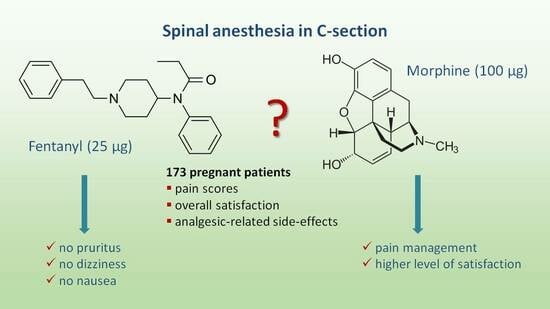
Perioperative Analgesia and Patients’ Satisfaction in Spinal Anesthesia for Cesarean Section: Fentanyl Versus Morphine
Abstract
:1. Introduction
- 1.
- Regional neuraxial anesthesia—includes techniques such as spinal anesthesia, epidural anesthesia, or combined spinal–epidural anesthesia. These techniques provide effective pain relief during and after the surgery, spinal anesthesia being the technique of choice.
- 2.
- General anesthesia—is only used if the patient’s medical condition requires it, if it is the mother‘s choice, or in the case of an emergency with no time to perform a regional neuraxial block.
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables under Examination
2.3. Sample Size
2.4. Medical Procedures’ Protocol
2.5. Data Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, B.; Cohen, S.E.; Lipman, S.S.; Fuller, A.; Mathusamy, A.D.; Macario, A. Patient Preferences for Anesthesia Outcomes Associated with Cesarean Delivery. Anesth. Analg. 2005, 101, 1182–1187. [Google Scholar] [CrossRef]
- Yurashevich, M.; Carvalho, B.; Butwick, A.J.; Ando, K.; Flood, P.D. Determinants of Women’s Dissatisfaction with Anaesthesia Care in Labour and Delivery. Anaesthesia 2019, 74, 1112–1120. [Google Scholar] [CrossRef]
- Weibel, S.; Neubert, K.; Jelting, Y.; Meissner, W.; Wöckel, A.; Roewer, N.; Kranke, P. Incidence and Severity of Chronic Pain after Caesarean Section: A Systematic Review with Meta-Analysis. Eur. J. Anaesthesiol. 2016, 33, 853–865. [Google Scholar] [CrossRef]
- Landau, R.; Bollag, L.; Ortner, C. Chronic Pain after Childbirth. Int. J. Obstet. Anesth. 2013, 22, 133–145. [Google Scholar] [CrossRef]
- Eisenach, J.C.; Pan, P.H.; Smiley, R.; Lavand’homme, P.; Landau, R.; Houle, T.T. Severity of Acute Pain after Childbirth, but Not Type of Delivery, Predicts Persistent Pain and Postpartum Depression. Pain 2008, 140, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sultan, P.; Ando, K.; Sultan, E.; Hawkins, J.E.; Chitneni, A.; Sharawi, N.; Sadana, N.; Blake, L.E.A.; Singh, P.M.; Flood, P.; et al. A Systematic Review of Patient-Reported Outcome Measures to Assess Postpartum Pain Using Consensus Based Standards for the Selection of Health Measurement Instruments (COSMIN) Guidelines. Br. J. Anaesth. 2021, 127, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; Evans, P.; Arfeen, Z.; Misra, U. A Comparison of Intrathecal Fentanyl and Diamorphine as Adjuncts in Spinal Anaesthesia for Caesarean Section. Anaesthesia 2005, 60, 453–457. [Google Scholar] [CrossRef]
- Uppal, V.; Retter, S.; Casey, M.; Sancheti, S.; Matheson, K.; McKeen, D.M. Efficacy of Intrathecal Fentanyl for Cesarean Delivery: A Systematic Review and Meta-Analysis of Randomized Controlled Trials With Trial Sequential Analysis. Anesth. Analg. 2020, 130, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, C.M.; Sinatra, R.S. Postoperative Analgesia: Epidural and Spinal Techniques. In Obstetric Anesthesia Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2004; pp. 472–503. [Google Scholar]
- Karaman, S.; Günüsen, I.; Uyar, M.; Biricik, E.; Fırat, V. The Effects of Morphine and Fentanyl Alone or in Combination Added to Intrathecal Bupivacaine in Spinal Anesthesia for Cesarean Section. Agri 2011, 23, 57–63. [Google Scholar] [PubMed]
