Lower Urinary Tract Symptoms (LUTS) as a New Clinical Presentation of Histamine Intolerance: A Prevalence Study of Genetic Diamine Oxidase Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Lower Urinary Tract Symptoms and Questionnaires
2.4. Histamine Intolerance
2.5. AOC1 Variant Genotyping
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. DAO Deficiency
3.3. Histamine Intolerance and LUTS
3.4. LUTS and Genetic DAO Deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur. Urol. 2006, 50, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Hilmer, S.N.; Gnjidic, D. The anticholinergic burden: From research to practice. Aust. Prescr. 2022, 45, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Sexton, C.C.; Notte, S.M.; Maroulis, C.; Dmochowski, R.R.; Cardozo, L.; Subramanian, D.; Coyne, K.S. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: A systematic review of the literature. Int. J. Clin. Pract. 2011, 65, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Lee, H.Y.; Park, J.J.; Kim, J.H. Persistence and adherence of anticholinergics and beta-3 agonist for the treatment of overactive bladder: Systematic review and meta-analysis, and network meta-analysis. J. Urol. 2021, 205, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- McGhan, W.F. Cost effectiveness and quality of life considerations in the treatment of patients with overactive bladder. Am. J. Manag. Care 2001, 7 (Suppl. S2), S62–S75. [Google Scholar] [PubMed]
- Murray, B.; Hessami, S.H.; Gultyaev, D.; Lister, J.; Dmochowski, R.; Gillard, K.K.; Stanisic, S.; Tung, A.; Boer, R.; Kaplan, S. Cost-effectiveness of overactive bladder treatments: From the US payer perspective. J. Comp. Eff. Res. 2019, 8, 61–71. [Google Scholar] [CrossRef]
- Taub, D.A.; Wei, J.T. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 2006, 7, 272–281. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine intolerance: The current state of the art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- McGrath, A.P.; Hilmer, K.M.; Collyer, C.A.; Shepard, E.M.; Elmore, B.O.; Brown, D.E.; Dooley, D.M.; Guss, J.M. Structure and inhibition of human diamine oxidase. Biochemistry 2009, 48, 9810–9822. [Google Scholar] [CrossRef]
