Aging and Vascular Disease: A Multidisciplinary Overview
Abstract
:1. Introduction
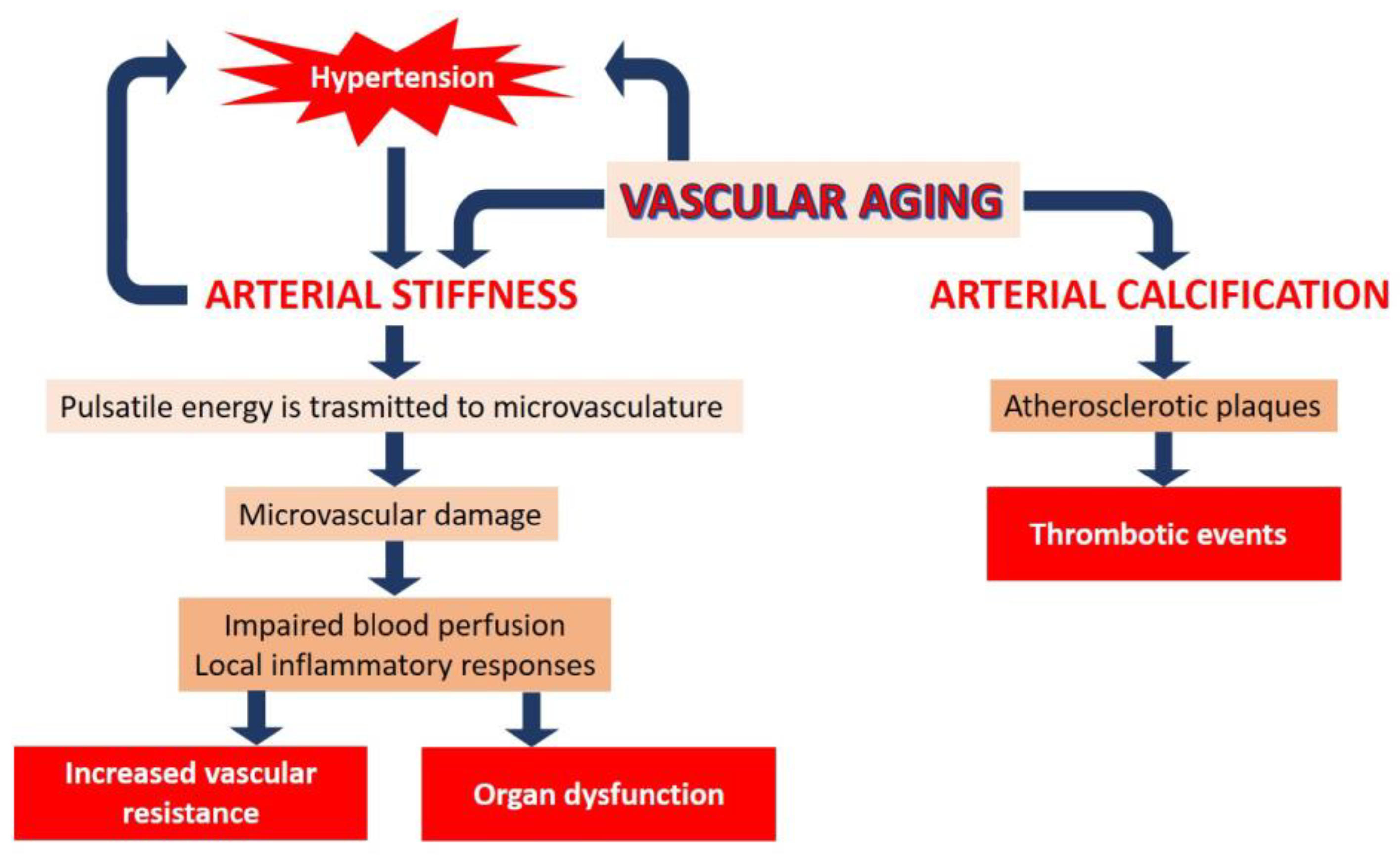
2. Vascular Aging
2.1. Biological vs. Chronological Aging: Lessons from Progeria
2.2. Sex Differences in Cardiovascular Aging
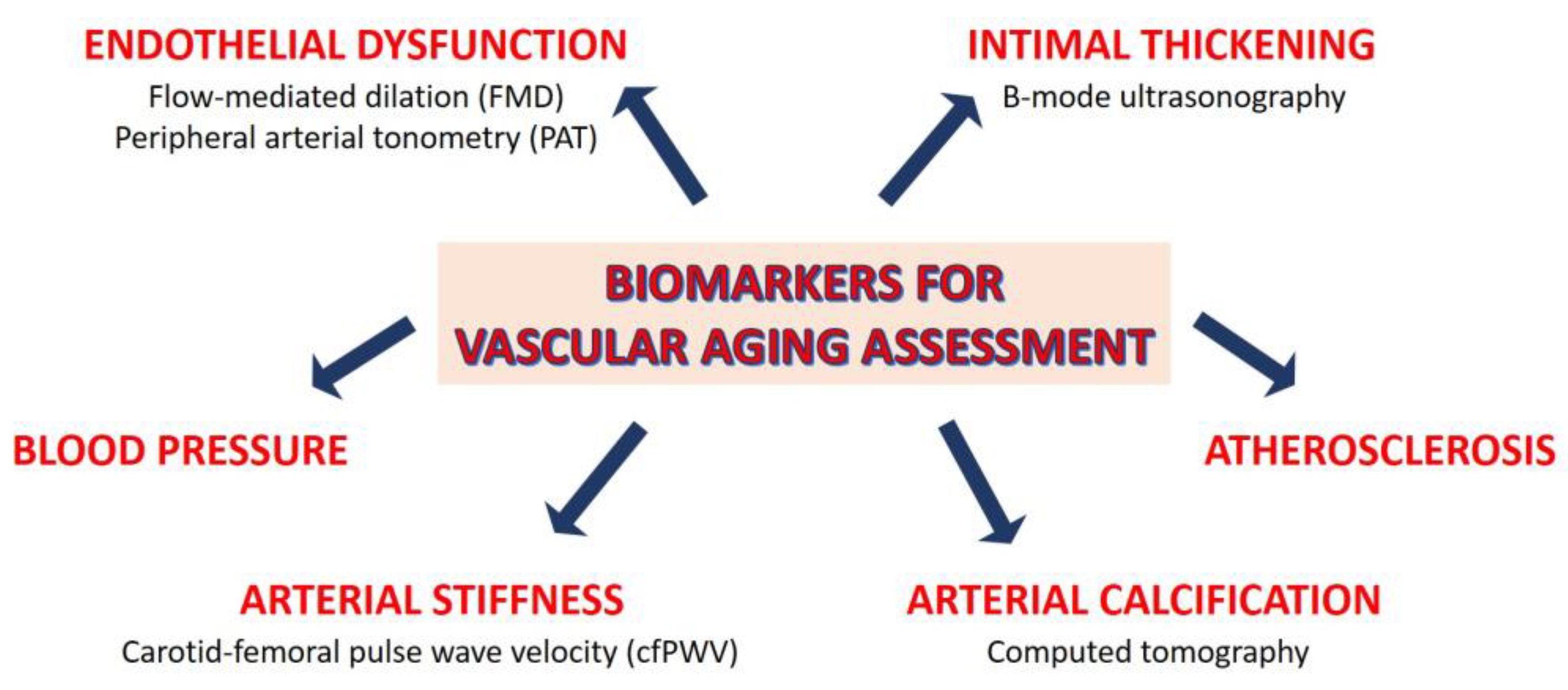
2.3. Assessment of Vascular Aging
3. Social Determinants in Vascular Aging
3.1. Social Isolation and Loneliness
3.2. Socioeconomic Status
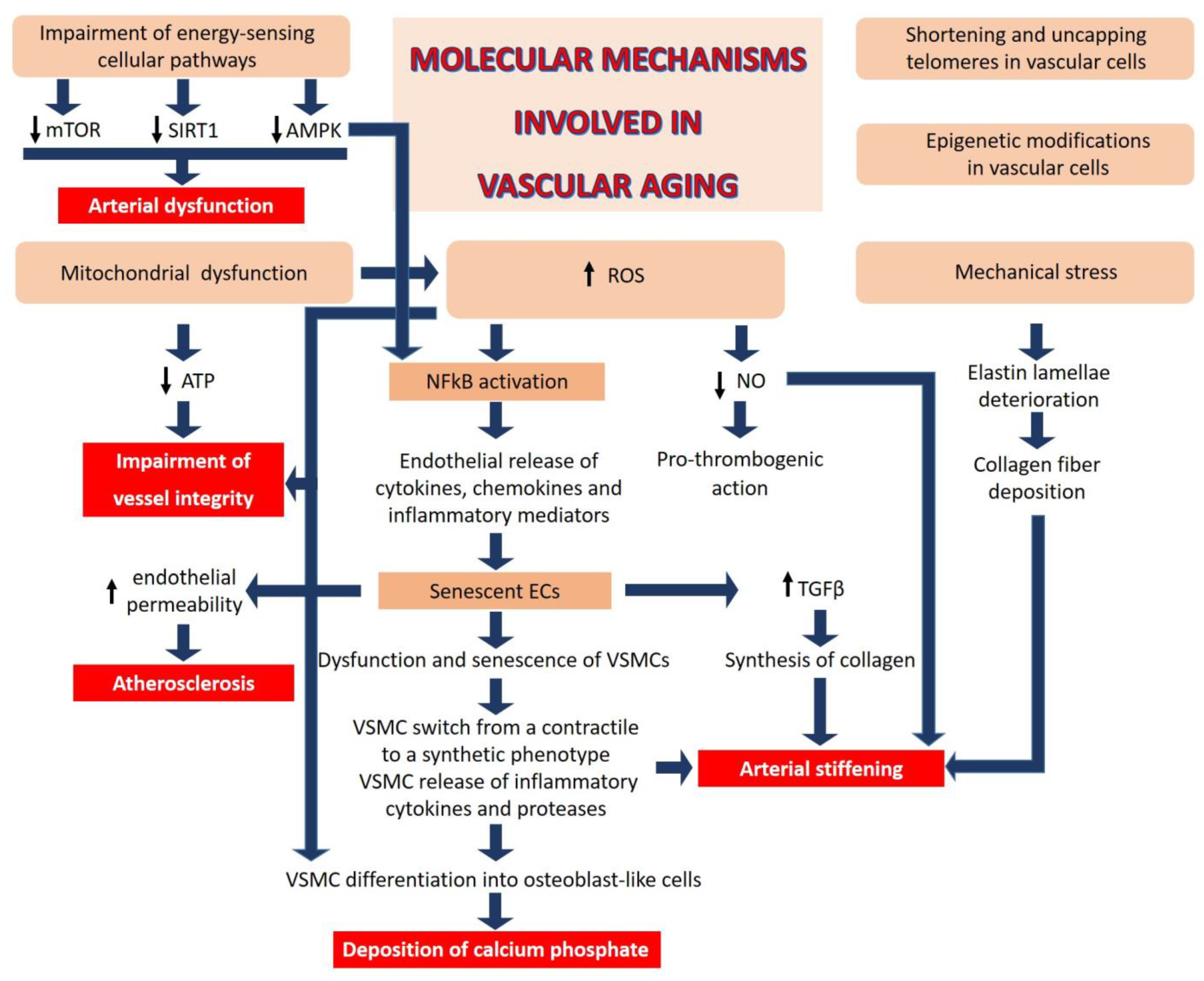
4. Cellular and Molecular Mechanisms Involved in Vascular Aging
- (1)
- Mitochondrial dysfunction: Impaired mitochondrial function in vascular cells reduces adenosine triphosphate (ATP) generation and increases reactive oxygen species (ROS), events that undermine the critical role of these cells in maintaining the integrity of the blood vessels.
- (2)
