CRB-65 for Risk Stratification and Prediction of Prognosis in Pulmonary Embolism
Abstract
:1. Introduction
2. Methods
2.1. Study Endpoints and In-Hospital Adverse Events
2.2. Definitions
2.3. Ethical Aspects and Study Oversight
2.4. Statistics
3. Results
4. Discussion
- (i)
- Annual numbers of PE cases increased slowly from 2005 to 2020.
- (ii)
- The proportion of high-risk patients according to the CRB-65 score (≥1 points) was widely stable over time.
- (iii)
- Established risk stratification parameters such as syncope and right ventricular dysfunction, as well as tachycardia and sPESI, were more prevalent in the high-risk patients according to the CRB-65 score (≥1 points).
- (iv)
- In-hospital case fatality rate was 15.6%, and MACCE rate 17.3% higher in PE patients of the high-risk group according to the CRB-65 score (≥1 points) compared to the low-risk group (= 0 points). In addition, stroke, acute kidney injury, pneumonia, and all bleeding events occurred more often in the high-risk group according to the CRB-65 score (≥1 points).
- (v)
- Systemic thrombolysis as well as surgical embolectomy were both more often used in the high-risk vs. low-risk group defined according to the CRB-65 score.
- (vi)
- An increase in CRB-65 score by 1 was independently related to a 3.8-fold higher risk for in-hospital death and a 3.4-fold higher risk for MACCE.
- (vii)
- The CRB-65 high-risk class was independently and strongly associated with in-hospital death as well as MACCE.
- (viii)
- The prognostic performance of the CRB-65 score was better as sPESI, wherby the sPESI was developed for risk stratification of haemodynamically stable PE patients.
- (ix)
- Systemic thrombolysis and surgical embolectomy were both independently more often used in the CRB-65 high-risk group.
- (x)
- The CRB-65 high-risk group was also independently associated with all bleeding events.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konstantinides, S.; Goldhaber, S.Z. Pulmonary embolism: Risk assessment and management. Eur. Heart J. 2012, 33, 3014–3022. [Google Scholar] [CrossRef]
- Keller, K.; Beule, J.; Schulz, A.; Coldewey, M.; Dippold, W.; Balzer, J.O. Right ventricular dysfunction in hemodynamically stable patients with acute pulmonary embolism. Thromb. Res. 2014, 133, 555–559. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Ebner, M.; Kresoja, K.-P.; Münzel, T.; Konstantinides, S.V.; Lankeit, M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur. Heart J. 2020, 41, 522–529. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Münzel, T.; Ostad, M.A. Impact of concomitant deep or superficial venous thrombosis of the legs on survival of patients with pulmonary embolism. Int. J. Cardiol. 2020, 315, 92–98. [Google Scholar] [CrossRef]
- Klok, F.; van der Hulle, T.; Exter, P.D.; Lankeit, M.; Huisman, M.; Konstantinides, S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev. 2014, 28, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G. The 2019 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2019, 40, 3453–3455. [Google Scholar] [CrossRef]
- Goldhaber, S.Z. Assessing the prognosis of acute pulmonary embolism: Tricks of the trade. Chest 2008, 133, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; Uresandi, F.; Otero, R.; Lobo, J.L.; Monreal, M.; Martí, D.; Zamora, J.; Muriel, A.; Aujesky, D.; Yusen, R.D. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: Systematic review and metaanalysis. Chest 2009, 136, 974–982. [Google Scholar] [CrossRef]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; Janssens, U.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar]