- Chassande, O.; Renard, S.; Barbry, P.; Lazdunski, M. The human gene for diamine oxidase, an amiloride binding protein. Molecular cloning, sequencing, and characterization of the promoter. J. Biol. Chem. 1994, 269, 14484–14489. [Google Scholar] [CrossRef]
- Novotny, W.F.; Chassande, O.; Baker, M.; Lazdunski, M.; Barbry, P. Diamine oxidase is the amiloride-binding protein and is inhibited by amiloride analogues. J. Biol. Chem. 1994, 269, 9921–9925. [Google Scholar] [CrossRef]
- Ayuso, P.; García-Martín, E.; Martínez, C.; Agúndez, J.A. Genetic variability of human diamine oxidase: Occurrence of three nonsynonymous polymorphisms and study of their effect on serum enzyme activity. Pharmacogenet. Genom. 2007, 17, 687–693. [Google Scholar] [CrossRef]
- García-Martín, E.; Ayuso, P.; Martínez, C.; Agúndez, J.A. Improved analytical sensitivity reveals the occurrence of gender-related variability in diamine oxidase enzyme activity in healthy individuals. Clin. Biochem. 2007, 40, 1339–1341. [Google Scholar] [CrossRef]
- Maintz, L.; Yu, C.F.; Rodríguez, E.; Baurecht, H.; Bieber, T.; Illig, T.; Weidinger, S.; Novak, N. Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities. Allergy 2011, 66, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, O.; Sjaastad, O.V. Urinary histamine excretion in migraine and cluster headache. Further observations. J. Neurol. 1977, 216, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Koshibu, T. Measurement of plasma histamine, urinary N-methylhistamine and peripheral eosinophil counts in food provocation tests in infants and children with atopic dermatitis. Arerugi 1994, 43, 787–795. [Google Scholar] [PubMed]
- Kaliner, M.; Shelhamer, J.H.; Ottesen, E.A. Effects of infused histamine: Correlation of plasma histamine levels and symptoms. J. Allergy Clin. Immunol. 1982, 69, 283–289. [Google Scholar] [CrossRef]
- Todd, J.K.; Mack, A.J. A study of human bladder detrusor muscle. Br. J. Urol. 1969, 41, 448–454. [Google Scholar] [CrossRef]
- Khanna, O.P.; DeGregorio, G.J.; Sample, R.C.; McMichael, R.F. Histamine receptors in urethrovesical smooth muscle. Urology 1977, 10, 375–381. [Google Scholar] [CrossRef]
- Barker, L.A.; Ebersole, B.J. Histamine H2-receptors on guinea-pig ileum myenteric plexus neurons mediate the release of contractile agents. J. Pharmacol. Exp. Ther. 1982, 221, 69–75. [Google Scholar]
