Management of Hypertension in Diabetic Kidney Disease
Abstract
:1. Introduction
2. Angiotensin Converting Enzyme Inhibitors and Angiotensin-Receptor Blockers
2.1. Selection of a RAS Inhibition Agent and Dose
2.2. Other Considerations: When to Stop RAS Inhibition
3. Mineralocorticoid Receptor Antagonists
Other Considerations: Preventing and Managing Hyperkalemia
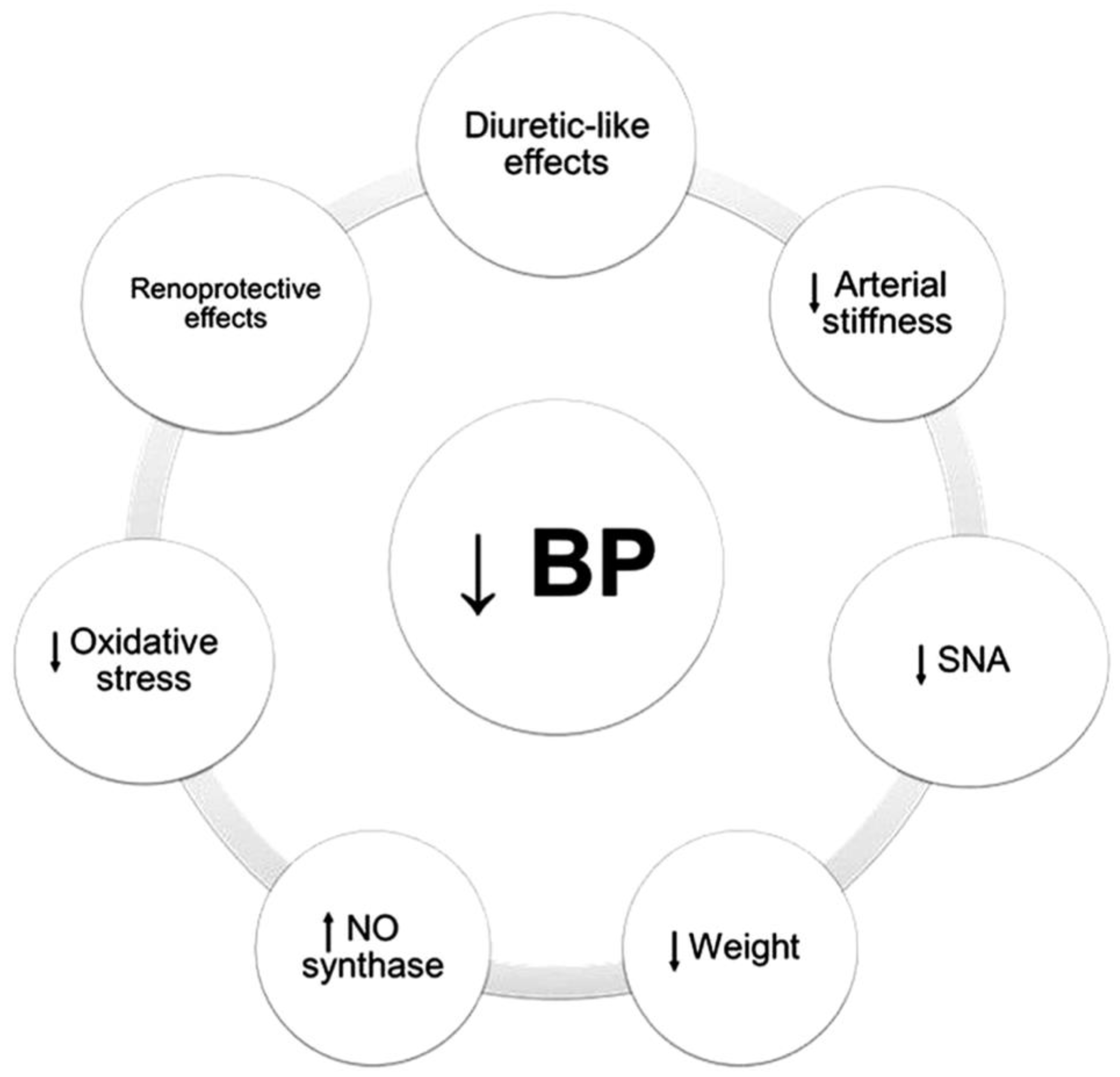
4. Sodium-Glucose Co-Transporter 2 Inhibitors
4.1. Magnitude of Effect and Patient Selection Considerations
4.2. Other Considerations: Safety of SGLT2i
5. Combining SGLT2 and Non-Steroidal MRAs
6. Guidelines on SGLT2 and Non-Steroidal MRAs on Hypertension Management
7. Novel Therapies
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.A.; Blumenthal, S.; Cordonnier, D.J.; De Alvaro, F.; Deferrari, G.; Eisner, G.; Esmatjes, E.; Gilbert, R.E.; Hunsicker, L.G.; de Faria, J.B.; et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J. Am. Soc. Nephrol. 2005, 16, 3027–3037. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Weir, M.R.; Shanifar, S.; Zhang, Z.; Douglas, J.; van Dijk, D.J.; Brenner, B.M.; RENAAL Study Group. Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Arch. Intern. Med. 2003, 163, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Sanidas, E.A.; Papadopoulos, D.P.; Hatziagelaki, E.; Grassos, C.; Velliou, M.; Barbetseas, J. Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors Across the Spectrum of Hypertension. Am. J. Hypertens. 2020, 33, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 1997, 349, 1857–1863. [Google Scholar] [CrossRef]
- Jafar, T.H. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. Ann. Intern. Med. 2001, 135, 73. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; De Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal cardiovascular outcomes in patients with type 2 diabetes nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Alsalemi, N.; Sadowski, C.A.; Elftouh, N.; Louis, M.; Kilpatrick, K.; Houle, S.K.; Lafrance, J.P. The effect of renin-angiotensin-aldosterone system inhibitors on continuous and binary kidney outcomes in subgroups of patients with diabetes: A meta-analysis of randomized clinical trials. BMC Nephrol. 2022, 23, 161. [Google Scholar] [CrossRef]
- Elrggal, M.E.; Ahmed SM, S.; El Nahas, M. Renin-Angiotensin-Aldosterone system blockade in diabetic kidney disease: A critical and contrarian point of view. Saudi J. Kidney Dis. Transpl. 2016, 27, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Gerstein, H.C.; Pogue, J.; Bosch, J.; Yusuf, S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann. Intern. Med. 2001, 134, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000, 355, 253–259. [Google Scholar] [CrossRef]
- Strippoli, G.F.; Bonifati, C.; Craig, M.; Navaneethan, S.D.; Craig, J.C.; Cochrane Kidney and Transplant Group. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst. Rev. 2006, 2006, CD006257. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-angiotensin system inhibitors kidney cardiovascular outcomes in patients with CKD: ABayesian network meta-analysis of randomized clinical trials. Am. J. Kidney Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef]