- Weigl, W.; Bieryło, A.; Wielgus, M.; Krzemień-Wiczyńska, Ś.; Kołacz, M.; Dąbrowski, M.J. Perioperative Analgesia after Intrathecal Fentanyl and Morphine or Morphine Alone for Cesarean Section: A Randomized Controlled Study. Medicine 2017, 96, e8892. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Shiga, T.; Mihara, T.; Hoshijima, H.; Hosokawa, Y.; Hyuga, S.; Fujita, T.; Koshika, K.; Okada, R.; Kurose, H.; et al. Effects of Intrathecal Opioids on Cesarean Section: A Systematic Review and Bayesian Network Meta-Analysis of Randomized Controlled Trials. J. Anesth. 2021, 35, 911–927. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.E.; Shobaki, G.; Shivakumar, C.; Steele, J. Adjusting Sample Size for Anticipated Dropouts in Clinical Trials. Psychopharmacol. Bull. 1998, 34, 25–33. [Google Scholar]
- Stewart, W.C.; Jackson, A.L.; Jenkins, J.N. Dropout Rates for Intent-to-Treat and per Protocol Analyses. Am. J. Ophthalmol. 2004, 137, 639–645. [Google Scholar] [CrossRef]
- Wu, Y.W.; Seah, Y.S.; Chung, K.T.; Liu, M.D. Postoperative Pain Relief in Primigravida Caesarean Section Patients--Combination of Intrathecal Morphine and Epinephrine. Acta Anaesthesiol. Sin. 1999, 37, 111–114. [Google Scholar] [PubMed]
- El Aish, K.A.; Tafish, R.; Zourob, H. Morphine versus Fentanyl for Spinal Post-Caesarean Analgesia: A Randomised Controlled Trial. Lancet 2018, 391 (Suppl. S2), S20. [Google Scholar] [CrossRef] [PubMed]
- Sibilla, C.; Albertazz, P.; Zatelli, R.; Martinello, R. Perioperative Analgesia for Caesarean Section: Comparison of Intrathecal Morphine and Fentanyl Alone or in Combination. Int. J. Obstet. Anesth. 1997, 6, 43–48. [Google Scholar] [CrossRef]
- Dahl, J.B.; Jeppesen, I.S.; Jørgensen, H.; Wetterslev, J.; Møiniche, S. Intraoperative and Postoperative Analgesic Efficacy and Adverse Effects of Intrathecal Opioids in Patients Undergoing Cesarean Section with Spinal Anesthesia: A Qualitative and Quantitative Systematic Review of Randomized Controlled Trials. Anesthesiology 1999, 91, 1919–1927. [Google Scholar] [CrossRef]
- Culebras, X.; Gaggero, G.; Zatloukal, J.; Kern, C.; Marti, R.A. Advantages of Intrathecal Nalbuphine, Compared with Intrathecal Morphine, after Cesarean Delivery: An Evaluation of Postoperative Analgesia and Adverse Effects. Anesth. Analg. 2000, 91, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.A.; Frias, J.A.F.; Braga, F.S.; Potério, G.B.; Hirata, E.S.; Torres, N.A. Spinal Anesthesia for Cesarean Section. Use of Hyperbaric Bupivacaine (10 mg) Combined with Different Adjuvants. Rev. Bras. Anestesiol. 2012, 62, 775–787. [Google Scholar] [CrossRef]
- Carvalho, B.; Drover, D.R.; Ginosar, Y.; Cohen, S.E.; Riley, E.T. Intrathecal Fentanyl Added to Bupivacaine and Morphine for Cesarean Delivery May Induce a Subtle Acute Opioid Tolerance. Int. J. Obstet. Anesth. 2012, 21, 29–34. [Google Scholar] [CrossRef]
- Abouleish, E.; Rawal, N.; Fallon, K.; Hernandez, D. Combined Intrathecal Morphine and Bupivacaine for Cesarean Section. Anesth. Analg. 1988, 67, 370–374. [Google Scholar] [CrossRef]
- Fournier, R.; Van Gessel, E.; Macksay, M.; Gamulin, Z. Onset and Offset of Intrathecal Morphine versus Nalbuphine for Postoperative Pain Relief after Total Hip Replacement. Acta Anaesthesiol. Scand. 2000, 44, 940–945. [Google Scholar] [CrossRef]