- Rubinstein, R.; Nissenkorn, I.; Cohen, S. Acetylcholine mediation of the contractile response to histamine in human bladder detrusor muscle. Eur. J. Pharmacol. 1987, 142, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.B.; Westfall, D.P. Potentiation of purinergic neurotransmission in guinea pig urinary bladder by histamine. J. Urol. 1994, 151, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Weimann, A.; Stolzenburg, J.U.; Dawood, W.; Schwalenberg, T.; Dorschner, W. Histamine receptors in human detrusor smooth muscle cells: Physiological properties and immunohistochemical representation of subtypes. World J. Urol. 2006, 24, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Oberbach, A.; Schwalenberg, T.; Stolzenburg, J.U. Cultured smooth muscle cells of the human vesical sphincter are more sensitive to histamine than are detrusor smooth muscle cells. Urology 2006, 67, 1086–1092. [Google Scholar] [CrossRef]
- Neuhaus, J.; Schulte-Baukloh, H.; Stolzenburg, J.U.; Speroni di Fenizio, P.; Horn, L.C.; Rüffert, H.; Hartenstein, S.; Burger, M.; Schulze, M.; Schwalenberg, T. Individual receptor profiling as a novel tool to support diagnosis of bladder pain syndrome/interstitial cystitis (BPS/IC). World J. Urol. 2012, 30, 693–700. [Google Scholar] [CrossRef]
- Moro, C.; Uchiyama, J.; Chess-Williams, R. Urothelial/lamina propria spontaneous activity and the role of M3 muscarinic receptors in mediating rate responses to stretch and carbachol. Urology 2011, 78, 1442.e9–1442.e15. [Google Scholar] [CrossRef]
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef]
- Yamada, T.; Murayama, T.; Mita, H.; Akiyama, K. Subtypes of bladder mast cells in interstitial cystitis. Int. J. Urol. 2000, 7, 292–297. [Google Scholar] [CrossRef]
- Apostolidis, A.; Jacques, T.S.; Freeman, A.; Kalsi, V.; Popat, R.; Gonzales, G.; Datta, S.N.; Ghazi-Noori, S.; Elneil, S.; Dasgupta, P.; et al. Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur. Urol. 2008, 53, 1245–1253. [Google Scholar] [CrossRef]
- Stromberga, Z.; Chess-Williams, R.; Moro, C. Histamine modulation of urinary bladder urothelium, lamina propria and detrusor contractile activity via H1 and H2 receptors. Sci. Rep. 2019, 9, 3899. [Google Scholar] [CrossRef]
