Corticosteroids in ARDS
Abstract
:1. Introduction
1.1. Sepsis—Definition, Epidemiology and Pathophysiology
1.2. Acute Respiratory Distress Syndrome [ARDS]—Definition, Epidemiology and Pathophysiology
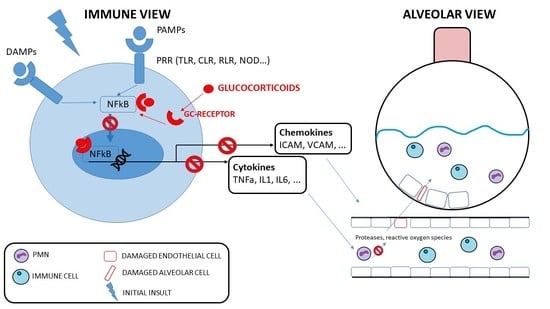
1.3. Corticosteroids
2. Corticosteroids in ARDS
2.1. Corticosteroids in Early Stage ARDS [Table 1]
2.2. Corticosteroids in Late-Stage ARDS
2.3. Dose and Type of Corticosteroid
2.4. Pathogens
2.5. Adverse Events
| Author, Reference | Type | Sample Size | Study Population | Treatment | Results |
|---|---|---|---|---|---|
| Bernard et al. [63] | RCT, multicenter | 99 | ARDS as Partial pressure of oxygen ≤ 70 mm Hg on > 40% oxygen, PaO2/PAO2 ratio < 0.3, bilateral lung infiltrates, pulmonary artery wedge pressure ≤ 18 mm Hg | MPS 30 mg/kg IV 6 hourly for 24 h vs. placebo | PEP mortality MPS 30/50 (60%); Pl 31/49 (63.2) OR 0.75 [0.4 to 1.57] p = 0.74 |
| Meduri et al. [60] | RCT multicenter | 24 | ARDS 1994 7 days of mechanical ventilation with an LIS of 2.5 or greater and less than a 1-point reduction from day 1 of ARDS, and no evidence of untreated infection. | MPS Loading dose of 2 mg/kg; then 2 mg/kg/d from day 1 to day 14, 1 mg/kg/d from day 15 to day 21, 0.5 mg/kg/d from day 22 to day 28, 0.25 mg/kg/d on days 29 and 30, 0.125 mg/kg/d on days 31 and 32. vs. placebo | PEP Lung injury and mortality day 10 MPS 1.7 [0.1]; Pl 3.0 [0.2]; p < 0.001 SEP: Mortality MPS 0/16 (0%); Pl 5/8 (62%) p = 0.002 Mortality in hospital MPS 2/16 (12.5%); Pl 5/8 (62.5%) OR 0.41 [0.06 to 99] p = 0.03 |
| Steinberg et al., ARDSnetwork, [67] | RCT Multicenter | 132/180 | ARDS 1994 in early and late stage At least 7 days duration ARDS; p/F < 200 Intubated, mechanical ventilation | MPS Loading dose of 2 mg/kg of predicted body weight followed by 0.5 mg/kg 6 hourly for 14 days; 0.5 mg/kg 12 hourly for 7 days; and then tapering of the dose. | In early ARDS (7–13 d) PEP mortality at 60 days MPS (36%); Pl (27%) p = 0.26 |
| Annane et al. [47] | post Hoc RCT | 129/300 177 ARDS: 129 non responders, 48 responder | ARDS 1994 bilateral infiltrate on chest radiography, PaO2/FiO2 < 200 mm Hg and Pulmonary occlusion pressure ≤ 18 mm Hg or no clinical evidence of left atrial hypertension | HSHC 50 mg IV 6 hourly and 9-alpha fludrocortisone once a day for 7 days. | PEP: mortality at 28-day In the non-responder subgroup HSHC + FC 33/62 (53%); Pl 50/67 (75%) RR = 0.71; 95% CI [0.54 to 0.94] p = 0.013 OR = 0.35; 95% CI [0.15 to 0.82], p = 0.016). In the responder group No significant result HSHC + FC 16/23 (70%); PL 12/25 (48%) RR = 1.4; 95% CI [0.89 to 2.36] p = 0.130 OR = 2.29; 95% CI [0.49 to 10.64] p = 0.290 |
| Meduri et al. [61] | RCT multicenter | 91 | ARDS 1994 Intubated and Mechanical ventilation ARDS ≤ 72 H of study entry | MPS Loading dose of 1 mg/kg Then 1 mg/kg/d from day 1 to day 14, 0.5 mg/kg/d from day 15 to day 21, 0.25 mg/kg/d from day 22 to day 25, 0.125 mg/kg/d from day 26 to day 28. | PEP 1-point reduction in LIS or MPS 69.8% vs. Pl 35.7%; p = 0.002 successful extubation 7-day MPS 53.9% vs. Pl 25.0%; p = 0.01 |
| Tongyoo et al. [91] | RCT Single center | 197 | Severe sepsis or septic shock receiving IMV for hypoxemic respiratory failure within 12 H of study entry + ARDS 1994 then reclassified accordingly to ARDS 2012 | HSHC 50 mg every 6 h or placebo | PEP 28 day all-cause mortality HSHC (22.5%) vs. Pl (27.3%) RR 0.82 [0.50 to 1.34] p = 0.51 HR 0.80, 95% CI [0.46 to 1.41]; p = 0.44 |