- ONTARGET Investigators. Telmisartan ramipril or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.F.; Emanuele, N.; Zhang, J.H.; Brophy, M.; Conner, T.A.; Duckworth, W.; Leehey, D.J.; McCullough, P.A.; O’Connor, T.; Palevsky, P.M.; et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013, 369, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Clase, C.M.; Barzilay, J.; Gao, P.; Smyth, A.; Schmieder, R.E.; Tobe, S.; Teo, K.K.; Yusuf, S.; Mann, J.F. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int. 2017, 91, 683–690. [Google Scholar] [CrossRef]
- Leon, S.J.; Whitlock, R.; Rigatto, C.; Komenda, P.; Bohm, C.; Sucha, E.; Bota, S.E.; Tuna, M.; Collister, D.; Sood, M.; et al. Hyperkalemia-Related Discontinuation of Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in CKD: A Population-Based Cohort Study. Am. J. Kidney Dis. 2022, 80, 164–173. [Google Scholar] [CrossRef]
- Weir, M.R.; Bakris, G.L.; Bushinsky, D.A.; Mayo, M.R.; Garza, D.; Stasiv, Y.; Wittes, J.; Christ-Schmidt, H.; Berman, L.; Pitt, B. Patiromer in patients with kidney disease hyperkalemia receiving RAAS inhibitors. N. Engl. J. Med. 2015, 372, 211–221. [Google Scholar] [CrossRef]
- Spinowitz, B.S.; Fishbane, S.; Pergola, P.E.; Roger, S.D.; Lerma, E.V.; Butler, J.; von Haehling, S.; Adler, S.H.; Zhao, J.; Singh, B.; et al. Sodium Zirconium Cyclosilicate among Individuals with Hyperkalemia: A 12-Month Phase 3 Study. Clin. J. Am. Soc. Nephrol. 2019, 14, 798–809. [Google Scholar] [CrossRef]
- Bhandari, S.; Mehta, S.; Khwaja, A.; Cleland, J.G.; Ives, N.; Brettell, E.; Chadburn, M.; Cockwell, P. Renin-Angiotensin System Inhibition in Advanced Chronic Kidney Disease. N. Engl. J. Med. 2022, 387, 2021–2032. [Google Scholar] [CrossRef]
- Bakris, G.L.; Weir, M.R.; Secic, M.; Campbell, B.; Weis-McNulty, A. Differential effects of calcium antagonist subclasses on markers of nephropathy progression. Kidney Int. 2004, 65, 1991–2002. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M.; et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Currie, G.; Taylor, A.H.; Fujita, T.; Ohtsu, H.; Lindhardt, M.; Rossing, P.; Boesby, L.; Edwards, N.C.; Ferro, C.J.; Townend, J.N.; et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2016, 17, 127. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Agarwal, R.; Joseph, A.; Anker, S.D.; Filippatos, G.; Rossing, P.; Ruilope, L.M.; Pitt, B.; Kolkhof, P.; Scott, C.; Lawatscheck, R.; et al. Hyperkalemia Risk with Finerenone: Results from the FIDELIO-DKD Trial. J. Am. Soc. Nephrol. 2022, 33, 225–237. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Reed, J.W. Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure. Vasc. Health Risk Manag. 2016, 12, 393–405. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Kravtsova, O.; Bohovyk, R.; Levchenko, V.; Palygin, O.; Klemens, C.A.; Rieg, T.; Staruschenko, A. SGLT2 inhibition effect on salt-induced hypertension, RAAS, and Na+ transport in Dahl SS rats. Am. J. Physiol. Renal Physiol. 2022, 322, F692–F707. [Google Scholar] [CrossRef]