- Grundy, L.; Caldwell, A.; Garcia Caraballo, S.; Erickson, A.; Schober, G.; Castro, J.; Harrington, A.M.; Brierley, S.M. Histamine induces peripheral and central hypersensitivity to bladder distension via the histamine H1 receptor and TRPV1. Am. J. Physiol. Ren. Physiol. 2020, 318, F298–F314. [Google Scholar] [CrossRef]
- Espuña Pons, M.; Puig Clota, M.; Rebollo Alvarez, P. Validation of the Spanish version of the “Bladder control Self-Assessment Questionnarie” (B-SAQ). A new screening instrument for lower urinary tract dysfunction. Actas Urol. Esp. 2006, 30, 1017–1024. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Badía, X.; García-Losa, M.; Dal-Ré, R.; Carballido, J.; Serra, M. Validation of a harmonized Spanish version of the IPSS: Evidence of equivalence with the original American scale. International Prostate Symptom Score. Urology 1998, 52, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
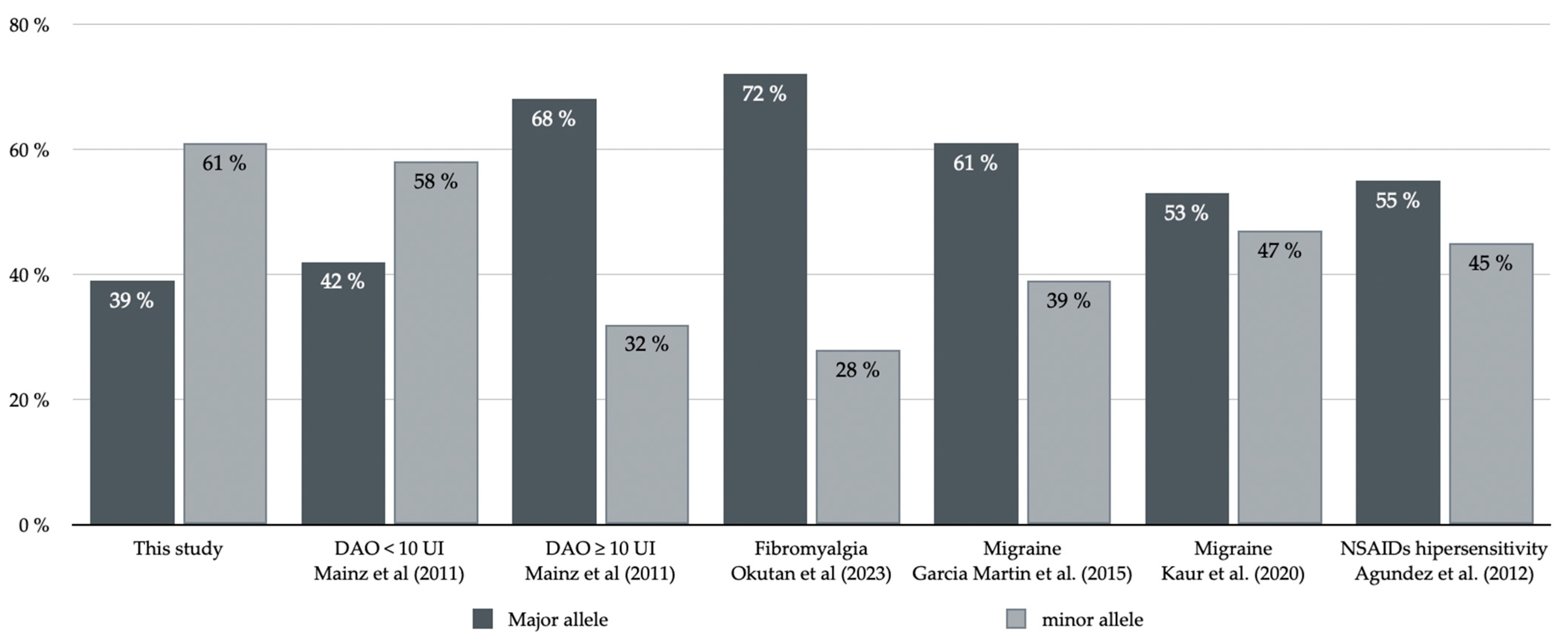
- García-Martín, E.; Martínez, C.; Serrador, M.; Alonso-Navarro, H.; Ayuso, P.; Navacerrada, F.; Agúndez, J.A.; Jiménez-Jiménez, F.J. Diamine oxidase rs10156191 and rs2052129 variants are associated with the risk for migraine. Headache 2015, 55, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Okutan, G.; Ruiz Casares, E.; Perucho Alcalde, T.; Sánchez Niño, G.M.; Penadés, B.F.; Terrén Lora, A.; Torrente Estríngana, L.; López Oliva, S.; San Mauro Martín, I. Prevalence of genetic diamine oxidase (DAO) deficiency in female patients with fibromyalgia in Spain. Biomedicines 2023, 11, 660. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Vidal-Carou, M.C.; Soler-Singla, L. Low serum diamine oxidase (DAO) activity levels in patients with migraine. J. Physiol. Biochem. 2018, 74, 93–99. [Google Scholar] [CrossRef]
- García-Martin, E.; Mendoza, J.L.; Martínez, C.; Taxonera, C.; Urcelay, E.; Ladero, J.M.; de la Concha, E.G.; Díaz-Rubio, M.; Agúndez, J.A. Severity of ulcerative colitis is associated with a polymorphism at diamine oxidase gene but not at histamine N-methyltransferase gene. World J. Gastroenterol. 2006, 28, 615–620. [Google Scholar] [CrossRef]
- Agúndez, J.A.; Ayuso, P.; Cornejo-García, J.A.; Blanca, M.; Torres, M.J.; Doña, I.; Salas, M.; Blanca-López, N.; Canto, G.; Rondon, C.; et al. The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLoS ONE 2012, 7, e47571. [Google Scholar] [CrossRef]
