Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies
Abstract
1. Introduction
Potential Treatment Strategies
2. Objectives of the Review and Search Strategy
3. Drug Penetration through the Ocular Surface
4. Major Routes for Drug Delivery
5. Limitations of Drug Formulations
6. Ocular Inserts
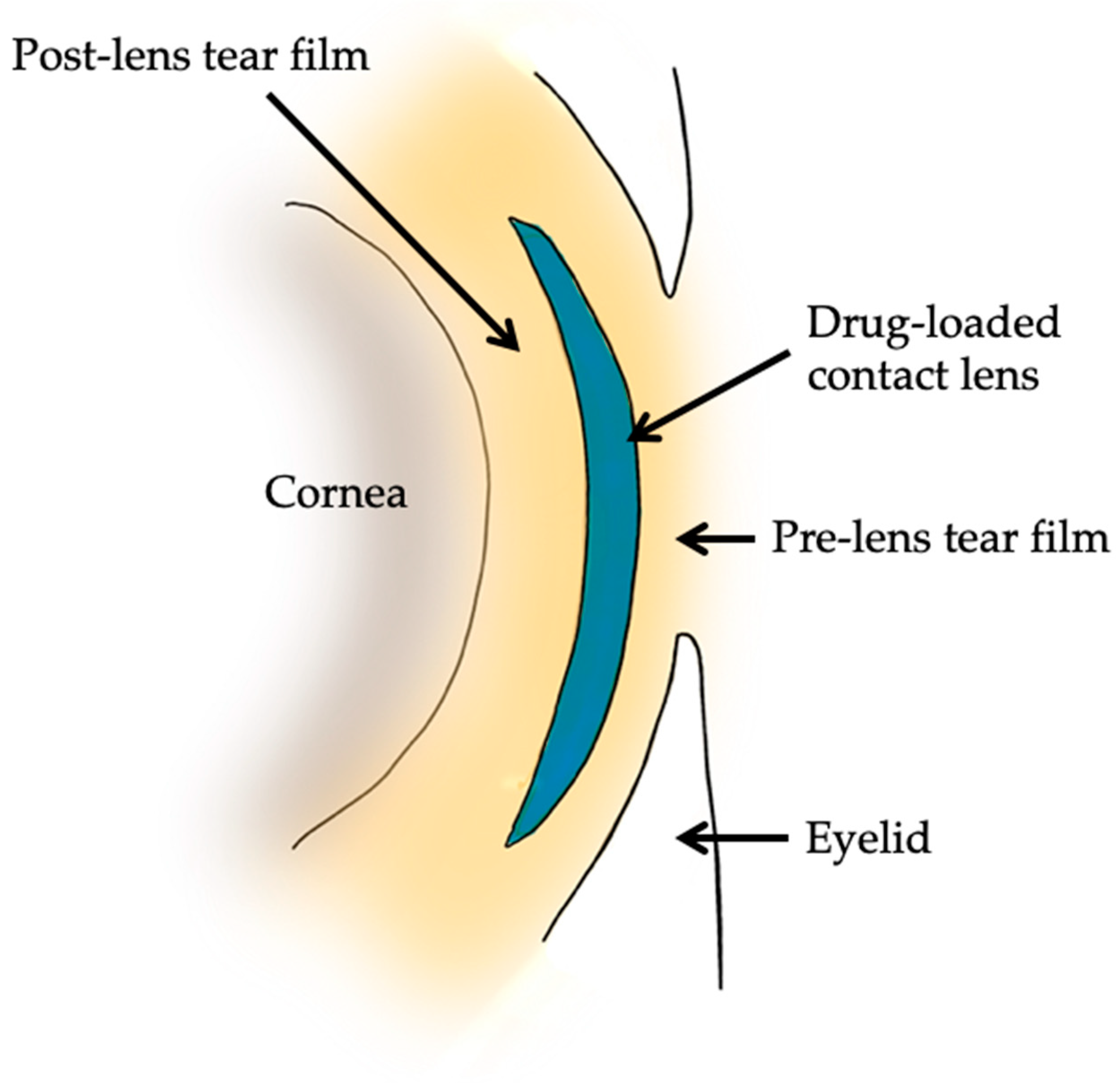
7. Contact Lenses
8. Hydrogels
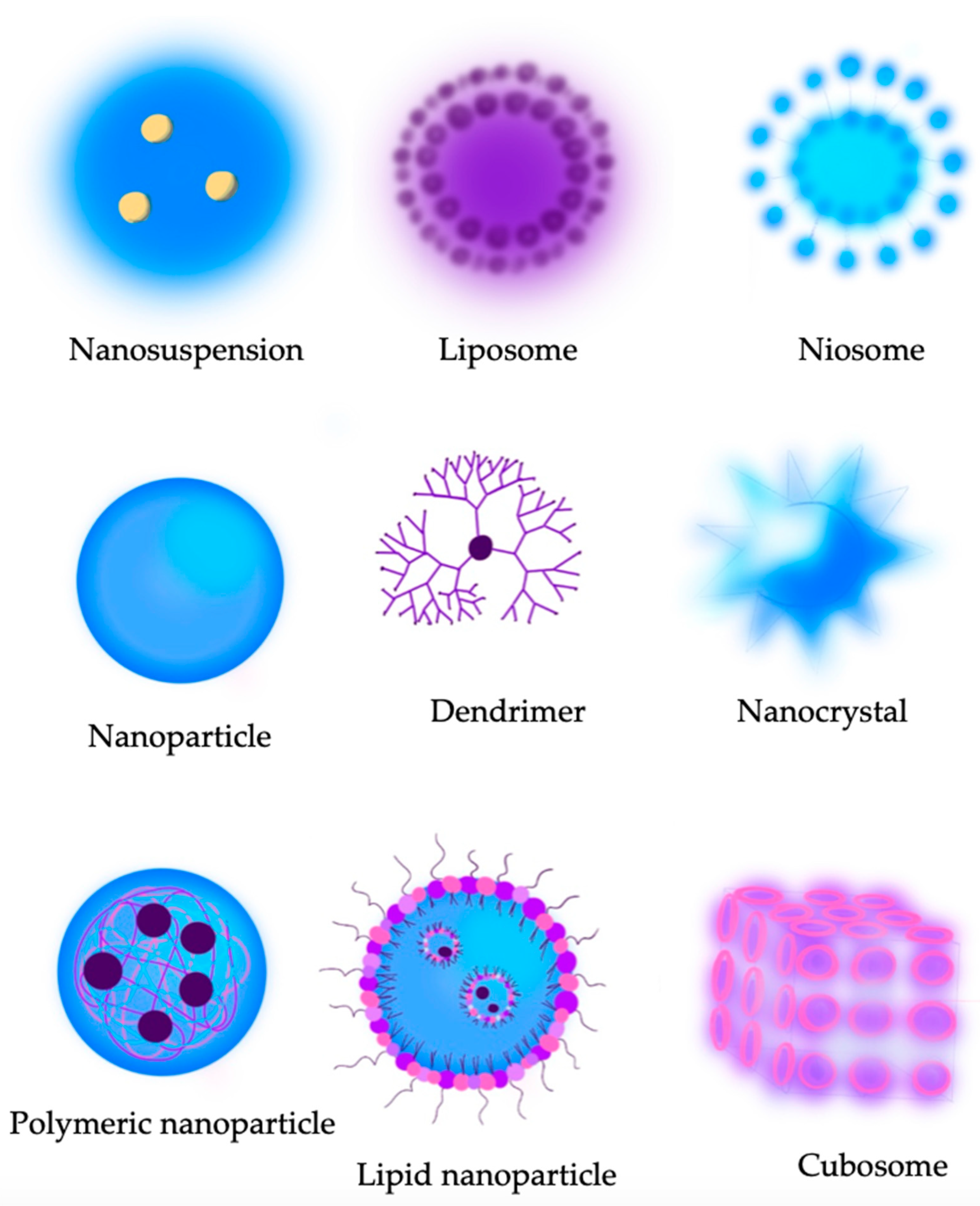
9. Novel Technologies
9.1. Liposomes
9.2. Niosomes
9.3. Nanoemulsions
9.4. Nanosuspensions and Nanocrystals
9.5. Nanomicelles
9.6. Polymeric Nanoparticles
9.7. Solid Lipid Nanoparticles
9.8. Nanostructured Lipid Carriers
9.9. Dendrimers
9.10. Cubosomes
9.11. Olaminosomes
9.12. Bilosomes
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACZ | Acetazolamide |
| BAK | Benzalkonium chloride |
| COPD | Chronic obstructive pulmonary disease |
| EDTA | Ethylenediaminetetraacetic acid |
| IOP | Increased intraocular pressure |
| LUVs | Large unilamellar vesicles |
| MLVs | Multilamellar vesicles |
| NCs | Nanocapsules |
| NLCs | Nanostructured lipid carriers |
| NPs | Nanoparticles |
| NSs | Nanospheres |
| OIs | Ocular inserts |
| OMDI | Omidenepag isopropyl |
| PBS | Phosphate-buffered saline |
| PLGA | Poly(lactic-co- glycolic acid) |
| PVA | Polyvinyl alcohol |
| SLNs | Solid lipid nanoparticles |
| SUVs | Small unilamellar vesicles |
| WHO | World Health Organization |
References
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults—Screening, Diagnosis, and Management. JAMA 2021, 325, 164. [Google Scholar] [CrossRef]
- Leske, M.C.; Wu, S.-Y.; Hennis, A.; Honkanen, R.; Nemesure, B. Risk Factors for Incident Open-angle Glaucoma. Ophthalmology 2008, 115, 85–93. [Google Scholar] [CrossRef]
- Badawi, A.H.; Al-Muhaylib, A.A.; Al Owaifeer, A.M.; Al-Essa, R.S.; Al-Shahwan, S.A. Primary congenital glaucoma: An updated review. Saudi J. Ophthalmol. 2019, 33, 382–388. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Jin, J. Screening for Primary Open-Angle Glaucoma. JAMA 2022, 327, 2030. [Google Scholar] [CrossRef]
- Ozaki, M. Mechanisms of Glaucoma in Exfoliation Syndrome. J. Glaucoma 2018, 27, S83–S86. [Google Scholar] [CrossRef]
- Moroi, S.E.; Lark, K.K.; Sieving, P.A.; Nouri-Mahdavi, K.; Schlötzer-Schrehardt, U.; Katz, G.J.; Ritch, R. Long anterior zonules and pigment dispersion. Am. J. Ophthalmol. 2003, 136, 1176–1178. [Google Scholar] [CrossRef]
- Li, Y.; Mitchell, W.; Elze, T.; Zebardast, N. Association Between Diabetes, Diabetic Retinopathy, and Glaucoma. Curr. Diab Rep. 2021, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.E.H.; Mathew, R.G.; Khaw, P.T.; Henein, C. Corneal Endothelial Cell Density Loss after Glaucoma Surgery Alone or in Combination with Cataract Surgery. Ophthalmology 2022, 129, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- Lim, R. The surgical management of glaucoma: A review. Clin. Exp. Ophthalmol. 2022, 50, 213–231. [Google Scholar] [CrossRef]
- Stamper, R.L.; Lieberman, M.F.; Drake, M.V. Prostaglandins. Becker-Shaffer’s Diagnosis and Therapy of the Glaucomas; Elsevier: Amsterdam, The Netherlands, 2009; pp. 359–375. [Google Scholar] [CrossRef]
- Konstas, A.G.P.; Katsanos, A.; Quaranta, L.; Mikropoulos, D.G.; Tranos, P.G.; Teus, M.A. Twenty-four hour efficacy of glaucoma medications. Prog. Brain Res. 2015, 221, 297–318. [Google Scholar] [CrossRef]
- Hoste, A.M. Beta-Blockers. In Glaucoma; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 548–558. [Google Scholar] [CrossRef]