| Villar et al., DEXA-ARDS, [64] | RCT multicenter | 277/314 stopped low enrollment 88% | ARDS 2012 (but PEEP ≥ 10) Moderate to severe ARDS < 24 h (but PEEP ≥ 10) | DXM IV 20 mg once daily day 1 to 5 then 10 mg once daily day 6 to 10 | PEP N° ventilator-free from day of randomization to day 28 Between-group difference 4.8 days 95% CI [2.57 to 7.03]; p < 0.0001). |
| Horby et al., RECOVERY, [82] | RCT multicenter | 6425 | Hospitalized patients with suspected or laboratory confirmed COVID-19 | DXM 6 mg (IV or orally) during 10 days vs. usual care | PEP 28 d mortality Overall: DXM 482/2104 (22.9%); Pl 1110/4321 (25.7%) (age-adjusted RR 0.83; 95% CI [0.75 to 0.93]; p < 0.001) >sub group mechanical ventilation (1007): 29.3% vs. 41.4%; RR 0.64; 95% CI [0.51 to 0.81] |
| Tomazini et al., CoDEX, [84] | RCT multicenter | 299/350 | COVID-19 infection suspected or confirmed, receiving IMV within 48H of meeting criteria for moderate to severe ARDS 2012 | DXM 20 mg daily for 5 days followed by 10 mg daily for 5 days | PEP Ventilator-free days (alive + free from IMV) DXM 6.6 95% CI [5.0 to 8.2) vs. Pl 4.0 95% CI [2.9 to 5.4] difference 4.0 95% CI [2.9 to 5.4] |
| Dequin et al., CAPE COVID [92] | RCT multicenter | 149/290 | Confirmed or suspected SARS-CoV-2 + 1 severity criteria IMV (PEEP > 5 cm H2O), p/F < 300 HFOT > 50% Fi, PaO2/FiO2 < 300 FMOT (specified charts), PSI > 130 | HSHC 200 mg daily for 4 to 7 then 100 mg daily for 2 to 4 days then 50 mg daily for 2 to 3 days total 8 days | PEP: 21-day treatment failure (death or persistent dependency on mechanical ventilation or high-flow oxygen therapy HSHC 42.1% vs. pl 50.7% Difference −8.6% [95.48% CI, −24.9% to 7.7%]; p = 0.29) |
| Angus et al., REMAP CAP- [85] | RCT multicenter | 384 | COVID-19 suspected or confirmed, severe ICU for Respiratory failure (invasive or non-invasive IMV or HFN flow rate > 30 L/m, and FI > 40% Cardiovascular failure: vaopressor/inotrope | 3 randomization arms Fixed: HSHC 50 mg every 6 h daily for 7 days Shock: HSHC 50 mg/6 h for 7 days while in shock No HSHC Or 200 mg/6 h for 7 days | PEP Composite of hospital mortality and ICU organ support-free days to day 21 Fixed 0 QR, −1 to 15; OR 1.43 95% CI [0.91 to 2.27] Shock 0 IQR, –1 to 13; OR1.22 95% CI [0.76–1.94] None 0 0 (IQR, −1 to 11) |
| Barros et al., MetCOVID [86] | RCT single center | 246 | Clinical-radiological suspicion of COVID-19 Sat ≤ 94% in room air or Requiring O2 or IMV | MPS IV 0.5 mg/kg every 12 h × 5 days | PEP pulmonary function testing at day 120 follow-up visit. (Pulmonary function and maximal respiratory pressure testing, DASI, 6MWT) FEV1 (2.6, [0.7], p = 0.01) and FVC (3.2, [0.8], p = 0.01 |