- Oxidative stress: The generation of ROS, beyond the cellular anti-oxidant capacity, leads to inactivation of NO, a key regulator of vascular homeostasis. NO relaxes VSMCs, inhibits their proliferation, and exerts anti-thrombogenic actions [65]. In addition, aberrant ROS generation can impair the function of proteins, lipids, and DNA by inducing oxidative post-translational modifications, negatively affecting vascular homeostasis [66].
- (3)
- Chronic low-grade inflammation: ROS activate nuclear factor (NF) kB, a transcription factor that orchestrates inflammation, resulting in endothelial release of cytokines, chemokines, and other inflammatory mediators. Indeed, age-related activation of inflammatory processes plays a key role in various macro- and micro-vascular disorders. Inflammatory cytokines re-shape endothelial function and promote senescence [67,68]. Moreover, endothelial cells (ECs) are one of the first cell types to become senescent with advancing age [67], promoting dysfunction and senescence of neighboring vascular cells, including VSMCs. Senescent ECs secrete transforming growth factor (TGF) β, which stimulates the synthesis of collagen and matrix metalloproteases [68], contributing to pathological remodeling of the vascular wall. Similarly, senescent VSMCs release pro-inflammatory factors such as IL6, leading to the activation of the IL6/STAT3 pathway which, in the setting of oxidative stress, stimulates the switch of VMSCs from a contractile to a synthetic phenotype [69,70] and consequent extracellular deposition of calcium phosphate. It has been estimated that VSMC-related mechanisms contribute ~50% to aortic wall stiffness with aging via an increase in the material stiffness of the aortic wall and/or vaso-actively regulating the aortic diameter.