- Jiménez, D.; Díaz, G.; Molina, J.; Martí, D.; Del Rey, J.; García-Rull, S.; Escobar, C.; Vidal, R.; Sueiro, A.; Yusen, R.D. Troponin I and risk stratification of patients with acute nonmassive pulmonary embolism. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2008, 31, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.; Trinquart, L.; Caille, V.; Couturaud, F.; Pacouret, G.; Meneveau, N.; Verschuren, F.; Roy, P.M.; Parent, F.; Righini, M.; et al. Prognostic factors for pulmonary embolism: The prep study, a prospective multicenter cohort study. Am. J. Respir. Crit. Care Med. 2010, 181, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Wallmann, D.; Carone, A.; Windecker, S.; Meier, B.; Hess, O.M. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur. Heart J. 2003, 24, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; Aujesky, D.; Moores, L.; Gómez, V.; Lobo, J.L.; Uresandi, F.; Otero, R.; Monreal, M.; Muriel, A.; Yusen, R.D.; et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch. Intern. Med. 2010, 170, 1383–1389. [Google Scholar] [CrossRef]
- Righini, M.; Roy, P.; Meyer, G.; Verschuren, F.; Aujesky, D.; Le Gal, G. The Simplified Pulmonary Embolism Severity Index (PESI): Validation of a clinical prognostic model for pulmonary embolism. J. Thromb. Haemost. 2011, 9, 2115–2117. [Google Scholar] [CrossRef]
- Donze, J.; Le Gal, G.; Fine, M.J.; Roy, P.M.; Sanchez, O.; Verschuren, F.; Cornuz, J.; Meyer, G.; Perrier, A.; Righini, M.; et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb. Haemost. 2008, 100, 943–948. [Google Scholar]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.-M.; Fine, M.J. Derivation and validation of a prognostic model for pulmonary embolism. Am. J. Respir. Crit. Care Med. 2005, 172, 1041–1046. [Google Scholar] [CrossRef]
- Keller, K.; Coldewey, M.; Dippold, W.; Beule, J.; Balzer, J.O. Simplified CRB-65 for risk stratification and predicting prognosis in acute pulmonary embolism. Phlebologie 2015, 44, 192–199. [Google Scholar]
- Ozsu, S.; Abul, Y.; Orem, A.; Oztuna, F.; Bulbul, Y.; Yaman, H.; Ozlu, T. Predictive value of troponins and simplified pulmonary embolism severity index in patients with normotensive pulmonary embolism. Multidiscip. Respir. Med. 2013, 8, 34. [Google Scholar] [CrossRef]
- Moores, L.; Aujesky, D.; Jiménez, D.; Díaz, G.; Gómez, V.; Martí, D.; Briongos, S.; Yusen, R. Pulmonary Embolism Severity Index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. J. Thromb. Haemost. JTH 2010, 8, 517–522. [Google Scholar] [CrossRef]
- Lankeit, M.; Gómez, V.; Wagner, C.; Aujesky, D.; Recio, M.; Briongos, S.; Moores, C.L.K.; Yusen, R.D.; Konstantinides, S.; Jiménez, D. A strategy combining imaging and laboratory biomarkers in comparison with a simplified clinical score for risk stratification of patients with acute pulmonary embolism. Chest 2012, 141, 916–922. [Google Scholar] [CrossRef]
- Hariharan, P.; Takayesu, J.K.; Kabrhel, C. Association between the Pulmonary Embolism Severity Index (PESI) and short-term clinical deterioration. Thromb. Haemost. 2011, 105, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Uresandi, F.; Otero, R.; Cayuela, A.; Cabezudo, M.A.; Jimenez, D.; Laserna, E.; Conget, F.; Oribe, M.; Nauffal, D. A clinical prediction rule for identifying short-term risk of adverse events in patients with pulmonary thromboembolism. Arch. Bronconeumol. 2007, 43, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Ben, S.Q.; Chen, H.L.; Ni, S.S. The prognostic value of pulmonary embolism severity index in acute pulmonary embolism: A meta-analysis. Respir. Res. 2012, 13, 111. [Google Scholar] [CrossRef]