| Dequin et al., CAPE COD, [54] | RCT multicenter | 795 | Severe community-acquired pneumoniae, defined by the presence of at least one of four following criteria The initiation of MV (invasive or noninvasive) with a positive end-expiratory pressure level ≥ 5 cm of water The initiation of the administration of oxygen through a HFOT with a ratio of PaO2:FiO2 < 300, with a FiO2 of 50% or more; For patients wearing a non-rebreathing mask, an estimated PaO2:FiO2 ratio < 300, or a score of more than 130 on the Pulmonary Severity Index, which classifies patients with community-acquired pneumonia into five groups according to increasing severity, with a score of more than 130 defining group V | HSHC continuous IV 200 mg/day during the first 4 days. On day 4, regarding medical decision based on predefined criteria, following administration for a total of 8 or 14 days | PEP mortality at day 28 HSHC 25 of 400 patients 6.2%; 95% CI, [3.9 to 8.6] vs. placebo 47 of 395 patients 11.9%; 95% CI, [8.7 to 15.1] (Absolute difference, −5.6 percentage points; 95% CI, [−9.6 to −1.7]; p = 0.006). |
| Dose | Author, Reference | Type | Sample Size | Study Population | Treatment | Results |
|---|---|---|---|---|---|---|
| High Dose | Bone et al. [68] | RCT multicenter | 382 (304 randomized) | Sepsis define as fever or hypothermia rectal T° >38.3 or <35.5 °C tachypnea (>20 bpm), tachycardia (>90 bpm) one of the following indices of organ dysfunction: a change in mental status, hypoxemia, elevated lactate levels or oliguria | MPS, 30 mg/kg, every six hours for 48 h vs. placebo | PEP: ARDS development MPS 50/152 32% Pl: 38/152 25% (p = 0.1) SEP: Reverse ARDS MPS 15/50 (31%); Pl: 23/38 (61%) (p = 0.005) 14 d mortality MPS 26/50 (52%); Pl 8/22 (%) (p = 0.004) |
| Bernard et al. [63] | RCT multicenter | 99 | ARDS Partial pressure of oxygen ≤70 mm Hg on >40% oxygen, PaO2/PAO2 ratio <0.3, bilateral lung infiltrates, pulmonary artery wedge pressure ≤18 mm Hg | MPS 30 mg/kg IV 6 hourly for 24 h vs. placebo | PEP mortality MPS 30/50 (60%); Pl 31/49 (63.2) OR 0.75 [0.4 to 1.57] p = 0.74 SEP reversal of ARDS MPS 18/50 (39%); Pl 19/49 (36%) (p = 0.07) | |
| High- Moderate Dose | Tomazini et al., CoDEX, [84] | RCT multicenter | 299/350 | COVID-19 moderate to severe ARDS according to Berlin, mechanical ventilation | DXM 20 mg daily for 5 days followed by 10 mg daily for 5 days | PEP Ventilator-free days (alive + free from IMV) DXM 6.6 95% CI% [5.0 to 8.2] vs. Pl 4.0 95% CI [2.9 to 5.4] difference 4.0 95% CI [2.9 to 5.4] SEP 28 d mortality all cause no difference |
| Barros et al., MetCOVID, [86] | RCT single center | 246 | In hospital COVID-19 Requiring O2 or IMV | MPS IV 0.5 mg/kg every 12 h × 5 days | PEP pulmonary function testing at day 120 follow-up visit. (pulmonary function and maximal respiratory pressure testing, DASI, 6MWT) FEV1 (2.6, [0.7], p = 0.01) and FVC (3.2, [0.8], p = 0.01 | |
| Munch et al., COVIDSTEROID, [87] | RCT multicenter | 982/1000 | Confirmed COVID-19 with > 10 L/mn O2 or IMV | DXM 12 mg/d vs. DXM 6 mg/d for 10 days | PEP number of days alive without life support at 28 d 22 d vs. 20.5 d adjusted mean difference, 1.3 days 95% CI [0 to 2.6 days]; p = 0.07 | |
| Bouadma et al., COVIDICUS, [88] | RCT multicenter | 546 | Admitted to ICU within 48H for confirmed or highly suspected COVID-19 AHRF (PaO2 < 70 mm Hg, SpO2 < 90%, tachypnea > 30 mn, labored breathing, respiratory distress, or need of O2 flow ≥ 6 L/mn) | DXM 6 mg/d for 10 days (or placebo prior to RECOVERY result communication) or Dexamethasone 20 mg/d for 5 days followed by Dexamethasone 10 mg/d for 5 days | PEP day 60 all-cause mortality Low 26.8% vs. High 25.9% Absolute risk difference −0.8% 95% CI [−8.3 to 6.5] HR, 0.96 95% CI [0.69 to 1.33]; (p = 0.79) | |