- Tikkanen, I.; Narko, K.; Zeller, C.; Green, A.; Salsali, A.; Broedl, U.C.; Woerle, H.J.; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015, 38, 420–428. [Google Scholar] [CrossRef]
- Weber, M.A.; Mansfield, T.A.; Cain, V.A.; Iqbal, N.; Parikh, S.; Ptaszynska, A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016, 4, 211–220. [Google Scholar] [CrossRef]
- Ferdinand, K.C.; Izzo, J.L.; Lee, J.; Meng, L.; George, J.; Salsali, A.; Seman, L. Antihyperglycemic and Blood Pressure Effects of Empagliflozin in Black Patients with Type 2 Diabetes Mellitus and Hypertension. Circulation 2019, 139, 2098–2109. [Google Scholar] [CrossRef]
- Yu, Z.; Coresh, J.; Qi, G.; Grams, M.; Boerwinkle, E.; Snieder, H.; Teumer, A.; Pattaro, C.; Köttgen, A.; Chatterjee, N.; et al. A bidirectional Mendelian randomization study supports causal effects of kidney function on blood pressure. Kidney Int. 2020, 98, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Li, X.; Gou, Q.; Liu, K.; Chen, X. Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials. Diab Vasc. Dis. Res. 2020, 17, 1479164120953625. [Google Scholar] [CrossRef] [PubMed]
- Hollander, P.; Bays, H.E.; Rosenstock, J.; Frustaci, M.E.; Fung, A.; Vercruysse, F.; Erondu, N. Coadministration of Canagliflozin and Phentermine for Weight Management in Overweight and Obese Individuals without Diabetes: A Randomized Clinical Trial. Diabetes Care 2017, 40, 632–639. [Google Scholar] [CrossRef]
- Kario, K.; Okada, K.; Kato, M.; Nishizawa, M.; Yoshida, T.; Asano, T.; Uchiyama, K.; Niijima, Y.; Katsuya, T.; Urata, H.; et al. Twenty-Four-Hour Blood Pressure-Lowering Effect of a Sodium-Glucose Cotransporter 2 Inhibitor in Patients with Diabetes and Uncontrolled Nocturnal Hypertension: Results From the Randomized, Placebo-Controlled SACRA Study. Circulation 2019, 139, 2089–2097. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Fitchett, D.; Ofstad, A.P.; Kraus, B.J.; Wanner, C.; Zwiener, I.; Zinman, B.; Lauer, S.; George, J.T.; Rossignol, P.; et al. Empagliflozin for Patients with Presumed Resistant Hypertension: A Post Hoc Analysis of the EMPA-REG OUTCOME Trial. Am. J. Hypertens. 2020, 33, 1092–1101. [Google Scholar] [CrossRef]
- Williams, B.; MacDonald, T.M.; Morant, S.; Webb, D.J.; Sever, P.; McInnes, G.; Ford, I.; Cruickshank, J.K.; Caulfield, M.J.; Salsbury, J.; et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): A randomised, double-blind, crossover trial. Lancet 2015, 386, 2059–2068. [Google Scholar] [CrossRef]