- Baraka, A.; Noueihid, R.; Hajj, S. Intrathecal Injection of Morphine for Obstetric Analgesia. Anesthesiology 1981, 54, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.; Hanumanthaiah, D.; O’Leary, R.A.; Iohom, G. Effects of Fentanyl Added to a Mixture of Intrathecal Bupivacaine and Morphine for Spinal Anaesthesia in Elective Caesearean Section. Rom. J. Anaesth. Intensive Care 2015, 22, 97–102. [Google Scholar]
- Hunt, C.O.; Naulty, J.S.; Bader, A.M.; Hauch, M.A.; Vartikar, J.V.; Datta, S.; Hertwig, L.M.; Ostheimer, G.W. Perioperative Analgesia with Subarachnoid Fentanyl-Bupivacaine for Cesarean Delivery. Anesthesiology 1989, 71, 535–540. [Google Scholar] [CrossRef]
- Milner, A.R.; Bogod, D.G.; Harwood, R.J. Intrathecal Administration of Morphine for Elective Caesarean Section. A Comparison between 0.1 Mg and 0.2 Mg. Anaesthesia 1996, 51, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.M.; Carvalho, J.C.; Amaro, A.R.; Prado, A.A.; Cappelli, E.L. Small Doses of Intrathecal Morphine Combined with Systemic Diclofenac for Postoperative Pain Control after Cesarean Delivery. Anesth. Analg. 1998, 86, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Mallick-Searle, T.; Fillman, M. The Pathophysiology, Incidence, Impact, and Treatment of Opioid-Induced Nausea and Vomiting. J. Am. Assoc. Nurse Pract. 2017, 29, 704–710. [Google Scholar] [CrossRef]
- Girgin, N.K.; Gurbet, A.; Turker, G.; Aksu, H.; Gulhan, N. Intrathecal Morphine in Anesthesia for Cesarean Delivery: Dose-Response Relationship for Combinations of Low-Dose Intrathecal Morphine and Spinal Bupivacaine. J. Clin. Anesth. 2008, 20, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Papava, I.; Dehelean, L.; Romosan, R.S.; Bondrescu, M.; Dimeny, C.Z.; Domuta, E.M.; Bratosin, F.; Bogdan, I.; Grigoras, M.L.; Tigmeanu, C.V.; et al. The Impact of Hyper-Acute Inflammatory Response on Stress Adaptation and Psychological Symptoms of COVID-19 Patients. Int. J. Environ. Res. Public Health 2022, 19, 6501. [Google Scholar] [CrossRef] [PubMed]
- Luncan, M.; Huniadi, A.; Bimbo-Szuhai, E.; Botea, M.; Zaha, I.; Stefan, L.; Beiusanu, C.; Romanescu, D.; Pallag, A.; Bodog, A.; et al. The Effectiveness of Intrauterine Antibiotic Infusion versus Oral Antibiotic Therapy in the Treatment of Chronic Endometritis in Patients during IVF (in Vitro Fertilization) Procedures. BMC Womens Health 2022, 22, 529. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, G.; Hultstrand, C.; Jakobsson, J.; Norman, M.; Eriksson, E.W.; Martin, H. Intrathecal Sufentanil, Fentanyl, or Placebo Added to Bupivacaine for Cesarean Section. Anesth. Analg. 1997, 85, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, N.M.; Guimarães, G.M.N.; Pontes, J.P.J.; de Araújo Azi, L.M.T.; de Ávila Oliveira, R. Safety and Effectiveness of Adding Fentanyl or Sufentanil to Spinal Anesthesia: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Braz. J. Anesthesiol. 2023, 73, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Huniadi, A.; Bimbo-Szuhai, E.; Botea, M.; Zaha, I.; Beiusanu, C.; Pallag, A.; Stefan, L.; Bodog, A.; Șandor, M.; Grierosu, C. Fertility Predictors in Intrauterine Insemination (IUI). J. Pers. Med. 2023, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://Ec.Europa.Eu/Eurostat/Databrowser/View/Hlth_co_proc3/Default/Table?Lang=en (accessed on 17 September 2023).