- (4)
- Telomere attrition and epigenetic alterations: All somatic cells have a limited lifespan because of the shortening of telomeres, an event which impairs end replication [23]. Of interest, short telomeres in vascular cells within the atherosclerotic plaque have been linked to a higher risk of CVD [71]. It is also emerging that telomere uncapping, i.e., the breakdown of their loop structure, is a better marker than telomere length in defining vascular aging [72]. Moreover, mounting evidence supports the role of epigenetics in vascular aging. Modifications of DNA and histones as well as non-coding RNA result in aberrant transcription and, therefore, in vascular cell dysfunction, vascular homeostatic imbalance and pathological remodeling [73,74].
- (5)
- Deregulated nutrient sensing: Diet has a significant impact on aging [75], and aging impairs the cellular pathways implicated in energy sensing [76], including mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), and sirtuins (SIRT), which control cellular behavior in response to nutrient availability, thereby influencing cell fate [76]. The mTOR inhibitor rapamycin reverses age-associated arterial dysfunction and decreases vascular stiffness and oxidative stress [77]. Similarly, AMPK confers vasoprotective effects by ameliorating endothelial function and inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) and, consequently, inflammation [78]. SIRT and, in particular, SIRT1 exerts beneficial effects on the vasculature through its anti-inflammatory and anti-oxidant actions [79], decreasing obesity-induced vascular stiffness.
- (6)
- Mechanical stress is an additional contributor to vascular aging. Unlike collagen, which can be actively synthesized over a lifetime, elastin, the fundamental component of large artery elastic compliance, is synthesized only during embryonic development and it has a very long half-life (~40 years) [80]. However, the cyclic strain from cardiac contraction deteriorates elastin lamellae over time. Moreover, calcium deposits in the media promote the destruction of elastic fibers. Collagen fibers are then deposited and/or structurally rearranged in response to fragmented elastin, leading to intrinsically stiffer elastic fibers. Decline in NO bioactivity with age further contributes to aortic wall stiffening via excessive extracellular matrix (ECM) protein crosslinking, in part by the activation of transglutaminase-2 and other enzymes [81].
5. Hints for a Healthy Vasculature
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ukraintseva, S.; Arbeev, K.; Duan, M.; Akushevich, I.; Kulminski, A.; Stallard, E.; Yashin, A. Decline in biological resilience as key manifestation of aging: Potential mechanisms and role in health and longevity. Mech. Ageing Dev. 2021, 194, 111418. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Population Division World Population Ageing: 1950–2050; United Nations: New York, NY, USA, 2001; ISBN 9210510925. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef]
- Tsao, C.W.; Himali, J.J.; Beiser, A.S.; Larson, M.G.; DeCarli, C.; Vasan, R.S.; Mitchell, G.F.; Seshadri, S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 2016, 86, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Woodard, T.; Sigurdsson, S.; Gotal, J.D.; Torjesen, A.A.; Inker, L.A.; Aspelund, T.; Eiriksdottir, G.; Gudnason, V.; Harris, T.B.; Launer, L.J.; et al. Mediation analysis of aortic stiffness and renal microvascular function. J. Am. Soc. Nephrol. 2015, 26, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Pescatore, L.A.; Gamarra, L.F.; Liberman, M. Multifaceted Mechanisms of Vascular Calcification in Aging. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1307–1316. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Chao, C.-T.; Huang, J.-W.; Huang, K.-C. Vascular Calcification as an Underrecognized Risk Factor for Frailty in 1783 Community-Dwelling Elderly Individuals. J. Am. Heart Assoc. 2020, 9, e017308. [Google Scholar] [CrossRef]
- Evrard, S.; Delanaye, P.; Kamel, S.; Cristol, J.-P.; Cavalier, E. Vascular calcification: From pathophysiology to biomarkers. Clin. Chim. Acta 2015, 438, 401–414. [Google Scholar] [CrossRef]