- Aujesky, D.; Roy, P.-M.; Le Manach, C.P.; Verschuren, F.; Meyer, G.; Obrosky, D.S.; Stone, R.A.; Cornuz, J.; Fine, M.J. Validation of a model to predict adverse outcomes in patients with pulmonary embolism. Eur. Heart J. 2006, 27, 476–481. [Google Scholar] [CrossRef]
- Leidi, A.; Bex, S.; Righini, M.; Berner, A.; Grosgurin, O.; Marti, C. Risk Stratification in Patients with Acute Pulmonary Embolism: Current Evidence and Perspectives. J. Clin. Med. 2022, 11, 2533. [Google Scholar] [CrossRef] [PubMed]
- Hobohm, L.; Becattini, C.; Konstantinides, S.V.; Casazza, F.; Lankeit, M. Validation of a fast prognostic score for risk stratification of normotensive patients with acute pulmonary embolism. Clin. Res. Cardiol. 2020, 109, 1008–1017. [Google Scholar] [CrossRef]
- Bova, C.; Vanni, S.; Prandoni, P.; Morello, F.; Dentali, F.; Bernardi, E.; Mumoli, N.; Bucherini, E.; Barbar, S.; Picariello, C.; et al. A prospective validation of the Bova score in normotensive patients with acute pulmonary embolism. Thromb. Res. 2018, 165, 107–111. [Google Scholar] [CrossRef]
- McNally, M.; Curtain, J.; O’Brien, K.; Dimitrov, B.; Fahey, T. Validity of British Thoracic Society guidance (the CRB-65 rule) for predicting the severity of pneumonia in general practice: Systematic review and meta-analysis. Br. J. Gen. Pract. J. R. Coll. Gen. Pract. 2010, 60, e423–e433. [Google Scholar] [CrossRef]
- Ewig, S.; Welte, T. CRB-65 for the assessment of pneumonia severity: Who could ask for more? Thorax 2008, 63, 665–666. [Google Scholar] [CrossRef]
- Bauer, T.T.; Ewig, S.; Marre, R.; Suttorp, N.; Welte, T.; The CAPNETZ Study Group. CRB-65 predicts death from community-acquired pneumonia. J. Intern. Med. 2006, 260, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Höffken, G.; Lorenz, J.; Kern, W.; Welte, T.; Bauer, T.; Dalhoff, K.; Dietrich, E.; Ewig, S.; Gastmeier, P.; Grabein, B.; et al. Epidemiology, diagnosis, antimicrobial therapy and management of community-acquired pneumonia and lower respiratory tract infections in adults. Guidelines of the Paul-Ehrlich-Society for Chemotherapy, the German Respiratory Society, the German Society for Infectiology and the Competence Network CAPNETZ Germany. Pneumologie 2009, 63, e1–e68. [Google Scholar]
- Kwok, C.S.; Loke, Y.K.; Woo, K.; Myint, P.K. Risk prediction models for mortality in community-acquired pneumonia: A systematic review. BioMed Res. Int. 2013, 2013, 504136. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.K.; Kwok, C.S.; Niruban, A.; Myint, P.K. Value of severity scales in predicting mortality from community-acquired pneumonia: Systematic review and meta-analysis. Thorax 2010, 65, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Marti, C.; Garin, N.; Grosgurin, O.; Poncet, A.; Combescure, C.; Carballo, S.; Perrier, A. Prediction of severe community-acquired pneumonia: A systematic review and meta-analysis. Crit. Care 2012, 16, R141. [Google Scholar] [CrossRef] [PubMed]
- Reinöhl, J.; Kaier, K.; Reinecke, H.; Schmoor, C.; Frankenstein, L.; Vach, W.; Cribier, A.; Beyersdorf, F.; Bode, C.; Zehender, M. Effect of Availability of Transcatheter Aortic-Valve Replacement on Clinical Practice. N. Engl. J. Med. 2015, 373, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Le Jeune, I.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64 (Suppl. 3), iii1–iii55. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Barco, S.; Konstantinides, S. External validation of the VTE-BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb. Haemost. 2017, 117, 1164–1170. [Google Scholar] [CrossRef]
- Klok, F.A.; Barco, S.; Konstantinides, S.V. Evaluation of VTE-BLEED for predicting intracranial or fatal bleeding in stable anticoagulated patients with venous thromboembolism. Eur. Respir. J. 2018, 51, 1800077. [Google Scholar] [CrossRef]