- Petre, I.; Barna, F.; Cîtu, C.; Gorun, F.; Gorun, O.-M.; Tomescu, L.C.; Apostol, A.; Bordianu, A.; Furau, C.; Petre, I. Development of a Framework for On-Demand Caesarean Section in Romania. Int. J. Environ. Res. Public. Health 2023, 20, 2705. [Google Scholar] [CrossRef]
- Boatin, A.A.; Schlotheuber, A.; Betran, A.P.; Moller, A.-B.; Barros, A.J.D.; Boerma, T.; Torloni, M.R.; Victora, C.G.; Hosseinpoor, A.R. Within Country Inequalities in Caesarean Section Rates: Observational Study of 72 Low and Middle Income Countries. BMJ 2018, 360, k55. [Google Scholar] [CrossRef]
- Betrán, A.P.; Temmerman, M.; Kingdon, C.; Mohiddin, A.; Opiyo, N.; Torloni, M.R.; Zhang, J.; Musana, O.; Wanyonyi, S.Z.; Gülmezoglu, A.M.; et al. Interventions to Reduce Unnecessary Caesarean Sections in Healthy Women and Babies. Lancet 2018, 392, 1358–1368. [Google Scholar] [CrossRef]
- Josi, R. Caesarean Section Epidemic: Tackling the Rise of Unnecessary Cuts. Eur. J. Midwifery 2019, 3, 6. [Google Scholar] [CrossRef]




| Characteristic/ Variable | All Patients (N = 173) | Morphine Group (N = 94) | Fentanyl group (N = 79) | p-Value (a),(b) |
|---|---|---|---|---|
| Age in years (a) | 31.88 ± 4.64 | 31.93 ± 4.43 | 31.84 ± 4.92 | 0.899 |
| Weight in kg (a) | 76.69 ± 13.43 | 78.00 ± 14.00 | 75.14 ± 11.18 | 0.153 |
| Height in cm (a) | 164.79 ± 5.72 | 165.00 ± 6.20 | 164.53 ± 5.11 | 0.587 |
| Smoker (b) | 24 (13.9%) | 17 (18.1%) | 7 (8.9%) | 0.039 * |
| Gesta (b) | 0.245 | |||
| 0 | 93 (53.8%) | 50 (53.2%) | 43 (54.4%) | |
| 1 | 59 (34.1%) | 32 (34%) | 27 (34.2%) | |
| 2 | 12 (6.9%) | 4 (4.3%) | 8 (10.1%) | |
| 3 | 9 (5.2%) | 8 (8.5%) | 1 (1.3%) | |
| Para (b) | 0.409 | |||
| 0 | 104 (60.1%) | 56 (59.6%) | 48 (60.8%) | |
| 1 | 58 (33.5%) | 32 (34%) | 26 (32.9%) | |
| 2 | 9 (5.2%) | 4 (4.3%) | 5 (6.3%) | |
| 3 | 2 (1.2%) | 2 (2.1%) | – | |
| Preoperative contractions (b) | 33 (19.1%) | 17 (18.1%) | 16 (20.3%) | 0.144 |
| Characteristic/ Variable | All Patients (N = 173) | Morphine Group (N = 94) | Fentanyl Group (N = 79) | p-Value (a) |
|---|---|---|---|---|
| Time to sensory block, in minutes (a) | 3.58 ± 0.96 | 3.52 ± 1.03 | 3.65 ± 0.86 | 0.234 |
| Time from anesthesia to incision, in minutes (a) | 6.50 ± 1.69 | 6.71 ± 1.82 | 6.25 ± 1.49 | 0.165 |
| Characteristic/ Variable | All Patients (N = 173) | Morphine Group (N = 94) | Fentanyl Group (N = 79) | p-Value (a) |
|---|---|---|---|---|
| AUPS72hR(a) | 47.07 ± 73.35 | 17.95 ± 39.47 | 81.72 ± 88.14 | <0.001 ** |
| AUPS72Hm (a) | 174.00 ± 103.53 | 124.35 ± 81.23 | 233.08 ± 96.25 | <0.001 ** |
| PS0 (a) | 0.13 ± 0.64 | 0.13 ± 0.74 | 0.13 ± 0.52 | 0.234 |
| PS1 (a) | 0.09 ± 0.36 | 0.06 ± 0.35 | 0.11 ± 0.36 | 0.075 |
| PS4hR (a) | 1.04 ± 2.05 | 0.21 ± 0.65 | 2.03 ± 2.63 | <0.001 ** |
| PS4hM (a) | 2.39 ± 2.54 | 1.21 ± 1.48 | 3.80 ± 2.81 | <0.001 ** |
| PS6hR (a) | 1.46 ± 2.28 | 0.31 ± 0.83 | 2.84 ± 7.11 | <0.001 ** |
| PS6hM (a) | 4.21 ± 2.78 | 2.60 ± 2.01 | 6.14 ± 2.30 | <0.001 ** |