- Stanley, R.G. Ocular Clinical Pharmacology. In Small Animal Clinical Pharmacology; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 557–573. [Google Scholar] [CrossRef]
- Bylund, D.B. Alpha Adrenergic Receptor Agents☆. In Reference Module in Biomedical Sciences; W.B. Saunders: Philadelphia, PA, USA, 2016. [Google Scholar] [CrossRef]
- Reardon, G.; Kotak Schwartz, G. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: A systematic review. Patient Prefer. Adherence 2011, 5, 441–463. [Google Scholar] [CrossRef]
- Sleath, B.; Robin, A.L.; Covert, D.; Byrd, J.E.; Tudor, G.; Svarstad, B. Patient-Reported Behavior and Problems in Using Glaucoma Medications. Ophthalmology 2006, 113, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Schoenwald, R.D. Ocular pharmacokinetic models of clonidine-3H hydrochloride. J. Pharmacokinet. Biopharm. 1986, 14, 175–211. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. The blood-ocular barriers. Surv. Ophthalmol. 1979, 23, 279–296. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190. [Google Scholar] [CrossRef]
- Zavala, J.; López Jaime, G.R.; Rodríguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Wels, M.; Roels, D.; Raemdonck, K.; De Smedt, S.C.; Sauvage, F. Challenges and strategies for the delivery of biologics to the cornea. J. Control. Release 2021, 333, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y. Molecular Design for Enhancement of Ocular Penetration. J. Pharm. Sci. 2008, 97, 2462–2496. [Google Scholar] [CrossRef]
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv. Ther. 2020, 37, 155–199. [Google Scholar] [CrossRef]
- Carreon, T.A.; Edwards, G.; Wang, H.; Bhattacharya, S.K. Segmental outflow of aqueous humor in mouse and human. Exp. Eye Res. 2017, 158, 59–66. [Google Scholar] [CrossRef]
- Vranka, J.A.; Acott, T.S. Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork. Exp. Eye Res. 2017, 158, 67–72. [Google Scholar] [CrossRef]
- Yucel, Y.; Gupta, N. Lymphatic drainage from the eye. Prog. Brain Res. 2015, 220, 185–198. [Google Scholar] [CrossRef] [PubMed]
- del Amo, E.M.; Urtti, A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp. Eye Res. 2015, 137, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, S.J.; Kim, E.S.; Geroski, D.H.; McCarey, B.E.; Edelhauser, H.F. Trans-Scleral Permeability of Oregon Green 488®. J. Ocul. Pharmacol. Ther. 2008, 24, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Sponsel, W.E.; Terry, S.; Khuu, H.D.; Lam, K.-W.; Frenzel, H. Periocular Accumulation of Timolol and Betaxolol in Glaucoma Patients Under Long-term Therapy. Surv. Ophthalmol. 1999, 43, S210–S213. [Google Scholar] [CrossRef]
- Hollό, G.; Whitson, J.T.; Faulkner, R.; McCue, B.; Curtis, M.; Wieland, H.; Chastain, J.; Sanders, M.; DeSantis, L.; Przydryga, J.; et al. Concentrations of Betaxolol in Ocular Tissues of Patients with Glaucoma and Normal Monkeys after 1 Month of Topical Ocular Administration. Investig. Opthalmol. Vis. Sci. 2006, 47, 235. [Google Scholar] [CrossRef]
- Lavik, E.; Kuehn, M.H.; Kwon, Y.H. Novel drug delivery systems for glaucoma. Eye 2011, 25, 578–586. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- El-Emam, G.A.; Girgis, G.N.; El-Sokkary, M.M.A.; El-Azeem Soliman, O.A.; Abd El Gawad, A.E.G.H. Ocular Inserts of Voriconazole-Loaded Proniosomal Gels: Formulation, Evaluation and Microbiological Studies. Int. J. Nanomed. 2020, 15, 7825–7840. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef]