- Kaur, S.; Ali, A.; Siahbalaei, Y.; Ahmad, U.; Nargis, F.; Pandey, A.K.; Singh, B. Asso-ciation of diamine oxidase (DAO) variants with the risk for migraine from North Indian population. Meta Gene 2020, 24, 100619. [Google Scholar] [CrossRef]
| Variables | Total, n = 100 | Men, n = 46 | Women, n = 54 | p Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 56.9 (14.6) | 60.4 (13.4) | 52.6 (14.7) | 0.011 |
| Age segments, n (%) | ||||
| <40 years | 13 (13.0) | 3 (6.5%) | 10 (18.5%) | 0.141 |
| 40–50 years | 24 (24.0%) | 9 (19.6%) | 15 (27.8%) | |
| 51–60 years | 19 (19.0%) | 9 (19.6%) | 10 (18.5%) | |
| 61–70 years | 21 (21.0%) | 10 (21.7%) | 11 (20.4%) | |
| >70 years | 23 (23.0%) | 15 (32.5%) | 8 (14.8%) | |
| B-SAQ score, mean (SD) | 11.5 (6.2) | 10.1 (6.3) | 12.5 (5.9) | 0.052 |
| IPSS score, mean (SD) | 17.2 (6.9) | 18.6 (7.1) | 16.0 (6.5) | 0.074 |
| S-IPSS score | 8.0 (3.2) | 7.6 (3.3) | 8.3 (3.1) | 0.174 |
| V-IPSS score | 9.2 (5.6) | 11.0 (5.5) | 7.6 (5.2) | 0.003 |
| IPSS severity, n (%) | ||||
| Mild | 7 (7.0) | 2 (4.3) | 5 (9.3) | 0.120 |
| Moderate | 58 (58.0) | 23 (50.0) | 35 (64.8) | |
| Severe | 35 (35.0) | 21 (45.7) | 14 (25.9) | |
| S-IPSS severity, n (%) | ||||
| Moderate | 69 (69.0) | 36 (78.3) | 33 (61.1) | 0.065 |
| Severe | 31 (31.0) | 10 (21.7) | 21 (38.9) | |
| V-IPSS severity, n (%) | ||||
| Moderate | 89 (89.0) | 38 (82.6) | 51 (94.4) | 0.059 |
| Severe | 11 (11.0) | 8 (17.4) | 3 (5.6) |
| Genetic Profile | Total Patients (n = 100) | Men (n = 46) | Women (n = 54) | p Value | |
|---|---|---|---|---|---|
| SNPs minor allele, n (%) | |||||
| 0 | 12 (12.0) | 4 (8.7) | 8 (14.8) | 0.808 | |
| 1 | 20 (20.0) | 8 (17.4) | 12 (22.2) | ||
| 2 | 22 (22.0) | 11 (23.9) | 11 (20.4) | ||
| 3 | 15 (15.0) | 7 (15.2) | 8 (14.8) | ||
| 4 | 31 (31.0) | 16 (34.8) | 15 (27.8) | ||
| SNPs genotype, n (%) | |||||
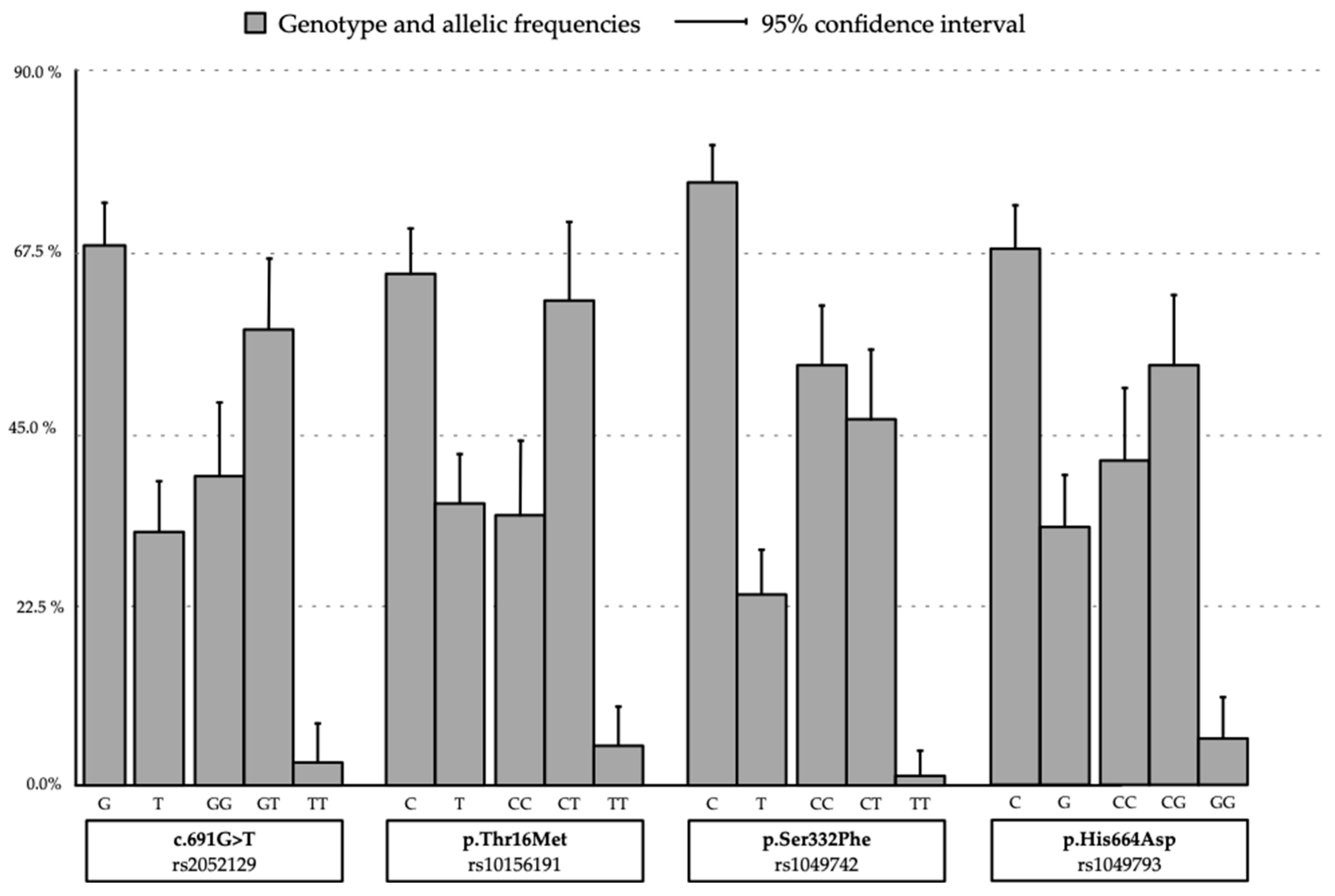
| rs2052129 (c.691G>T) | GG | 39 (39.0) | 17 (36.9) | 22 (40.7) | 0.816 |
| GT | 58 (58.0) | 28 (60.9) | 30 (55.6) | ||
| TT | 3 (3.0) | 1 (2.2) | 2 (3.7) | ||
| rs10156191 (p.Thr16Met) | CC | 34 (34.0) | 14 (30.4) | 20 (37.0) | 0.742 |
| CT | 61 (61.0) | 30 (65.2) | 31 (57.4) | ||
| TT | 5 (5.0) | 2 (4.3) | 3 (5.6) | ||