| PS12hR (a) | 0.88 ± 2.05 | 0.29 ± 0.67 | 2.25 ± 2.57 | <0.001 ** |
| PS12hM (a) | 3.77 ± 2.65 | 2.31 ± 1.84 | 5.51 ± 2.42 | <0.001 ** |
| PS24hR (a) | 1.03 ± 1.67 | 0.51 ± 1.15 | 1.65 ± 1.96 | <0.001 ** |
| PS24hM (a) | 3.44 ± 2.29 | 2.46 ± 1.77 | 4.61 ± 2.30 | <0.001 ** |
| PS48hR (a) | 0.46 ± 1.05 | 0.20 ± 0.76 | 0.76 ± 1.25 | <0.001 ** |
| PS48hM (a) | 2.09 ± 1.68 | 1.70 ± 1.68 | 2.56 ± 1.56 | <0.001 ** |
| PS72hR (a) | 0.10 ± 0.43 | 0.06 ± 0.38 | 0.15 ± 0.48 | 0.068 |
| PS72hM (a) | 0.83 ± 1.05 | 0.65 ± 0.97 | 1.04 ± 1.12 | 0.007 ** |
| Characteristic/ Variable | All Patients (N = 173) | Morphine Group (N = 94) | Fentanyl Group (N = 79) | p-Value (a),(b) |
|---|---|---|---|---|
| Pruritus on a 0–4 scale (a) | 0.68 ± 0.68 | 0.90 ± 0.73 | 0.41 ± 0.49 | <0.001 ** |
| Pruritus moderate to severe (b) | 14 (8.1%) | 14 (14.9%) | – | <0.001 ** |
| Nausea (b) | 25 (14.5%) | 19 (20.2%) | 6 (7.6%) | 0.019 * |
| OR = 0.324; 95%CI (0.123;0.858) | ||||
| Vomiting (b) | 12 (6.9%) | 12 (12.8%) | – | 0.001 |
| Sedation (b) | – | – | – | – |
| Respiratory depression (b) | – | – | – | – |
| Dizziness on mobilization (b) | 71 (41%) | 51 (55.3%) | 19 (24.1%) | <0.001 ** |
| OR = 0.256; 95%CI (0.133;0.493) | ||||
| Constant dizziness (b) | 3 (1.7%) | 3 (3.2%) | – | 0.251 |
| Characteristic/ Variable | All Patients (N = 173) | Morphine Group (N = 94) | Fentanyl Group (N = 79) | p-Value (a) |
|---|---|---|---|---|
| Rescue medication (a) | 13 (7.5%) | 3 (3.2%) | 10 (12.7%) | 0.019 * |
| OR = 4.396; 95%CI (1.165;16.582) | ||||
| Characteristic/ Variable | All Patients (N = 173) | Morphine Group (N = 94) | Fentanyl Group (N = 79) | p-Value (a),(b),(c) |
|---|---|---|---|---|
| Analgesia was effective (a) | 155 (89.9) | 94 (100%) | 61 (77.2%) | <0.001 ** |
| Satisfaction on a 1–5 Likert-type scale (b),(c) | 4.75 ± 0.58 | 5 (constant) | 4.46 ± 0.77 | <0.001 ** |
| 5 (5 – 5) | 5 (constant) | 5 (4 – 5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botea, M.O.; Lungeanu, D.; Petrica, A.; Sandor, M.I.; Huniadi, A.C.; Barsac, C.; Marza, A.M.; Moisa, R.C.; Maghiar, L.; Botea, R.M.; et al. Perioperative Analgesia and Patients’ Satisfaction in Spinal Anesthesia for Cesarean Section: Fentanyl Versus Morphine. J. Clin. Med. 2023, 12, 6346. https://doi.org/10.3390/jcm12196346
Botea MO, Lungeanu D, Petrica A, Sandor MI, Huniadi AC, Barsac C, Marza AM, Moisa RC, Maghiar L, Botea RM, et al. Perioperative Analgesia and Patients’ Satisfaction in Spinal Anesthesia for Cesarean Section: Fentanyl Versus Morphine. Journal of Clinical Medicine. 2023; 12(19):6346. https://doi.org/10.3390/jcm12196346
Chicago/Turabian StyleBotea, Mihai O., Diana Lungeanu, Alina Petrica, Mircea I. Sandor, Anca C. Huniadi, Claudiu Barsac, Adina M. Marza, Ramona C. Moisa, Laura Maghiar, Raluca M. Botea, and et al. 2023. "Perioperative Analgesia and Patients’ Satisfaction in Spinal Anesthesia for Cesarean Section: Fentanyl Versus Morphine" Journal of Clinical Medicine 12, no. 19: 6346. https://doi.org/10.3390/jcm12196346