- Nasser, S.A.; Arora, N.; Ferdinand, K.C. Addressing Cardiovascular Disparities in Racial/Ethnic Populations: The Blood Pressure-Lowering Effects of SGLT2 Inhibitors. Rev. Cardiovasc. Med. 2022, 23, 411. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Alqudsi, M.; Velez, J.C.Q.; Navarrete, J. Medical management of resistant hypertension: The role of sodium-glucose cotransporter 2 inhibitors (SGLT2i). Curr. Opin. Cardiol. 2021, 36, 420–428. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Puchades, M.J.; Garofalo, C.; Jongs, N.; D’Marco, L.; Andreucci, M.; De Nicola, L.; Gorriz, J.L.; Heerspink, H.J.L.; ROTATE-3 Study Group; et al. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J. Am. Soc. Nephrol. 2022, 33, 1569–1580. [Google Scholar] [CrossRef]
- Kolkhof, P.; Hartmann, E.; Freyberger, A.; Pavkovic, M.; Mathar, I.; Sandner, P.; Droebner, K.; Joseph, A.; Hüser, J.; Eitner, F. Effects of Finerenone Combined with Empagliflozin in a Model of Hypertension-Induced End-Organ Damage. Am. J. Nephrol. 2021, 52, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Mancia Chairperson, G.; Kreutz Co-Chair, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J. Hypertens. 2023, 28, 1462–1536. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Hesp, A.C.; Schaub, J.A.; Prasad, P.V.; Vallon, V.; Laverman, G.D.; Bjornstad, P.; van Raalte, D.H. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020, 98, 579–589. [Google Scholar] [CrossRef]
- Strutz, F.; Heeg, M.; Kochsiek, T.; Siemers, G.; Zeisberg, M.; Müller, G.A. Effects of pentoxifylline, pentifylline and gamma-interferon on proliferation, differentiation, and matrix synthesis of human renal fibroblasts. Nephrol. Dial. Transpl. 2000, 15, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; Muros de Fuentes, M.; Chahin, J.; Méndez, M.L.; Gallego, E.; Macía, M.; del Castillo, N.; Rivero, A.; Getino, M.A.; et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J. Am. Soc. Nephrol. 2015, 26, 220–229. [Google Scholar] [CrossRef]
- Raun, K.; von Voss, P.; Gotfredsen, C.F.; Golozoubova, V.; Rolin, B.; Knudsen, L.B. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 2007, 56, 8–15. [Google Scholar] [CrossRef]
- Rossing, P.; Baeres, F.M.M.; Bakris, G.; Bosch-Traberg, H.; Gislum, M.; Gough, S.C.L.; Idorn, T.; Lawson, J.; Mahaffey, K.W.; Mann, J.F.E.; et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol. Dial. Transpl. 2023, 38, 2041–2051. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Greasley, P.J.; Ahlström, C.; Althage, M.; Dwyer, J.P.; Law, G.; Wijkmark, E.; Lin, M.; Mercier, A.K.; Sunnåker, M.; et al. Efficacy and safety of zibotentan and dapagliflozin in patients with chronic kidney disease: Study design and baseline characteristics of the ZENITH-CKD trial. Nephrol. Dial. Transpl. 2023, gfad183. [Google Scholar] [CrossRef] [PubMed]
| Study Title | Trial Identification | Enrolled | Primary Outcomes |
|---|---|---|---|
| Pentoxifylline in DKD | NCT03625648 PTXRx | 2510 patients Pentoxifylline vs. Placebo | Time to ESRD or Death |
| A Phase 3 Study of Bardoxolone Methyl in Patients with DKD | NCT03550443 AYAME | 1323 patients Bardoxolone vs. Placebo | Time to onset of a >30% dec in EGFR from baseline or ESRD |