- Al-Qaysi, Z.K.; Beadham, I.G.; Schwikkard, S.L.; Bear, J.C.; Al-Kinani, A.A.; Alany, R.G. Sustained release ocular drug delivery systems for glaucoma therapy. Expert. Opin. Drug Deliv. 2023, 20, 905–919. [Google Scholar] [CrossRef]
- Pelusi, L.; Mandatori, D.; Mastropasqua, L.; Agnifili, L.; Allegretti, M.; Nubile, M.; Pandolfi, A. Innovation in the Development of Synthetic and Natural Ocular Drug Delivery Systems for Eye Diseases Treatment: Focusing on Drug-Loaded Ocular Inserts, Contacts, and Intraocular Lenses. Pharmaceutics 2023, 15, 625. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Sall, K.; DuBiner, H.; Benza, R.; Alster, Y.; Walker, G.; Semba, C.P.; Budenz, D.; Day, D.; Flowers, B.; et al. Six-Month Intraocular Pressure Reduction with a Topical Bimatoprost Ocular Insert. Ophthalmology 2016, 123, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Eaton, J.S. Medical anti-glaucoma therapy: Beyond the drop. Vet. Ophthalmol. 2021, 24, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; DuBiner, H.B.; Benza, R.; Sall, K.N.; Walker, G.A.; Semba, C.P.; Budenz, D.; Day, D.; Flowers, B.; Lee, S.; et al. Long-term Safety and Efficacy of a Sustained-Release Bimatoprost Ocular Ring. Ophthalmology 2017, 124, 1565–1566. [Google Scholar] [CrossRef]
- Dabral, K.; Uniyal, Y. Ocular inserts: Novel approach for drug delivery into eyes. GSC Biol. Pharm. Sci. 2019, 7, 001–007. [Google Scholar] [CrossRef]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained drug release by contact lenses for glaucoma treatment—A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Gupta, H.; Aqil, M. Contact lenses in ocular therapeutics. Drug Discov. Today 2012, 17, 522–527. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Dowling, J.; Ryan, R.; McLoughlin, P.; Fitzhenry, L. Pharmaceutical-loaded contact lenses as an ocular drug delivery system: A review of critical lens characterization methodologies with reference to ISO standards. Contact Lens Anterior Eye 2021, 44, 101487. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, C.; Ma, Y.; Ma, Y.; Mao, Y.; Meng, Z. Sustained bimatoprost release using gold nanoparticles laden contact lenses. J. Biomater. Sci. Polym. Ed. 2021, 32, 1618–1634. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control Release 2016, 226, 47–56. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, L.M.C.; De Guzman, G.Q.; Borromeo, E.C. Brinzolamide-loaded soft contact lens for ophthalmic delivery. Ther. Deliv. 2022, 13, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, J.B.; Stefanescu, C.F.; Ross, A.E.; Salvador-Culla, B.; Cortez, P.; Ford, E.M.; Wymbs, K.A.; Sprague, S.L.; Mascoop, D.R.; Rudina, S.S.; et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014, 35, 432–439. [Google Scholar] [CrossRef]
- Kim, H.J.; Zhang, K.; Moore, L.; Ho, D. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano 2014, 8, 2998–3005. [Google Scholar] [CrossRef]
- Sekar, P.; Chauhan, A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. J. Colloid. Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef]
- Wei, N.; Dang, H.; Huang, C.; Sheng, Y. Timolol loaded microemulsion laden silicone contact lens to manage glaucoma: In Vitro and in vivo studies. J. Dispers. Sci. Technol. 2021, 42, 742–750. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Ross, A.E.; Tulsan, R.; Watts, A.C.; Wang, R.F.; Zurakowski, D.; Serle, J.B.; Kohane, D.S. Latanoprost-Eluting Contact Lenses in Glaucomatous Monkeys. Ophthalmology 2016, 123, 2085–2092. [Google Scholar] [CrossRef]