- Klok, F.A.; Hösel, V.; Clemens, A.; Yollo, W.D.; Tilke, C.; Schulman, S.; Lankeit, M.; Konstantinides, S.V. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur. Respir. J. 2016, 48, 1369–1376. [Google Scholar] [CrossRef]
- Klok, F.A.; Kooiman, J.; Huisman, M.V.; Konstantinides, S.; Lankeit, M. Predicting anticoagulant-related bleeding in patients with venous thromboembolism: A clinically oriented review. Eur. Respir. J. 2015, 45, 201–210. [Google Scholar] [CrossRef]
- Klok, F.A.; Niemann, C.; Dellas, C.; Hasenfuß, G.; Konstantinides, S.; Lankeit, M. Performance of five different bleeding-prediction scores in patients with acute pulmonary embolism. J. Thromb. Thrombolysis 2016, 41, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, M.; Hedström, U.; Sjunnesson, M.; Lärfars, G.; Jorup-Rönström, C. Initial symptoms in pulmonary embolism differ from those in pneumonia: A retrospective study during seven years. Eur. J. Emerg. Med. 2006, 13, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, N.; Kanbay, A.; Bukan, N.; Ekim, N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin. Appl. Thromb. 2011, 17, 519–525. [Google Scholar] [CrossRef] [PubMed]


| CRB-65 Point Score | ICD or OPS Codes | |
|---|---|---|
| Confusion | +1 point | ICD code R40 |
| Respiratory failure | +1 point | ICD code J96 and/or OPS codes 8–71 or 8–72 |
| Unstable pulmonary embolism (CPR or shock) | +1 point | ICD code R57 and/or OPS code 8–77 |
| Age ≥65 years | +1 point | |
Graduation of patients according to CRB-65 score:
| ||
| Parameters | PE Patients with CRB-65 Score = 0 (n = 321,901; 23.4%) | PE Patients with CRB-65 Score ≥ 1 (n = 1,051,244; 76.6%) | p-Value |
|---|---|---|---|
| Age | 53.0 (44.0–59.0) | 76.0 (69.0–82.0) | <0.001 |
| Age ≥65 years | 0 (0.0%) | 921,165 (87.6%) | <0.001 |
| Female sex * | 140,497 (43.6%) | 586,987 (55.8%) | <0.001 |
| In-hospital stay (days) | 7.0 (4.0–12.0) | 10.0 (6.0–17.0) | <0.001 |
| Traditional cardiovascular risk factors | |||
| Obesity | 35,267 (11.0%) | 95,375 (9.1%) | <0.001 |
| Essential arterial hypertension | 91,902 (28.5%) | 510,857 (48.6%) | <0.001 |
| Diabetes mellitus | 30,219 (9.4%) | 226,017 (21.5%) | <0.001 |
| Hyperlipidaemia | 24,257 (7.5%) | 147,887 (14.1%) | <0.001 |
| Classical risk factors for venous thromboembolism and proportion of DVT | |||
| Cancer | 68,567 (21.3%) | 210,606 (20.0%) | <0.001 |
| Any surgery | 154,930 (48.1%) | 556,953 (53.0%) | <0.001 |
| Thrombophilia | 7853 (2.4%) | 8218 (0.8%) | <0.001 |
| Deep venous thrombosis or thrombophlebitis | 138,595 (43.1%) | 350,439 (33.3%) | <0.001 |
| Comorbidities | |||
| Charlson comorbidity index | 2.0 (0.0–3.0) | 5.0 (4.0–7.0) | <0.001 |
| Coronary artery disease | 16,262 (5.1%) | 171,331 (16.3%) | <0.001 |
| Heart failure | 24,245 (7.5%) | 276,552 (26.3%) | <0.001 |
| Peripheral artery disease | 4092 (1.3%) | 35,586 (3.4%) | <0.001 |
| Atrial fibrillation/flutter | 12,069 (3.7%) | 194,995 (18.5%) | <0.001 |
| Chronic obstructive pulmonary disease | 14,190 (4.4%) | 124,215 (11.8%) | <0.001 |
| Acute and chronic kidney disease | 19,514 (6.1%) | 274,962 (26.2%) | <0.001 |
| Risk stratification markers of VTE | |||
| Unstable PE (CPR or shock) | 0 (0.0%) | 123,180 (11.7%) | <0.001 |
| Shock | 0 (0.0%) | 56,644 (5.4%) | <0.001 |
| Syncope | 4673 (1.5%) | 28,643 (2.7%) | <0.001 |
| Right ventricular dysfunction | 54,433 (16.9%) | 326,828 (31.1%) | <0.001 |
| Tachycardia | 6955 (2.2%) | 33,964 (3.2%) | <0.001 |
| Respiratory failure | 0 (0.0%) | 394,858 (37.6%) | <0.001 |
| Confusion | 0 (0.0%) | 25,385 (2.4%) | <0.001 |