| Prognostic Imaging Biomarkers for DKD | NCT03716401 iBEAt | 500 patients Biopsy vs. MRI follow-up, Microvascular | Cross-sectional. MRI biomarkers will be combined with fluid-based biomarkers |
| Comparison Between the Efficacy of SGLT2i vs. ACEi in DKD | NCT05373004 SGLT2i vs. ACEi | 212 patients Empagliflozin vs. Enalapril | eGFR rate, UACR measurement |
| Decision Impact Trial of KidneyIntelX | NCT04791358 | 1500 patients KidneyIntelX test | Blood pressure, HBA1c, ACEi/ARB, SGLT2i/GLP1, UACR |
| SGLT2i Prophylaxis Against Post-contrast AKI in DKD | NCT04853615 | 800 patients Normal Saline vs. Allopurinol/linagliptin vs. SGLT2i vs. SGLT2i/allopurinol | SGLT2i proves protective, non-inferiority to allopurinol |
| PREvention of CardIovascular and DKD in Type 2 DM | NCT05390892 PRECIDENTD | 9000 participants SGLT2i vs. GLP1 vs. SGLT2i/GLP1 | Total CV, Kidney, and Death events |
| Efficacy of a High-Intensity Physical Activity Program on Renal Function | NCT03184662 ACTIDIANE | 300 patients Twice weekly activity session vs. Counseling alone | Renal Function Decline |
| Repository of Novel Analytes Leading to Autoimmune, Inflammatory, and DKD | NCT01802034 RENAL AID | 2000 patients Biopsy tissue repository vs. nonuse of RENAL AID | Change in disease progression |
| Atrasentan in Patients with Proteinuric Glomerular Disease | NCT04573920 AFFINITY | 100 patients 0.75 mg atrasentan and 1.5 mg atrasentan (FSGS) | Change in proteinuria, albuminuria (DKD) |
| Efficacy, Safety, and Tolerability of AZD9977 and Dapagliflozin in Participants with HF and CKD | NCT04595370 MIRACLE | 500 patients AZD9977 Dosings + Dapagliflozin | Percent change in UACR at 12 weeks |
| BI 690517 Alone vs. Combination with Empagliflozin in CKD | NCT05182840 | 714 patients Empagliflozin + BI 690517 vs. Placebo combinations | Change in UACR |
| Finerenone and Empagliflozin Combination vs. Alone | NCT05254002 CONFIDENCE | 807 patients Finerenone/Empagliflozin, vs. Empagliflozin, vs. Finerenone | Change in UACR at 180 days |
| Ocedurenone KBP-5074 | NCT04968184 Clarion-CKD | 600 patients KBP-5074 vs. placebo | Change in SBP at 12 weeks and SBP at 48 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colbert, G.B.; Elrggal, M.E.; Gaddy, A.; Madariaga, H.M.; Lerma, E.V. Management of Hypertension in Diabetic Kidney Disease. J. Clin. Med. 2023, 12, 6868. https://doi.org/10.3390/jcm12216868
Colbert GB, Elrggal ME, Gaddy A, Madariaga HM, Lerma EV. Management of Hypertension in Diabetic Kidney Disease. Journal of Clinical Medicine. 2023; 12(21):6868. https://doi.org/10.3390/jcm12216868
Chicago/Turabian StyleColbert, Gates B., Mohamed E. Elrggal, Anna Gaddy, Hector M. Madariaga, and Edgar V. Lerma. 2023. "Management of Hypertension in Diabetic Kidney Disease" Journal of Clinical Medicine 12, no. 21: 6868. https://doi.org/10.3390/jcm12216868
APA StyleColbert, G. B., Elrggal, M. E., Gaddy, A., Madariaga, H. M., & Lerma, E. V. (2023). Management of Hypertension in Diabetic Kidney Disease. Journal of Clinical Medicine, 12(21), 6868. https://doi.org/10.3390/jcm12216868