- Das, S.; Saha, D.; Majumdar, S.; Giri, L. Imaging Methods for the Assessment of a Complex Hydrogel as an Ocular Drug Delivery System for Glaucoma Treatment: Opportunities and Challenges in Preclinical Evaluation. Mol. Pharm. 2022, 19, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Fea, A.M.; Novarese, C.; Caselgrandi, P.; Boscia, G. Glaucoma Treatment and Hydrogel: Current Insights and State of the Art. Gels 2022, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liu, X.; Zhuang, X.; Liu, Y.; Li, S. Co-delivery of dexamethasone and melatonin by drugs laden PLGA nanoparticles for the treatment of glaucoma. J. Drug Deliv. Sci. Technol. 2020, 60, 102086. [Google Scholar] [CrossRef]
- Khan, N.; Ameeduzzafar Khanna, K.; Bhatnagar, A.; Ahmad, F.J.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187. [Google Scholar] [CrossRef]
- Shinde, U.; Ahmed, M.H.; Singh, K. Development of Dorzolamide Loaded 6-O -Carboxymethyl Chitosan Nanoparticles for Open Angle Glaucoma. J. Drug Deliv. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Hsieh, A.-C. A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials 2012, 33, 2372–2387. [Google Scholar] [CrossRef]
- Fielding, R.M. Liposomal Drug Delivery. Clin. Pharmacokinet. 1991, 21, 155–164. [Google Scholar] [CrossRef]
- Ghareb, M.D.F. Development and in vitro/in vivo Evaluation of Liposomal Gels for the Sustained Ocular Delivery of Latanoprost. J. Clin. Exp. Ophthalmol. 2015, 6, 1000390. [Google Scholar] [CrossRef]
- Abd-El-Azim, H.; Ramadan, A.; Nafee, N.; Khalafallah, N. Entrapment efficiency of pyridoxine hydrochloride in unilamellar liposomes: Experimental versus model-generated data. J. Liposome Res. 2018, 28, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.V.; Ang, M.; Darwitan, A.; Chattopadhyay, S.; Wong, T.T.; Venkatraman, S.S. Nanomedicine for glaucoma: Liposomes provide sustained release of latanoprost in the eye. Int. J. Nanomed. 2012, 7, 123–131. [Google Scholar] [CrossRef][Green Version]
- Huang, H.; Yang, X.; Li, H.; Lu, H.; Oswald, J.; Liu, Y.; Zeng, J.; Jin, C.; Peng, X.; Liu, J.; et al. iRGD decorated liposomes: A novel actively penetrating topical ocular drug delivery strategy. Nano Res. 2020, 13, 3105–3109. [Google Scholar] [CrossRef]
- González-Cela-Casamayor, M.A.; López-Cano, J.J.; Bravo-Osuna, I.; Andrés-Guerrero, V.; Vicario-de-la-Torre, M.; Guzmán-Navarro, M.; Benítez-Del-Castillo, J.M.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Novel Osmoprotective DOPC-DMPC Liposomes Loaded with Antihypertensive Drugs as Potential Strategy for Glaucoma Treatment. Pharmaceutics 2022, 14, 1405. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pradhan, M.; Nag, M.; Singh, M.R. Vesicular system: Versatile carrier for transdermal delivery of bioactives. Artif. Cells Nanomed. Biotechnol. 2015, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Manchanda, S.; Sahoo, P.K. Topical delivery of acetazolamide by encapsulating in mucoadhesive nanoparticles. Asian J. Pharm. Sci. 2017, 12, 550–557. [Google Scholar] [CrossRef]
- Fathalla, D.; Fouad, E.A.; Soliman, G.M. Latanoprost niosomes as a sustained release ocular delivery system for the management of glaucoma. Drug Dev. Ind. Pharm. 2020, 46, 806–813. [Google Scholar] [CrossRef]
- Prabhu, P.; Nitish Kumar, R.; Koland, M.; Harish, N.M.; Vijayanarayan, K.; Dhondge, G.; Charyulu, R.N. Preparation and Evaluation of Nano-vesicles of Brimonidine Tartrate as an Ocular Drug Delivery System. J. Young Pharm. 2010, 2, 356–361. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kaur, I.P. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int. J. Pharm. 2005, 290, 155–159. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef]