- St Hilaire, C. Medial Arterial Calcification: A Significant and Independent Contributor of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Hamczyk, M.R.; del Campo, L.; Andrés, V. Aging in the Cardiovascular System: Lessons from Hutchinson-Gilford Progeria Syndrome. Annu. Rev. Physiol. 2018, 80, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Benedicto, I.; Dorado, B.; Andrés, V. Molecular and Cellular Mechanisms Driving Cardiovascular Disease in Hutchinson-Gilford Progeria Syndrome: Lessons Learned from Animal Models. Cells 2021, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Rivera-Torres, J.; Osorio, F.G.; Acín-Pérez, R.; Enriquez, J.A.; López-Otín, C.; Andrés, V. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 2013, 127, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Dela Justina, V.; Miguez, J.S.G.; Priviero, F.; Sullivan, J.C.; Giachini, F.R.; Webb, R.C. Sex Differences in Molecular Mechanisms of Cardiovascular Aging. Front. Aging 2021, 2, 725884. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kwan, A.C.; Chen, M.T.; Ouyang, D.; Ebinger, J.E.; Bell, S.P.; Niiranen, T.J.; Bello, N.A.; Cheng, S. Sex Differences in Myocardial and Vascular Aging. Circ. Res. 2022, 130, 566–577. [Google Scholar] [CrossRef]
- Karikkineth, A.C.; AlGhatrif, M.; Oberdier, M.T.; Morrell, C.; Palchamy, E.; Strait, J.B.; Ferrucci, L.; Lakatta, E.G. Sex Differences in Longitudinal Determinants of Carotid Intima Medial Thickening with Aging in a Community-Dwelling Population: The Baltimore Longitudinal Study on Aging. J. Am. Heart Assoc. 2020, 9, e015396. [Google Scholar] [CrossRef]
- Skaug, E.-A.; Aspenes, S.T.; Oldervoll, L.; Mørkedal, B.; Vatten, L.; Wisløff, U.; Ellingsen, O. Age and gender differences of endothelial function in 4739 healthy adults: The HUNT3 Fitness Study. Eur. J. Prev. Cardiol. 2013, 20, 531–540. [Google Scholar] [CrossRef]
- Cheng, S.; Xanthakis, V.; Sullivan, L.M.; Vasan, R.S. Blood pressure tracking over the adult life course: Patterns and correlates in the Framingham heart study. Hypertension 2012, 60, 1393–1399. [Google Scholar] [CrossRef]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.M.; Charlton, P.; Grillo, A.; et al. Vascular Ageing—Moving from Bench towards Bedside. Eur. J. Prev. Cardiol. 2023, 30, zwad028. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P.; Cífková, R.; Marie Maier, J.A.; Nemcsik, J.; Šabovič, M.; Jug, B.; Ježovnik, M.K.; Schernthaner, G.H.; Antignani, P.L.; Catalano, M.; et al. Preclinical atherosclerosis and cardiovascular events: Do we have a consensus about the role of preclinical atherosclerosis in the prediction of cardiovascular events? Atherosclerosis 2022, 348, 25–35. [Google Scholar] [CrossRef]
- Holder, S.M.; Bruno, R.M.; Shkredova, D.A.; Dawson, E.A.; Jones, H.; Hopkins, N.D.; Hopman, M.T.E.; Bailey, T.G.; Coombes, J.S.; Askew, C.D.; et al. Reference Intervals for Brachial Artery Flow-Mediated Dilation and the Relation with Cardiovascular Risk Factors. Hypertension 2021, 77, 1469–1480. [Google Scholar] [CrossRef]
- Schnall, R.P.; Sheffy, J.K.; Penzel, T. Peripheral arterial tonometry-PAT technology. Sleep Med. Rev. 2022, 61, 101566. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef]
- Sena, C.M.; Gonçalves, L.; Seiça, R. Methods to evaluate vascular function: A crucial approach towards predictive, preventive, and personalised medicine. EPMA J. 2022, 13, 209–235. [Google Scholar] [CrossRef]
- Babcock, M.C.; DuBose, L.E.; Witten, T.L.; Brubaker, A.; Stauffer, B.L.; Hildreth, K.L.; Moreau, K.L. Assessment of macrovascular and microvascular function in aging males. J. Appl. Physiol. 2021, 130, 96–103. [Google Scholar] [CrossRef]
- Alberro, A.; Iribarren-Lopez, A.; Sáenz-Cuesta, M.; Matheu, A.; Vergara, I.; Otaegui, D. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci. Rep. 2021, 11, 4358. [Google Scholar] [CrossRef]
- Machin, D.R.; Bloom, S.I.; Campbell, R.A.; Phuong, T.T.T.; Gates, P.E.; Lesniewski, L.A.; Rondina, M.T.; Donato, A.J. Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H531–H539. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.K.; Cepinskas, G.; Fraser, D.D. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front. Med. 2022, 9, 898592. [Google Scholar] [CrossRef] [PubMed]