| rs1049742 (p.Ser332Phe) | CC | 43 (43.0) | 24 (52.2) | 29 (53.7) | 0.912 |
| CT | 56 (56.0) | 22 (47.8) | 24 (44.4) | ||
| TT | 1 (1.0) | 0 | 1 (1.1) | ||
| rs1049743 (p.His664Asp) | CC | 41 (41.0) | 14 (30.4) | 27 (50.0) | 0.046 |
| CG | 53 (53.0) | 27 (58.7) | 26 (48.1) | ||
| GG | 6 (6.0) | 5 (10.9) | 1 (1.9) | ||
| SNPs Genotypes | Prevalence | B-SAQ Score Mean (SD) | p Value | IPSS Score Mean (SD) | p Value | |
|---|---|---|---|---|---|---|
| rs2052129 (c.691G>T) | GG | 39% | 11.3 (5.8) | 0.900 | 14.9 (5.1) | 0.021 |
| GT | 58% | 11.6 (6.4) | 18.8 (7.6) | |||
| TT | 3% | 10.7 (10.7) | 17.3 (3.1) | |||
| rs10156191 (p.Thr16Met) | CC | 34% | 11.6 (5.9) | 0.755 | 15.3 (3.0) | 0.047 |
| CT | 61% | 11.2 (6.3) | 18.1 (7.7) | |||
| TT | 5% | 13.2 (7.7) | 20.8 (4.7) | |||
| rs1049742 (p.Ser332Phe) | CC | 43% | 12.2 (5.8) | 0.319 | 15.9 (6.1) | 0.083 |
| CT | 56% | 10.6 (6.6) | 18.5 (7.4) | |||
| TT | 1% | 14 (.) | 28 (.) | |||
| rs1049743 (p.His664Asp) | CC | 41% | 12.9 (6.0) | 0.066 | 16.4 (6.2) | 0.070 |
| CG | 53% | 10.9 (6.0) | 17.2 (7.2) | |||
| GG | 6% | 6.5 (7.3) | 23.7 (6.4) | |||
| Variables | IPSS Score | Allele Positivity Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|---|
| Mild/Moderate <20, n (%) | Severe ≥20, n (%) | |||
| Gender | Male | 25 (54.3) | 21 (47.5) | 0.42 (0.18–0.97); p = 0.039 |
| Female | 40 (74.1) | 14 (25.9) | ||
| rs2052129 (c.691G>T) | GG | 30 (76.9) | 9 (23.1) | 2.48 (1.01–6.10); p = 0.046 |
| GT-TT | 35 (57.4) | 26 (42.6) | ||
| rs10156191 (p.Thr16Met) | CC | 26 (76.5) | 8 (23.5) | 2.25 (0.89–5.71); p = 0.084 |
| CT-TT | 39 (59.1) | 27 (40.9) | ||
| rs1049742 (p.Ser332Phe) | CC | 38 (71.7) | 15 (28.3) | 1.88 (0.82–4.31); p = 0.136 |
| CT-TT | 27 (57.4) | 20 (42.6) | ||
| rs1049743 (p.His664Asp) | CC | 30 (73.2) | 11 (26.8) | 1.87 (0.79–4.44); p = 0.153 |
| CG-GG | 35 (59.3) | 24 (40.7) | ||
| rs2052129 rs10156191 | No | 33 (78.6) | 9 (21.4) | 2.98 (1.21–7.33); p = 0.015 |
| Yes | 32 (55.2) | 26 (44.8) | ||
| SNPs | <4 SNPs | 50 (72.5) | 19 (27.5) | 2.81 (1.16–6.77); p = 0.020 |
| 4 SNPs | 15 (48.4) | 16 (51.6) | ||
| Homozygosity | No | 59 (67.8) | 38 (32.2) | 2.46 (0.76–8.00); p = 0.210 |
| Yes | 6 (46.2) | 7 (53.8) | ||
| Variables | S-IPSS Score | Allele Positivity Odds Ratio (95% Confidence Interval) | V-IPSS | Allele Positivity Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|---|---|---|