- Choradiya, B.R.; Patil, S.B. A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J. Mol. Liq. 2021, 339, 116751. [Google Scholar] [CrossRef]
- Tan, S.F.; Masoumi, H.R.F.; Karjiban, R.A.; Stanslas, J.; Kirby, B.P.; Basri, M.; Bin Basri, H. Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrason. Sonochem. 2016, 29, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Farhadian, N.; Golmohammadzadeh, S.; Biriaee, A.; Ebrahimi, M.; Karimi, M. Preparation, characterization and in-vivo evaluation of microemulsions containing tamoxifen citrate anti-cancer drug. Eur. J. Pharm. Sci. 2017, 96, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Daull, P.; Buggage, R.; Lambert, G.; Faure, M.-O.; Serle, J.; Wang, R.-F.; Garrigue, J.-S.; Ciolino, J.B.; Ross, A.E.; Tulsan, R.; et al. A Comparative Study of a Preservative-Free Latanoprost Cationic Emulsion (Catioprost) and a BAK-Preserved Latanoprost Solution in Animal Models. J. Ocul. Pharmacol. Ther. 2012, 28, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Daull, P.; Benita, S.; Buggage, R.; Garrigue, J.-S. Successfully Improving Ocular Drug Delivery Using the Cationic Nanoemulsion, Novasorb. J. Drug Deliv. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Morsi, N.; Ibrahim, M.; Refai, H.; El Sorogy, H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur. J. Pharm. Sci. 2017, 104, 302–314. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Drug nanocrystals—Versatile option for formulation of poorly soluble materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Chen, M.C.W.; Gupta, M.; Cheung, K.C. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed. Microdevices 2010, 12, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Järvinen, K.; et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, M.A.; Mansour, M.; El-Assal, M.I.A.; Ishak, R.A.H.; Mortada, N.D. Travoprost Liquid Nanocrystals: An Innovative Armamentarium for Effective Glaucoma Therapy. Pharmaceutics 2023, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, S.H.; Khang, D.; Lee, J.Y. Potential Therapeutic Usage of Nanomedicine for Glaucoma Treatment. Int. J. Nanomed. 2020, 15, 5745–5765. [Google Scholar] [CrossRef]
- Donia, M.; Osman, R.; Awad, G.A.S.; Mortada, N. Polypeptide and glycosaminoglycan polysaccharide as stabilizing polymers in nanocrystals for a safe ocular hypotensive effect. Int. J. Biol. Macromol. 2020, 162, 1699–1710. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the noninvasive delivery of ocular therapeutics. Expert. Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef]
- Durgun, M.E.; Güngör, S.; Özsoy, Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 323–341. [Google Scholar] [CrossRef]
- Pepić, I.; Jalšenjak, N.; Jalšenjak, I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int. J. Pharm. 2004, 272, 57–64. [Google Scholar] [CrossRef]
- Ozturk, M.-R.; Popa, M.; Rata, D.M.; Cadinoiu, A.N.; Parfait, F.; Delaite, C.; Atanase, L.I.; Solcan, C.; Daraba, O.M. Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. Int. J. Mol. Sci. 2022, 23, 9382. [Google Scholar] [CrossRef]
- Reimondez-Troitiño, S.; Csaba, N.; Alonso, M.J.; de la Fuente, M. Nanotherapies for the treatment of ocular diseases. Eur. J. Pharm. Biopharm. 2015, 95, 279–293. [Google Scholar] [CrossRef]