| Low Dose | Dequin et al., CAPE COVID, [92] | RCT multicenter | 149/290 | Confirmed or suspected SARS-CoV-2 + 1 severity criteria IMV (PEEP > 5 cm H2O), p/F < 300 HFOT > 50% Fi, PaO2/FiO2 < 300 FMOT (specified charts), PSI > 130 Excluded septic shock Low recruitment | HSHC 200 mg daily for 4 to 7 then 100 mg daily for 2 to 4 days then 50 mg daily for 2 to 3 days total 8 days | PEP: 21-day treatment failure (death or persistent dependency on mechanical ventilation or high-flow oxygen therapy HSHC 42.1% vs. pl 50.7% Difference −8.6% 95.48% CI [−24.9% to 7.7%]; p = 0.29) SEP 21 d mortality HSHC 14.7% vs. placebo 27.4% difference −12.7% 95% CI [−25.7% to 0.3%]; p = 0.06 |
| Angus et al., REMAP CAP, [85] | RCT multicenter | 384 | COVID-19 suspected or confirmed, severe ICU for Respiratory failure (invasive) or noninvasive MV or HFN flow rate > 30 L/m, and FI > 40% Cardiovascular failure: vasopressor/inotrope Stop ethical | 3 randomization arms Fixed: HSHC 50 mg every 6 h daily for 7 days Shock: HSHC 50 mg/6 h for 7 days while in shock No HSHC Or 200 mg/6 h for 7 days | PEP Composite of hospital mortality and ICU organ support-free days to day 21 Fixed 0 QR, −1 to 15; OR 1.43 95% CI [0.91 to 2.27] Shock 0 IQR, −1 to 13; OR1.22 95% CI [0.76 to 1.94] None 0 0 (IQR, −1 to 11) SEP 28 d mortality Fixed 30%; OR 1.03 95% CI [0.53 to 1.95] Shock 26%;1.10 95% CI [0.58 to 2.11] None 33% | |
| Horby et al., RECOVERY, [82] | RCT multicenter | 6425 | hospitalized patients with suspected or laboratory confirmed COVID-19 | DXM 6 mg (IV or orally) for 10 days vs. usual care | PEP 28 d mortality Overall: DXM 482/2104 (22.9%); Pl 1110/4321 (25.7%) (age-adjusted RR 0.83; 95% CI [0.75 to 0.93]; p < 0.001) >sub group mechanical ventilation (1007): 29.3% vs. 41.4%; RR 0.64; 95% CI [0.51 to 0.81) SEP Invasive mechanical ventilation or death RR 0.93; 95% CI [0.85 to 1.01] | |
| Corral- Gudino et al., GLUCOCOVID, [93] | RCT multicenter | 64/180 Low recruitment | Symptom > 7 days Radiological evidence of lung disease on chest X-ray or CT-scan Moderate to severe disease with abnormal gas exchange: (PaO2/FiO2 or PaFi) < 300 Or (SaO2/FiO2 or SaFi) < 400 Or ≥2 criteria of the BRESCIA-COVID Respiratory Severity Scale (BCRSS Evidence of a systemic inflammatory response: serum C-reactive protein > 15 mg/dL, D-dimer > 800 ng/mL, ferritin > 1000 mg/dL, or IL-6 levels > 20 pg/mL | MPS 40 mg IV q12 × 3 days and then 20 mg q12 h × 3 days | PEP: Composite endpoint (in-hospital all-cause mortality, escalation to ICU admission, or progression of respiratory insufficiency that required noninvasive ventilation) In ITT: MPS 40% vs. Pl 48% p = 0.25 In PP: RR 0.42 95% CI [0.20 to 0.89] |
3. Summary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The Pathogenesis of Sepsis. Annu. Rev. Pathol. 2011, 6, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Reinhart, K. Recognizing Sepsis as a Global Health Priority—A WHO Resolution|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/nejmp1707170 (accessed on 2 January 2023).
- Torio, C.M.; Andrews, R.M. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2006. Available online: http://www.ncbi.nlm.nih.gov/books/NBK169005/ (accessed on 2 January 2023).