| Mild/ Moderate <20, n (%) | Severe ≥20, n (%) | Mild/ Moderate <20, n (%) | Severe ≥20, n (%) | ||||
| Gender | Male | 36 (78.3) | 10 (21.7) | 2.29 (0.94–5.57); p = 0.065 | 38 (82.6) | 8 (17.4) | 0.28 (0.07–1.12); p = 0.059 |
| Female | 33 (61.1) | 21 (38.9) | 51 (94.4) | 3 (5.6) | |||
| rs2052129 (c.691G>T) | GG | 29 (74.4) | 10 (25.6) | 1.52 (0.62–3.71); p = 0.354 | 39 (100.0) | 0 | 17.0 (1.03–314.7); p = 0.006 * |
| GT-TT | 40 (65.6) | 21 (34.4) | 50 (82.0) | 11 (18.0) | |||
| rs10156191 (p.Thr16Met) | CC | 26 (76.5) | 8 (23.5) | 1.74 (0.68–4.45); p = 0.246 | 34 (100.0) | 0 | 14.3 (0.82–250.4); p = 0.014 * |
| CT-TT | 43 (65.2) | 23 (34.8) | 55 (83.3) | 11 (16.7) | |||
| rs1049742 (p.Ser332Phe) | CC | 38 (71.7) | 15 (28.3) | 1.31 (0.56–3.06); p = 0.536 | 50 (94.3) | 3 (5.7) | 3.42 (0.85–13.75); p = 0.070 |
| CT-TT | 31 (66.0) | 16 (34.0) | 39 (83.0) | 8 (17.0) | |||
| rs1049743 (p.His664Asp) | CC | 26 (63.4) | 15 (36.6) | 0.64 (0.27–1.52); p = 0.314 | 38 (92.7) | 3 (7.3) | 1.99 (0.49–7.99); p = 0.518 |
| CG-GG | 43 (72.9) | 16 (27.1) | 51 (86.4) | 3 (16.3) | |||
| rs2052129 rs10156191 | No | 31 (73.8) | 11 (26.2) | 1.48 (0.62–3.56); p = 0.376 | 42 (100.0) | 0 | 20.58 (1.86–359.9); p = 0.002 * |
| Yes | 38 (65.5) | 20 (34.5) | 47 (81.0) | 11 (19.0) | |||
| SNPs | < 4 SNPs | 50 (72.5) | 19 (27.5) | 1.66 (0.68–4.07); p = 0.264 | 65 (94.2) | 4 (5.8) | 4.74 (1.27–17.65); p = 0.032 |
| 4 SNPs | 19 (61.3) | 12 (38.7) | 15 (48.4) | 16 (51.6) | |||
| Homozygosity | No | 61 (70.1) | 26 (29.9) | 1.47 (0.44–4.90); p = 0.534 | 80 (92.0) | 7 (8.0) | 5.08 (1.24–20.77); p = 0.035 |
| Yes | 8 (61.5) | 5 (38.5) | 9 (69.2) | 4 (30.8) | |||
| Variables | V-IPSS Score in Men | Allele Positivity p Value | V-IPSS in Women | Allele Positivity p Value | |||
|---|---|---|---|---|---|---|---|
| Mild/ Moderate <17, n (%) | Severe ≥17, n (%) | Mild/ Moderate <17, n (%) | Severe ≥17, n (%) | ||||
| rs2052129 (c.691G>T) | GG | 17 (44.7) | 0 | 0.019 | 22 (43.1) | 0 | 0.262 |
| GT-TT | 21 (55.3) | 8 (100.0) | 29 (56.9) | 3 (100.0) | |||
| rs10156191 (p.Thr16Met) | CC | 14 (36.8) | 0 | 0.085 | 20 (39.2) | 0 | 0.287 |
| CT-TT | 24 (63.2) | 8 (100.0) | 31 (60.8) | 3 (100.0) | |||