- Swetledge, S.; Jung, J.P.; Carter, R.; Sabliov, C. Distribution of polymeric nanoparticles in the eye: Implications in ocular disease therapy. J. Nanobiotechnol. 2021, 19, 10. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.; Chowdhury, P.; Al-Saedi, Z.; Karla, P.; Boddu, S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Franco, Y.L.; Zhou, Y.; Chen, J. Nanotechnology in retinal drug delivery. Int. J. Ophthalmol. 2018, 11, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Ameeduzzafar Ali, J.; Fazil, M.; Qumbar, M.; Khan, N.; Ali, A. Colloidal drug delivery system: Amplify the ocular delivery. Drug Deliv. 2016, 23, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Leffler, C.T. Hybrid Dendrimer Hydrogel/Poly(Lactic-Co-Glycolic Acid) Nanoparticle Platform: An Advanced Vehicle for Topical Delivery of Antiglaucoma Drugs and a Likely Solution to Improving Compliance and Adherence in Glaucoma Management. J. Ocul. Pharmacol. Ther. 2013, 29, 166–172. [Google Scholar] [CrossRef]
- Lee, C.-H.; Li, Y.-J.; Huang, C.-C.; Lai, J.-Y. Poly(ε-caprolactone) nanocapsule carriers with sustained drug release: Single dose for long-term glaucoma treatment. Nanoscale 2017, 9, 11754–11764. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Helal, D.A.; Omar, M.M.; El Sisi, A.M. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int. J. Nanomed. 2019, 14, 2973–2983. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Q.-M.; Wang, X.; Liu, D.; Zhang, W.; Ye, T.; Yang, X.; Pan, W. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int. J. Pharm. 2015, 480, 128–136. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, R. Brinzolamide- and latanoprost-loaded nano lipid carrier prevents synergistic retinal damage in glaucoma. Acta Biochim. Pol. 2022, 69, 423–428. [Google Scholar] [CrossRef] [PubMed]
- El-Salamouni, N.S.; Farid, R.M.; El-Kamel, A.H.; El-Gamal, S.S. Nanostructured lipid carriers for intraocular brimonidine localisation: Development, in-vitro and in-vivo evaluation. J. Microencapsul. 2018, 35, 102–113. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Lancina, M.G.; Wang, J.; Williamson, G.S.; Yang, H. DenTimol as A Dendrimeric Timolol Analogue for Glaucoma Therapy: Synthesis and Preliminary Efficacy and Safety Assessment. Mol. Pharm. 2018, 15, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Umar, H.; Wahab, H.A.; Gazzali, A.M.; Tahir, H.; Ahmad, W. Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers 2022, 14, 3118. [Google Scholar] [CrossRef]
- Lee, K.; Nguyen, T.; Hanley, T.; Boyd, B. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef] [PubMed]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, W.H.; ElKasabgy, N.A. Mucoadhesive olaminosomes: A novel prolonged release nanocarrier of agomelatine for the treatment of ocular hypertension. Int. J. Pharm. 2019, 560, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Safo, I.A.; Dosche, C.; Özaslan, M. Effects of Capping Agents on the Oxygen Reduction Reaction Activity and Shape Stability of Pt Nanocubes. ChemPhysChem 2019, 20, 3010–3023. [Google Scholar] [CrossRef]
- Nemr, A.A.; El-Mahrouk, G.M.; Badie, H.A. Hyaluronic acid-enriched bilosomes: An approach to enhance ocular delivery of agomelatine via D-optimal design: Formulation, in vitro characterization, and in vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2022, 29, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Al-mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.G.; El-Zahaby, S.A.; Al-Mahallawi, A.M.; Ghorab, D.M. Fabrication of betaxolol hydrochloride-loaded highly permeable ocular bilosomes (HPOBs) to combat glaucoma: In vitro, ex vivo & in vivo characterizations. J. Drug Deliv. Sci. Technol. 2023, 82, 104363. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]