- Annane, D.; Sharshar, T. Cognitive decline after sepsis. Lancet Respir. Med. 2015, 3, 61–69. [Google Scholar] [CrossRef]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit. Care Med. 2017, 45, 2078–2088. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Arcaroli, J.; Fessler, M.B.; Abraham, E. Genetic polymorphisms and sepsis. Shock 2005, 24, 300. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Spooner, C.E.; Markowitz, N.P.; Saravolatz, L.D. The role of tumor necrosis factor in sepsis. Clin. Immunol. Immunopathol. 1992, 62, S11–S17. [Google Scholar] [CrossRef]
- Pruitt, J.H.; Copeland, E.M.; Moldawer, L.L. Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock 1995, 3, 235–251. [Google Scholar] [CrossRef]
- O’Brien, X.M.; Biron, B.M.; Reichner, J.S. Consequences of Extracellular Trap Formation in Sepsis. Curr. Opin. Hematol. 2017, 24, 66–71. [Google Scholar] [CrossRef]
- Singer, M.; De Santis, V.; Vitale, D.; Jeffcoate, W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 2004, 364, 545–548. [Google Scholar] [CrossRef]
- Dunn, A.J. Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 2000, 917, 608–617. [Google Scholar] [CrossRef]
- Polito, A.; Sonneville, R.; Guidoux, C.; Barrett, L.; Viltart, O.; Mattot, V.; Siami, S.; Lorin de la Grandmaison, G.; Chrétien, F.; Singer, M.; et al. Changes in CRH and ACTH synthesis during experimental and human septic shock. PLoS ONE 2011, 6, e25905. [Google Scholar] [CrossRef]
- Dendoncker, K.; Libert, C. Glucocorticoid resistance as a major drive in sepsis pathology. Cytokine Growth Factor Rev. 2017, 35, 85–96. [Google Scholar] [CrossRef]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Diamond, M.; Peniston, H.L.; Sanghavi, D.; Mahapatra, S. Acute Respiratory Distress Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK436002/ (accessed on 11 December 2022).
- Auriemma, C.L.; Zhuo, H.; Delucchi, K.; Deiss, T.; Liu, T.; Jauregui, A.; Ke, S.; Vessel, K.; Lippi, M.; Seeley, E.; et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020, 46, 1222–1231. [Google Scholar] [CrossRef]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef]
- Hodgson, C.L.; Higgins, A.M.; Bailey, M.J.; Mather, A.M.; Beach, L.; Bellomo, R.; Bissett, B.; Boden, I.J.; Bradley, S.; Burrell, A.; et al. Comparison of 6-Month Outcomes of Survivors of COVID-19 vs. Non–COVID-19 Critical Illness. Am. J. Respir. Crit. Care. Med. 2022, 205, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Shah, C.V.; Meyer, N.J.; Gaieski, D.F.; Lyon, S.; Miltiades, A.N.; Goyal, M.; Fuchs, B.D.; Bellamy, S.L.; Christie, J.D. The epidemiology of acute respiratory distress syndrome in patient presenting to the emergency department with severe sepsis. Shock 2013, 40, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Lung Cytokines and ARDS: Roger, S. Mitchell Lecture—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/10424558/ (accessed on 7 January 2023).
- Yang, S.-C.; Tsai, Y.-F.; Pan, Y.-L.; Hwang, T.-L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Comparison of the Berlin Definition for Acute Respiratory Distress Syndrome with Autopsy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23370917/ (accessed on 7 January 2023).
- Diamond, M.; Peniston, H.L.; Sanghavi, D.; Mahapatra, S. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 1903–1905. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- de Herder, W.W. Heroes in endocrinology: Nobel Prizes. Endocr. Connect. 2014, 3, R94–R104. [Google Scholar] [CrossRef]
- Heming, N.; Sivanandamoorthy, S.; Meng, P.; Bounab, R.; Annane, D. Immune Effects of Corticosteroids in Sepsis. Front. Immunol. 2018, 9, 1736. [Google Scholar] [CrossRef]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Czock, D.; Keller, F.; Rasche, F.M.; Häussler, U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 2005, 44, 61–98. [Google Scholar] [CrossRef]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- Cronstein, B.N.; Kimmel, S.C.; Levin, R.I.; Martiniuk, F.; Weissmann, G. A mechanism for the antiinflammatory effects of corticosteroids: The glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 1992, 89, 9991–9995. [Google Scholar] [CrossRef]