| sPESI ≥1 (sPESI high-risk class) | 113,092 (35.1%) | 741,874 (70.6%) | <0.001 |
| Adverse events during hospitalization | |||
| In-hospital death | 10,874 (3.4%) | 199,702 (19.0%) | <0.001 |
| MACCE | 16,318 (5.1%) | 235,908 (22.4%) | <0.001 |
| Systemic thrombolysis | 6645 (2.1%) | 50,532 (4.8%) | <0.001 |
| Surgical embolectomy | 417 (0.13%) | 1593 (0.15%) | 0.004 |
| Pneumonia | 73,215 (22.7%) | 257,620 (24.5%) | <0.001 |
| Acute kidney injury | 5423 (1.7%) | 84,936 (8.1%) | <0.001 |
| Stroke (ischaemic or haemorrhagic) | 4816 (1.5%) | 35,764 (3.4%) | <0.001 |
| Intracerebral bleeding | 1034 (0.3%) | 7531 (0.7%) | <0.001 |
| Gastrointestinal bleeding | 2335 (0.7%) | 18,594 (1.8%) | <0.001 |
| Transfusion of blood constituents | 21,462 (6.7%) | 138,194 (13.1%) | <0.001 |
| Univariable Regression Model | Multivariable Regression Model * | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| In-hospital death | 3.72 (3.69–3.74) | <0.001 | 3.81 (3.79–3.84) | <0.001 |
| MACCE | 3.37 (3.35–3.39) | <0.001 | 3.35 (3.32–3.37) | <0.001 |
| Pneumonia | 1.28 (1.27–1.28) | <0.001 | 1.45 (1.44–1.45) | <0.001 |
| Acute kidney injury | 2.96 (2.94–2.99) | <0.001 | 2.37 (2.34–2.39) | <0.001 |
| Stroke (ischaemic or haemorrhagic) | 1.70 (1.69–1.72) | <0.001 | 1.77 (1.74–1.79) | <0.001 |
| Intracerebral bleeding | 1.89 (1.85–1.94) | <0.001 | 2.35 (2.29–2.42) | <0.001 |
| Gastrointestinal bleeding | 1.72 (1.69–1.75) | <0.001 | 1.51 (1.48–1.54) | <0.001 |
| Transfusion of blood constituents | 1.89 (1.87–1.90) | <0.001 | 2.04 (2.02–2.05) | <0.001 |
| Univariable Regression Model | Multivariable Regression Model * | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
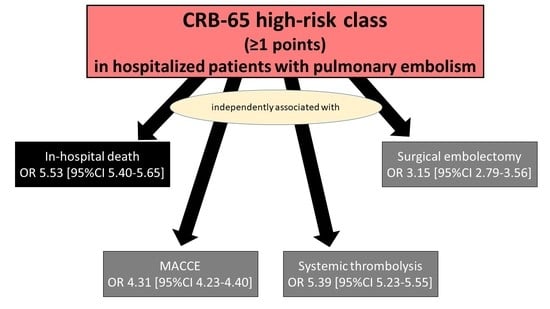
| In-hospital death | 6.71 (6.58–6.84) | <0.001 | 5.53 (5.40–5.65) | <0.001 |
| MACCE | 5.42 (5.33–5.51) | <0.001 | 4.31 (4.23–4.40) | <0.001 |
| Right ventricular dysfunction | 2.22 (2.20–2.24) | <0.001 | 2.42 (2.39–2.45) | <0.001 |
| Systemic thrombolysis | 2.40 (2.33–2.46) | <0.001 | 5.39 (5.23–5.55) | <0.001 |
| Surgical embolectomy | 1.17 (1.05–1.30) | <0.001 | 3.15 (2.79–3.56) | <0.001 |
| Pneumonia | 1.10 (1.09–1.11) | <0.001 | 1.49 (1.47–1.51) | <0.001 |
| Acute kidney injury | 5.13 (4.99–5.27) | <0.001 | 2.97 (2.86–3.09) | <0.001 |
| Stroke (ischaemic or haemorrhagic) | 2.32 (2.25–2.39) | <0.001 | 2.49 (2.40–2.58) | <0.001 |
| Intracerebral bleeding | 2.24 (2.10–2.39) | <0.001 | 3.97 (3.68–4.28) | <0.001 |
| Gastrointestinal bleeding | 2.46 (2.36–2.57) | <0.001 | 1.89 (1.79–1.99) | <0.001 |
| Transfusion of blood constituents | 2.12 (2.09–2.15) | <0.001 | 2.75 (2.70–2.81) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, K.; Schmitt, V.H.; Sagoschen, I.; Münzel, T.; Espinola-Klein, C.; Hobohm, L. CRB-65 for Risk Stratification and Prediction of Prognosis in Pulmonary Embolism. J. Clin. Med. 2023, 12, 1264. https://doi.org/10.3390/jcm12041264
Keller K, Schmitt VH, Sagoschen I, Münzel T, Espinola-Klein C, Hobohm L. CRB-65 for Risk Stratification and Prediction of Prognosis in Pulmonary Embolism. Journal of Clinical Medicine. 2023; 12(4):1264. https://doi.org/10.3390/jcm12041264
Chicago/Turabian StyleKeller, Karsten, Volker H. Schmitt, Ingo Sagoschen, Thomas Münzel, Christine Espinola-Klein, and Lukas Hobohm. 2023. "CRB-65 for Risk Stratification and Prediction of Prognosis in Pulmonary Embolism" Journal of Clinical Medicine 12, no. 4: 1264. https://doi.org/10.3390/jcm12041264
APA StyleKeller, K., Schmitt, V. H., Sagoschen, I., Münzel, T., Espinola-Klein, C., & Hobohm, L. (2023). CRB-65 for Risk Stratification and Prediction of Prognosis in Pulmonary Embolism. Journal of Clinical Medicine, 12(4), 1264. https://doi.org/10.3390/jcm12041264