| rs2052129 rs10156191 | No | 18 (47.4) | 0 | 0.015 | 24 (47.1) | 0 | 0.245 |
| Yes | 20 (52.6) | 8 (100.0) | 27 (52.9) | 3 (100.0) | |||
| SNPs | <4 SNPs | 26 (68.4) | 4 (50.0) | 0.421 | 39 (76.5) | 0 | 0.018 |
| 4 SNPs | 12 (31.6) | 4 (50.0) | 12 (23.5) | 3 (100.0) | |||
| Homozygosity | No | 33 (86.8) | 6 (75.0) | 0.597 | 47 (92.2) | 1 (33.3) | 0.030 |
| Yes | 5 (13.2) | 2 (25.0) | 4 (7.8) | 2 (66.7) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce Díaz-Reixa, J.; Aller Rodríguez, M.; Martínez Breijo, S.; Suanzes Hernández, J.; Ruiz Casares, E.; Perucho Alcalde, T.; Bohorquez Cruz, M.; Mosquera Seoane, T.; Sánchez Merino, J.M.; Freire Calvo, J.; et al. Lower Urinary Tract Symptoms (LUTS) as a New Clinical Presentation of Histamine Intolerance: A Prevalence Study of Genetic Diamine Oxidase Deficiency. J. Clin. Med. 2023, 12, 6870. https://doi.org/10.3390/jcm12216870
Ponce Díaz-Reixa J, Aller Rodríguez M, Martínez Breijo S, Suanzes Hernández J, Ruiz Casares E, Perucho Alcalde T, Bohorquez Cruz M, Mosquera Seoane T, Sánchez Merino JM, Freire Calvo J, et al. Lower Urinary Tract Symptoms (LUTS) as a New Clinical Presentation of Histamine Intolerance: A Prevalence Study of Genetic Diamine Oxidase Deficiency. Journal of Clinical Medicine. 2023; 12(21):6870. https://doi.org/10.3390/jcm12216870
Chicago/Turabian StylePonce Díaz-Reixa, Jose, Marcos Aller Rodríguez, Sara Martínez Breijo, Jorge Suanzes Hernández, Eva Ruiz Casares, Teresa Perucho Alcalde, Manuel Bohorquez Cruz, Teresa Mosquera Seoane, Jose M. Sánchez Merino, Jacobo Freire Calvo, and et al. 2023. "Lower Urinary Tract Symptoms (LUTS) as a New Clinical Presentation of Histamine Intolerance: A Prevalence Study of Genetic Diamine Oxidase Deficiency" Journal of Clinical Medicine 12, no. 21: 6870. https://doi.org/10.3390/jcm12216870
APA StylePonce Díaz-Reixa, J., Aller Rodríguez, M., Martínez Breijo, S., Suanzes Hernández, J., Ruiz Casares, E., Perucho Alcalde, T., Bohorquez Cruz, M., Mosquera Seoane, T., Sánchez Merino, J. M., Freire Calvo, J., Fernández Suárez, P., & Chantada Abal, V. (2023). Lower Urinary Tract Symptoms (LUTS) as a New Clinical Presentation of Histamine Intolerance: A Prevalence Study of Genetic Diamine Oxidase Deficiency. Journal of Clinical Medicine, 12(21), 6870. https://doi.org/10.3390/jcm12216870