- Barish, G.D.; Downes, M.; Alaynick, W.A.; Yu, R.T.; Ocampo, C.B.; Bookout, A.L.; Mangelsdorf, D.J.; Evans, R.M. A Nuclear Receptor Atlas: Macrophage activation. Mol. Endocrinol. 2005, 19, 2466–2477. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Brown, D.E.; Brewer, J.A.; Vogt, S.K.; Muglia, L.J. Macrophage glucocorticoid receptors regulate Toll-like receptor 4–mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 2007, 109, 4313–4319. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Feng, Y.; Velazquez, H.; Sessa, W.C. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 306–311. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, M.J.; Bae, J.; Lee, J.H.; Lee, H.A.R.; Mun, S.; Kim, Y.; Yune, C.J.; Chung, T.N.; Kim, K. Effects of Glucocorticoid Therapy on Sepsis Depend Both on the Dose of Steroids and on the Severity and Phase of the Animal Sepsis Model. Life 2022, 12, 421. [Google Scholar] [CrossRef]
- Fabian, T.C.; Patterson, R. Steroid therapy in septic shock. Survival studies in a laboratory model. Am. Surg. 1982, 48, 614–617. [Google Scholar]
- White, G.L.; Archer, L.T.; Beller, B.K.; Hinshaw, L.B. Increased survival with methylprednisolone treatment in canine endotoxin shock. J. Surg. Res. 1978, 25, 357–364. [Google Scholar] [CrossRef]
- Barber, A.E.; Coyle, S.M.; Marano, M.A.; Fischer, E.; Calvano, S.E.; Fong, Y.; Moldawer, L.L.; Lowry, S.F. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J. Immunol. 1993, 150, 1999–2006. [Google Scholar] [CrossRef]
- Briegel, J.; Jochum, M.; Gippner-Steppert, C.; Thiel, M. Immunomodulation in septic shock: Hydrocortisone differentially regulates cytokine responses. J. Am. Soc. Nephrol. 2001, 12 (Suppl. 17), S70–S74. [Google Scholar] [CrossRef]
- Annane, D.; Sébille, V.; Charpentier, C.; Bollaert, P.-E.; François, B.; Korach, J.-M.; Capellier, G.; Cohen, Y.; Azoulay, E.; Troché, G.; et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002, 288, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Sébille, V.; Bellissant, E.; Ger-Inf-05 Study Group. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit. Care Med. 2006, 34, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Renault, A.; Brun-Buisson, C.; Megarbane, B.; Quenot, J.-P.; Siami, S.; Cariou, A.; Forceville, X.; Schwebel, C.; Martin, C.; et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 2018, 378, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, B.; Finfer, S.; Cohen, J.; Rajbhandari, D.; Arabi, Y.; Bellomo, R.; Billot, L.; Correa, M.; Glass, P.; Harward, M.; et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018, 378, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Oczkowski, S.J.; Siemieniuk, R.A.C.; Agoritsas, T.; Belley-Cote, E.; D’Aragon, F.; Duan, E.; English, S.; Gossack-Keenan, K.; Alghuroba, M.; et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Bellissant, E.; Bollaert, P.E.; Briegel, J.; Keh, D.; Kupfer, Y.; Pirracchio, R.; Rochwerg, B. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst. Rev. 2019, 2019, CD002243. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Dequin, P.-F.; Meziani, F.; Quenot, J.-P.; Kamel, T.; Ricard, J.-D.; Badie, J.; Reignier, J.; Heming, N.; Plantefève, G.; Souweine, B.; et al. Hydrocortisone in Severe Community-Acquired Pneumonia. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef]
- Annane, D. Pro: The illegitimate crusade against corticosteroids for severe H1N1 pneumonia. Am. J. Respir. Crit. Care Med. 2011, 183, 1125–1126. [Google Scholar] [CrossRef]
- Matthay, M.A.; Liu, K.D. Con: Corticosteroids are not indicated for treatment of acute lung injury from H1N1 viral pneumonia. Am. J. Respir. Crit. Care Med. 2011, 183, 1127–1128. [Google Scholar] [CrossRef]
- Hong, S.; Jian, C.; Wang, H.; Wang, X.; Xing, L.; Qiao, L. Effects of different doses of methylprednisolone therapy on acute respiratory distress syndrome: Results from animal and clinical studies. BMC Pulm. Med. 2022, 22, 348. [Google Scholar] [CrossRef]
- Rocksén, D.; Lilliehöök, B.; Larsson, R.; Johansson, T.; Bucht, A. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin. Exp. Immunol. 2000, 122, 249–256. [Google Scholar] [CrossRef]
- Khilnani, G.C.; Hadda, V. Corticosteroids and ARDS: A review of treatment and prevention evidence. Lung India 2011, 28, 114–119. [Google Scholar] [CrossRef]