- Gaarder, M.; Seierstad, T. Measurements of carotid intima media thickness in non-invasive high-frequency ultrasound images: The effect of dynamic range setting. Cardiovasc. Ultrasound 2015, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Sarkola, T.; Manlhiot, C.; Slorach, C.; Bradley, T.J.; Hui, W.; Mertens, L.; Redington, A.; Jaeggi, E. Evolution of the arterial structure and function from infancy to adolescence is related to anthropometric and blood pressure changes. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- van den Munckhof, I.C.L.; Jones, H.; Hopman, M.T.E.; de Graaf, J.; Nyakayiru, J.; van Dijk, B.; Eijsvogels, T.M.H.; Thijssen, D.H.J. Relation between age and carotid artery intima-medial thickness: A systematic review. Clin. Cardiol. 2018, 41, 698–704. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.W.; Kimmerly, D.S.; Theou, O. Sex-specific frailty and chronological age normative carotid artery intima-media thickness values using the Canadian longitudinal study of aging. Vascular 2023, 31, 17085381231157124. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Boutouyrie, P.; Laurent, S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension 2009, 54, 3–10. [Google Scholar] [CrossRef]
- Laurent, S.; Chatellier, G.; Azizi, M.; Calvet, D.; Choukroun, G.; Danchin, N.; Delsart, P.; Girerd, X.; Gosse, P.; Khettab, H.; et al. SPARTE Study: Normalization of Arterial Stiffness and Cardiovascular Events in Patients with Hypertension at Medium to Very High Risk. Hypertension 2021, 78, 983–995. [Google Scholar] [CrossRef]
- Bruno, R.M.; Nilsson, P.M.; Engström, G.; Wadström, B.N.; Empana, J.-P.; Boutouyrie, P.; Laurent, S. Early and Supernormal Vascular Aging: Clinical Characteristics and Association with Incident Cardiovascular Events. Hypertension 2020, 76, 1616–1624. [Google Scholar] [CrossRef]
- McClelland, R.L.; Nasir, K.; Budoff, M.; Blumenthal, R.S.; Kronmal, R.A. Arterial age as a function of coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2009, 103, 59–63. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B.S.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Cuende, J.I.; Cuende, N.; Calaveras-Lagartos, J. How to calculate vascular age with the SCORE project scales: A new method of cardiovascular risk evaluation. Eur. Heart J. 2010, 31, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, H.; Kőrösi, B.; Batta, D.; Nemcsik-Bencze, Z.; László, A.; Tislér, A.; Cseprekál, O.; Torzsa, P.; Eörsi, D.; Nemcsik, J. Comparison of Different Cardiovascular Risk Score and Pulse Wave Velocity-Based Methods for Vascular Age Calculation. Heart Lung Circ. 2021, 30, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Vecsey-Nagy, M.; Szilveszter, B.; Kolossváry, M.; Boussoussou, M.; Vattay, B.; Merkely, B.; Maurovich-Horvat, P.; Radovits, T.; Nemcsik, J. Correlation between Coronary Artery Calcium- and Different Cardiovascular Risk Score-Based Methods for the Estimation of Vascular Age in Caucasian Patients. J. Clin. Med. 2022, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Rafnsson, S.B.; Orrell, M.; d’Orsi, E.; Hogervorst, E.; Steptoe, A. Loneliness, Social Integration, and Incident Dementia Over 6 Years: Prospective Findings from the English Longitudinal Study of Ageing. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, 114–124. [Google Scholar] [CrossRef]
- Shankar, A.; Hamer, M.; McMunn, A.; Steptoe, A. Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosom. Med. 2013, 75, 161–170. [Google Scholar] [CrossRef]
- Shankar, A.; McMunn, A.; Banks, J.; Steptoe, A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011, 30, 377–385. [Google Scholar] [CrossRef]
- Christiansen, J.; Lund, R.; Qualter, P.; Andersen, C.M.; Pedersen, S.S.; Lasgaard, M. Loneliness, Social Isolation, and Chronic Disease Outcomes. Ann. Behav. Med. 2021, 55, 203–215. [Google Scholar] [CrossRef]
- Bu, F.; Zaninotto, P.; Fancourt, D. Longitudinal associations between loneliness, social isolation and cardiovascular events. Heart 2020, 106, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Shankar, A.; Demakakos, P.; Wardle, J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc. Natl. Acad. Sci. USA 2013, 110, 5797–5801. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Philip, K.; Fancourt, D. Social isolation and loneliness as risk factors for hospital admissions for respiratory disease among older adults. Thorax 2020, 75, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Abell, J.; Zaninotto, P.; Fancourt, D. A longitudinal analysis of loneliness, social isolation and falls amongst older people in England. Sci. Rep. 2020, 10, 20064. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Pearce, E.; Ajnakina, O.; Johnson, S.; Lewis, G.; Mann, F.; Pitman, A.; Solmi, F.; Sommerlad, A.; Steptoe, A.; et al. The association between loneliness and depressive symptoms among adults aged 50 years and older: A 12-year population-based cohort study. Lancet Psychiatry 2021, 8, 48–57. [Google Scholar] [CrossRef]