| Authors | Materials | Fabrication Method | Drug-Loaded | Drug Loading Technique | Results |
|---|---|---|---|---|---|
| Mohamdeen et al. (2022) [55] | Polylactic acid (PLA) | 3D printing | Timolol maleate | Drug solution added to polymeric formulation | Sustained release of timolol maleate for 3 days |
| Li et al. (2021) [56] | Gold nanoparticles | Synthesis of gold nanoparticles through the reduction of chloroauric acid | Bimatoprost | Drug solution loaded into nanoparticles and nanoparticles implemented into contact lense matrix | Sustained bimatoprost release up to 72 h |
| Hsu et al. (2015) [57] | Vitamin E | Incorporation of vitamin E | Timolol and dorzolamide | Vitamin E-loaded lenses for timolol and dorzolamide loaded separately and then together in the same lens | Increased the release durations of both drugs to 2 days |
| Maulvi et al. (2016) [58] | Cellulose nanoparticles | Synthesis of ethyl cellulose nanoparticles | Timolol maleate | Soaking | Reduced IOP for eight days in a rabbit glaucoma model |
| C de Guzman et al. (2022) [59] | Silicone | Polymerization of monomers | Brinzolamide | Soaking | Enhanced drug loading capacity and release |
| Ciolino et al. (2014) [60] | PLGA | Encapsulating contact lenses with PLGA-Latanoprost film | Latanoprost | Drug-loaded platform embedded in hydrogel-based lenses | Sustained release of latanoprost for 1 month |
| Kim et al. (2014) [61] | Diamond particles | Lysozyme-dependent polysaccharide degradation-mediated diamond nanogel contact lenses development | Timolol maleate | Soaking | Enhanced drug loading capacity and release |
| Sekar et al. (2019) [62] | Vitamin E | Incorporation of vitamin E into ACUVUE®OASYS® and ACUVUE® TruEyeTM | Bimatoprost and latanoprost | Soaking | Increased the release durations of both drugs to 10 days |
| Wei et al. (2021) [63] | Microemulsion | Microemulsion soaking emulsion | Timolol | Imprinting and soaking | Enhanced drug loading capacity and release to 96 h |
| Ciolino et al. (2016) [64] | Methafilicon and methacrylic acid | Photopolymerization | Latanoprost | Soaking |
| Novel Technology | Possible Drugs Loaded | Advantages of the Technology | Disadvantages of the Technology |
|---|---|---|---|
| Liposomes | Latanoprost, brinzolamide, travoprost | Biocompatibility, biodegradability, lack of toxicity, prolonged drug presence on the ocular surface, potential to traverse the epithelium, reach the eye by effectively penetrating through the corneal stroma due to the small size, safety for the patients, a faster and longer-lasting reduction in intraocular pressure compared to other products, prolonged half-life of a drug | Might cause blurring after injection to the vitreous humour, limited storage conditions |
| Niosomes | Acetazolamide, brimonidine, timolol, | High formulation viscosity, excellent dispersion capacity on the corneal surface, increased resistance to drainage, low toxicity, non-immunogenic, ability to encapsulate hydrophilic and lipophilic active substances | Physical instability, aggregation, inefficient drug loading |
| Nanoemulsions | Acetazolamide, latanoprost, pilocarpine, dorzolamide, timolol | Good stability and penetatration throught the corneal surface, enhance the solubility and permeability of many poorly soluble drugs, controlled drug release | Lower permeability and drug bioavailability |