- Meduri, G.U.; Headley, A.S.; Golden, E.; Carson, S.J.; Umberger, R.A.; Kelso, T.; Tolley, E.A. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: A randomized controlled trial. JAMA 1998, 280, 159–165. [Google Scholar] [CrossRef]
- Meduri, G.U.; Golden, E.; Freire, A.X.; Taylor, E.; Zaman, M.; Carson, S.J.; Gibson, M.; Umberger, R. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest 2007, 131, 954–963. [Google Scholar] [CrossRef]
- Abdelsalam Rezk, N.; Mohamed Ibrahim, A. Effects of methyl prednisolone in early ARDS. Egypt. J. Chest Dis. Tuberc. 2013, 62, 167–172. [Google Scholar] [CrossRef]
- Bernard, G.R.; Luce, J.M.; Sprung, C.L.; Rinaldo, J.E.; Tate, R.M.; Sibbald, W.J.; Kariman, K.; Higgins, S.; Bradley, R.; Metz, C.A. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N. Engl. J. Med. 1987, 317, 1565–1570. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Meduri, G.U.; Belenchia, J.M.; Estes, R.J.; Wunderink, R.G.; Torky, M.E.; Leeper, K.V. Fibroproliferative Phase of ARDS: Clinical Findings and Effects of Corticosteroids. Chest 1991, 100, 943–952. [Google Scholar] [CrossRef]
- Wajanaponsan, N.; Reade, M.C.; Milbrandt, E.B. Steroids in late ARDS? Crit. Care 2007, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, K.P.; Hudson, L.D.; Goodman, R.B.; Hough, C.L.; Lanken, P.N.; Hyzy, R.; Thompson, B.T.; Ancukiewicz, M.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Fisher, C.J.; Clemmer, T.P.; Slotman, G.J.; Metz, C.A. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest 1987, 92, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Huang, Y.; Liu, S.; Yang, C.; Guo, F.; Qiu, H.; Yang, Y. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency. Zhonghua Nei Ke Za Zhi 2012, 51, 599–603. (In Chinese) [Google Scholar]
- Falagas, M.E.; Vouloumanou, E.K.; Baskouta, E.; Rafailidis, P.I.; Polyzos, K.; Rello, J. Treatment options for 2009 H1N1 influenza: Evaluation of the published evidence. Int. J. Antimicrob. Agents 2010, 35, 421–430. [Google Scholar] [CrossRef]
- Brun-Buisson, C.; Richard, J.-C.M.; Mercat, A.; Thiébaut, A.C.M.; Brochard, L.; REVA-SRLF A/H1N1v 2009 Registry Group. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2011, 183, 1200–1206. [Google Scholar] [CrossRef]
- Moreno, G.; Rodríguez, A.; Reyes, L.F.; Gomez, J.; Sole-Violan, J.; Díaz, E.; Bodí, M.; Trefler, S.; Guardiola, J.; Yébenes, J.C.; et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: A propensity score matching study. Intensive Care Med. 2018, 44, 1470–1482. [Google Scholar] [CrossRef]
- Tsang, K.W.; Ho, P.L.; Ooi, G.C.; Yee, W.K.; Wang, T.; Chan-Yeung, M.; Lam, W.K.; Seto, W.H.; Yam, L.Y.; Cheung, T.M.; et al. A Cluster of Cases of Severe Acute Respiratory Syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1977–1985. [Google Scholar] [CrossRef]
- Li, N.; Ma, J.; Nie, L.; Li, H.; Que, C.; Gao, Z.; Wang, G.; Xu, X.; Lu, H.; Wang, G. Retrospective analysis of the corticosteroids treatment on severe acute respiratory syndrome (SARS). Beijing Da Xue Xue Bao Yi Xue Ban 2003, 35, 16–18. [Google Scholar]
- Al Ghamdi, M.; Alghamdi, K.M.; Ghandoora, Y.; Alzahrani, A.; Salah, F.; Alsulami, A.; Bawayan, M.F.; Vaidya, D.; Perl, T.M.; Sood, G. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 2016, 16, 174. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hong, S.-B.; Yun, S.-C.; Choi, W.-I.; Ahn, J.-J.; Lee, Y.J.; Lee, H.-B.; Lim, C.-M.; Koh, Y.; Korean Society of Critical Care Medicine H1N1. Collaborative Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: Analytic strategy using propensity scores. Am. J. Respir. Crit. Care Med. 2011, 183, 1207–1214. [Google Scholar] [CrossRef]
- Yaqoob, H.; Greenberg, D.; Hwang, F.; Lee, C.; Vernik, D.; Manglani, R.; Wang, Z.; Murad, M.H.; Chandy, D.; Epelbaum, O. Comparison of pulse-dose and high-dose corticosteroids with no corticosteroid treatment for COVID-19 pneumonia in the intensive care unit. J. Med. Virol. 2022, 94, 349–356. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Peking Union Medical College Hospital. Glucocorticoid Therapy for Critically Ill Patients with Severe Acute Respiratory Infections Caused by COVID-19: A Prospective, Randomized Controlled Trial. clinicaltrials.gov. Report No.: NCT04244591. Available online: https://clinicaltrials.gov/ct2/show/NCT04244591 (accessed on 8 December 2022).
