [352; 696] |
| Ineffective Efforts (n) | 7 [4; 9] | 6 [2; 9] | 11 [1; 126] | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] |
| Auto-Triggered breaths (n) | 4 [2; 17] | 8 [0; 17] | 0 [1; 23] | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] |
| Double Ttriggered breaths (n) | 0 [0; 1] | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] | 0 [0; 0] |
| RTB (n) | 4 [2; 12] | 9 [3; 14] | 12 [4; 29] | 0 [1; 4] | 3 [0; 6] | 6 [0; 24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhini, F.; Simonte, R.; Vaschetto, R.; Navalesi, P.; Cammarota, G. Reverse Triggered Breath during Pressure Support Ventilation and Neurally Adjusted Ventilatory Assist at Increasing Propofol Infusion. J. Clin. Med. 2023, 12, 4857. https://doi.org/10.3390/jcm12144857
Longhini F, Simonte R, Vaschetto R, Navalesi P, Cammarota G. Reverse Triggered Breath during Pressure Support Ventilation and Neurally Adjusted Ventilatory Assist at Increasing Propofol Infusion. Journal of Clinical Medicine. 2023; 12(14):4857. https://doi.org/10.3390/jcm12144857
Chicago/Turabian StyleLonghini, Federico, Rachele Simonte, Rosanna Vaschetto, Paolo Navalesi, and Gianmaria Cammarota. 2023. "Reverse Triggered Breath during Pressure Support Ventilation and Neurally Adjusted Ventilatory Assist at Increasing Propofol Infusion" Journal of Clinical Medicine 12, no. 14: 4857. https://doi.org/10.3390/jcm12144857