| Nanosuspensions and nanocrystals | Forskolin, brinzolamide, travoprost, betaxolol, acetazolamide | Enhanced drug solubility, enhanced stability, improved drug permeation through ocular barriers, high drug loading, increased bioavailability | Accurate doses cannot be achieved unless suspended |
| Nanomicelles | Pilocarpine, dorzolamide | Lower toxicity, small particle size, high drug loading and water solubility, good structurla stability, protects drugs from environemntal conditions, controlled drug release | Occasional cytotoxicity, sometimes surface modifications are needed, low drug-loading capacity, can only be used for lipoholic drugs |
| Polymeric nanoparticles | Brimonidine, timolol, a combination of dexamethasone and melatonin, forskolin, acetazolamide | Can be used for both hydrophilic and hydrophobic drugs, high stability, controlled drug release | Insufficient data regarding toxicity |
| Solid lipid nanoparticles | Melatonin | Can be used for both hydrophilic and hydrophobic drugs, biocompatibile and well tolerated by the body, easily produced on a larger scale, controlled and extended drug retention in the body, long stability, lower cytotoxicity, improves drug bioavailability | Insufficient amount of clinical studies, drug expulsion during storage |
| Nanostructured lipid carriers | Brinzolamide, latanoprost, brimonidine | Higher drug loading capacity, low toxicity and cytotoxicity | Low drug loading and risk of gelation for SLNs |
| Dendrimers | Pilocarpine, tropicamide, timolol | Controlled pharmacokinetics, increased drug solubility, stability, and permeability, low toxicity, non-immunogenic, improved bioavailability, high purity and uniformity, controlled drug release | Inssuficient amount of clinical studies, difficulties in synthesizing large quantities pure enough to be used in clinical trials |
| Cubosomes | Latanoprost, acetazolamide | Ability to accommodate hydrophobic, hydrophilic, and amphiphilic drugs, biocompatibile, low toxicity, good thermodynamic stability | Large-scale production might be difficult due to the high viscosity |
| Olaminosomes | Agomelatine | Good safety profile, biocompatibility | Insufficient amount of research |
| Bilosomes | Acetazolamide, betaxolol hydrochloride | Ability to deliver hydrophilic molecules | Insufficient amount of research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zembala, J.; Forma, A.; Zembala, R.; Januszewski, J.; Zembala, P.; Adamowicz, D.; Teresiński, G.; Buszewicz, G.; Flieger, J.; Baj, J. Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies. J. Clin. Med. 2023, 12, 5798. https://doi.org/10.3390/jcm12185798
Zembala J, Forma A, Zembala R, Januszewski J, Zembala P, Adamowicz D, Teresiński G, Buszewicz G, Flieger J, Baj J. Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies. Journal of Clinical Medicine. 2023; 12(18):5798. https://doi.org/10.3390/jcm12185798
Chicago/Turabian StyleZembala, Julita, Alicja Forma, Roksana Zembala, Jacek Januszewski, Patryk Zembala, Dominik Adamowicz, Grzegorz Teresiński, Grzegorz Buszewicz, Jolanta Flieger, and Jacek Baj. 2023. "Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies" Journal of Clinical Medicine 12, no. 18: 5798. https://doi.org/10.3390/jcm12185798
APA StyleZembala, J., Forma, A., Zembala, R., Januszewski, J., Zembala, P., Adamowicz, D., Teresiński, G., Buszewicz, G., Flieger, J., & Baj, J. (2023). Technological Advances in a Therapy of Primary Open-Angle Glaucoma: Insights into Current Nanotechnologies. Journal of Clinical Medicine, 12(18), 5798. https://doi.org/10.3390/jcm12185798