- Shao, M.; Lin, X.; Jiang, D.; Tian, H.; Xu, Y.; Wang, L.; Ji, F.; Zhou, C.; Song, X.; Zhuo, C. Depression and cardiovascular disease: Shared molecular mechanisms and clinical implications. Psychiatry Res. 2020, 285, 112802. [Google Scholar] [CrossRef] [PubMed]
- Halaris, A. Inflammation-Associated Co-morbidity Between Depression and Cardiovascular Disease. Curr. Top. Behav. Neurosci. 2017, 31, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Demakakos, P.; Biddulph, J.P.; Bobak, M.; Marmot, M.G. Wealth and mortality at older ages: A prospective cohort study. J. Epidemiol. Community Health 2016, 70, 346–353. [Google Scholar] [CrossRef]
- Collinge, A.N.; Bath, P.A. Socioeconomic Background and Self-Reported Sleep Quality in Older Adults during the COVID-19 Pandemic: An Analysis of the English Longitudinal Study of Ageing (ELSA). Int. J. Environ. Res. Public Health 2023, 20, 4534. [Google Scholar] [CrossRef]
- Hamilton, O.S.; Steptoe, A. Socioeconomic determinants of inflammation and neuroendocrine activity: A longitudinal analysis of compositional and contextual effects. Brain Behav. Immun. 2023, 107, 276–285. [Google Scholar] [CrossRef]
- Zaninotto, P.; Lassale, C. Socioeconomic trajectories of body mass index and waist circumference: Results from the English Longitudinal Study of Ageing. BMJ Open 2019, 9, e025309. [Google Scholar] [CrossRef] [PubMed]
- Zaninotto, P.; Batty, G.D.; Stenholm, S.; Kawachi, I.; Hyde, M.; Goldberg, M.; Westerlund, H.; Vahtera, J.; Head, J. Socioeconomic Inequalities in Disability-free Life Expectancy in Older People from England and the United States: A Cross-national Population-Based Study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Zaninotto, P. Lower socioeconomic status and the acceleration of aging: An outcome-wide analysis. Proc. Natl. Acad. Sci. USA 2020, 117, 14911–14917. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef] [PubMed]
- Bachschmid, M.M.; Schildknecht, S.; Matsui, R.; Zee, R.; Haeussler, D.; Cohen, R.A.; Pimental, D.; van der Loo, B. Vascular aging: Chronic oxidative stress and impairment of redox signaling-consequences for vascular homeostasis and disease. Ann. Med. 2013, 45, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; Awad, E.M.; Oszwald, A.; Mayr, M.; Yin, X.; Waltenberger, B.; Stuppner, H.; Lipovac, M.; Uhrin, P.; Breuss, J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017, 7, 39501. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Chi, C.; Li, D.-J.; Jiang, Y.-J.; Tong, J.; Fu, H.; Wu, Y.-H.; Shen, F.-M. Vascular smooth muscle cell senescence and age-related diseases: State of the art. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1810–1821. [Google Scholar] [CrossRef]
- Kurozumi, A.; Nakano, K.; Yamagata, K.; Okada, Y.; Nakayamada, S.; Tanaka, Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone 2019, 124, 53–61. [Google Scholar] [CrossRef]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andrés, V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef]
- Morgan, R.G.; Donato, A.J.; Walker, A.E. Telomere uncapping and vascular aging. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1–H5. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-N.; Tang, X.; Chen, H.-Z.; Liu, D.-P. Epigenetic Regulation of Vascular Aging and Age-Related Vascular Diseases. Adv. Exp. Med. Biol. 2018, 1086, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ding, Q.; Li, X.; Feng, Y.; He, H.; Huang, C.; Zhu, Y. Targeting Epigenetic Mechanisms in Vascular Aging. Front. Cardiovasc. Med. 2021, 8, 806988. [Google Scholar] [CrossRef] [PubMed]
- Leitão, C.; Mignano, A.; Estrela, M.; Fardilha, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Effect of Nutrition on Aging-A Systematic Review Focusing on Aging-Related Biomarkers. Nutrients 2022, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Sabatini, D.M. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef]
- Lesniewski, L.A.; Zigler, M.C.; Durrant, J.R.; Donato, A.J.; Seals, D.R. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech. Ageing Dev. 2012, 133, 368–371. [Google Scholar] [CrossRef]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef]
- Santhanam, L.; Tuday, E.C.; Webb, A.K.; Dowzicky, P.; Kim, J.H.; Oh, Y.J.; Sikka, G.; Kuo, M.; Halushka, M.K.; Macgregor, A.M.; et al. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ. Res. 2010, 107, 117–125. [Google Scholar] [CrossRef]