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients with Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Angus, D.C.; Derde, L.; Al-Beidh, F.; Annane, D.; Arabi, Y.; Beane, A.; van Bentum-Puijk, W.; Berry, L.; Bhimani, Z.; Bonten, M.; et al. Effect of Hydrocortisone on Mortality and Organ Support in Patients with Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020, 324, 1317–1329. [Google Scholar] [CrossRef]
- Barros, C.M.S.S.; Freire, R.S.; Frota, E.; Rezende Santos, A.G.; Farias, M.E.L.; Rodrigues, M.G.A.; Silva, B.M.; Prado Jeronimo, C.M.; Netto, R.L.A.; Silva Borba, M.G.; et al. Short-Course of Methylprednisolone Improves Respiratory Functional Parameters After 120 Days in Hospitalized COVID-19 Patients (Metcovid Trial): A Randomized Clinical Trial. Front. Med. 2021, 8, 758405. [Google Scholar] [CrossRef]
- COVID STEROID 2 Trial Group; Munch, M.W.; Myatra, S.N.; Vijayaraghavan, B.K.T.; Saseedharan, S.; Benfield, T.; Wahlin, R.R.; Rasmussen, B.S.; Andreasen, A.S.; Poulsen, L.M.; et al. Effect of 12 mg vs. 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA 2021, 326, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Mekontso-Dessap, A.; Burdet, C.; Merdji, H.; Poissy, J.; Dupuis, C.; Guitton, C.; Schwebel, C.; Cohen, Y.; Bruel, C.; et al. High-Dose Dexamethasone and Oxygen Support Strategies in Intensive Care Unit Patients with Severe COVID-19 Acute Hypoxemic Respiratory Failure: The COVIDICUS Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.-S.; Macdonald, H.; Zeng, L.; et al. A living WHO guideline on drugs for COVID-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Sasaki, K.; Karkar, A.; Sharif, S.; Lewis, K.; Mammen, M.J.; Alexander, P.; Ye, Z.; Lozano, L.E.C.; Munch, M.W.; et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: A systematic review and meta-analysis. Intensive Care Med. 2021, 47, 521–537. [Google Scholar] [CrossRef]
- Tongyoo, S.; Permpikul, C.; Mongkolpun, W.; Vattanavanit, V.; Udompanturak, S.; Kocak, M.; Meduri, G.U. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: Results of a randomized controlled trial. Crit. Care 2016, 20, 329. [Google Scholar] [CrossRef]
- Dequin, P.-F.; Heming, N.; Meziani, F.; Plantefève, G.; Voiriot, G.; Badié, J.; François, B.; Aubron, C.; Ricard, J.-D.; Ehrmann, S.; et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1298–1306. [Google Scholar] [CrossRef]
- Corral-Gudino, L.; Bahamonde, A.; Arnaiz-Revillas, F.; Gómez-Barquero, J.; Abadía-Otero, J.; García-Ibarbia, C.; Mora, V.; Cerezo-Hernández, A.; Hernández, J.L.; López-Muñíz, G.; et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: An open-label randomized trial (GLUCOCOVID). Wien Klin. Wochenschr. 2021, 133, 303–311. [Google Scholar] [CrossRef]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef]
- Umberto Meduri, G.; Tolley, E.A.; Chinn, A.; Stentz, F.; Postlethwaite, A. Procollagen Types I and III Aminoterminal Propeptide Levels during Acute Respiratory Distress Syndrome and in Response to Methylprednisolone Treatment. Am. J. Respir. Crit. Care Med. 1998, 158, 1432–1441. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Roquencourt, C.; Moine, P.; Saffroy, G.; Carn, S.; Heming, N.; Fleuriet, J.; Salvator, H.; Naline, E.; Couderc, L.-J.; et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. EBioMedicine 2021, 63, 103154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuperminc, E.; Heming, N.; Carlos, M.; Annane, D. Corticosteroids in ARDS. J. Clin. Med. 2023, 12, 3340. https://doi.org/10.3390/jcm12093340
Kuperminc E, Heming N, Carlos M, Annane D. Corticosteroids in ARDS. Journal of Clinical Medicine. 2023; 12(9):3340. https://doi.org/10.3390/jcm12093340
Chicago/Turabian StyleKuperminc, Emmanuelle, Nicholas Heming, Miguel Carlos, and Djillali Annane. 2023. "Corticosteroids in ARDS" Journal of Clinical Medicine 12, no. 9: 3340. https://doi.org/10.3390/jcm12093340
APA StyleKuperminc, E., Heming, N., Carlos, M., & Annane, D. (2023). Corticosteroids in ARDS. Journal of Clinical Medicine, 12(9), 3340. https://doi.org/10.3390/jcm12093340