- Huynh, J.; Nishimura, N.; Rana, K.; Peloquin, J.M.; Califano, J.P.; Montague, C.R.; King, M.R.; Schaffer, C.B.; Reinhart-King, C.A. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011, 3, 112ra122. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Lüscher, T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014, 35, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.M.; Harrison, D.G. A T-Cell Small RNA With miRacle Effects on Aortic Stiffening. Circ. Res. 2020, 126, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zuo, Z.; Tian, J.; Ali, Q.; Lin, Y.; Lei, H.; Sun, Z. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension 2016, 68, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.L.; Al Sayah, L.; Weisbrod, R.M.; Van Roy, I.; Weng, X.; Cohen, R.A.; Bachschmid, M.M.; Seta, F. Vascular Smooth Muscle Sirtuin-1 Protects Against Diet-Induced Aortic Stiffness. Hypertension 2016, 68, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Abud, T.; Kounidas, G.; Martin, K.R.; Werth, M.; Cooper, K.; Myint, P.K. Determinants of healthy ageing: A systematic review of contemporary literature. Aging Clin. Exp. Res. 2022, 34, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; LaRocca, T.J.; Martens, C.R.; Seals, D.R. Healthy lifestyle-based approaches for successful vascular aging. J. Appl. Physiol. 2018, 125, 1888–1900. [Google Scholar] [CrossRef]
- Kresnajati, S.; Lin, Y.-Y.; Mündel, T.; Bernard, J.R.; Lin, H.-F.; Liao, Y.-H. Changes in Arterial Stiffness in Response to Various Types of Exercise Modalities: A Narrative Review on Physiological and Endothelial Senescence Perspectives. Cells 2022, 11, 3544. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lv, Y.; Su, Q.; You, Q.; Yu, L. The effect of aerobic exercise on pulse wave velocity in middle-aged and elderly people: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, 960096. [Google Scholar] [CrossRef]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef]
- Lan, Y.S.; Khong, T.K.; Yusof, A. Effect of Exercise on Arterial Stiffness in Healthy Young, Middle-Aged and Older Women: A Systematic Review. Nutrients 2023, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Lona, G.; Hauser, C.; Köchli, S.; Infanger, D.; Endes, K.; Faude, O.; Hanssen, H. Blood Pressure Increase and Microvascular Dysfunction Accelerate Arterial Stiffening in Children: Modulation by Physical Activity. Front. Physiol. 2020, 11, 613003. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Henein, M.Y. Caloric Restriction and Its Effect on Blood Pressure, Heart Rate Variability and Arterial Stiffness and Dilatation: A Review of the Evidence. Int. J. Mol. Sci. 2018, 19, 751. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Rossman, M.J.; Chonchol, M.; Seals, D.R. Strategies for Achieving Healthy Vascular Aging. Hypertension 2018, 71, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Whaley-Connell, A.T.; Sowers, J.R. Fructose and uric acid: Is there a role in endothelial function? Curr. Hypertens. Rep. 2014, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Serdula, M.K.; Liu, S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 492–499. [Google Scholar] [CrossRef]
- York, A. Your microbiome is what you eat. Nat. Rev. Microbiol. 2019, 17, 721. [Google Scholar] [CrossRef]
- Agnoletti, D.; Piani, F.; Cicero, A.F.G.; Borghi, C. The Gut Microbiota and Vascular Aging: A State-of-the-Art and Systematic Review of the Literature. J. Clin. Med. 2022, 11, 3557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, J.A.; Andrés, V.; Castiglioni, S.; Giudici, A.; Lau, E.S.; Nemcsik, J.; Seta, F.; Zaninotto, P.; Catalano, M.; Hamburg, N.M. Aging and Vascular Disease: A Multidisciplinary Overview. J. Clin. Med. 2023, 12, 5512. https://doi.org/10.3390/jcm12175512
Maier JA, Andrés V, Castiglioni S, Giudici A, Lau ES, Nemcsik J, Seta F, Zaninotto P, Catalano M, Hamburg NM. Aging and Vascular Disease: A Multidisciplinary Overview. Journal of Clinical Medicine. 2023; 12(17):5512. https://doi.org/10.3390/jcm12175512
Chicago/Turabian StyleMaier, Jeanette A., Vicente Andrés, Sara Castiglioni, Alessandro Giudici, Emily S. Lau, János Nemcsik, Francesca Seta, Paola Zaninotto, Mariella Catalano, and Naomi M. Hamburg. 2023. "Aging and Vascular Disease: A Multidisciplinary Overview" Journal of Clinical Medicine 12, no. 17: 5512. https://doi.org/10.3390/jcm12175512




