Cannabinoids and the Endocannabinoid System in Early SARS-CoV-2 Infection and Long COVID-19—A Scoping Review
Abstract
:1. Introduction
2. Cannabinoids for Early SARS-CoV-2 Infection
2.1. Cannabinoids and Viral Entry
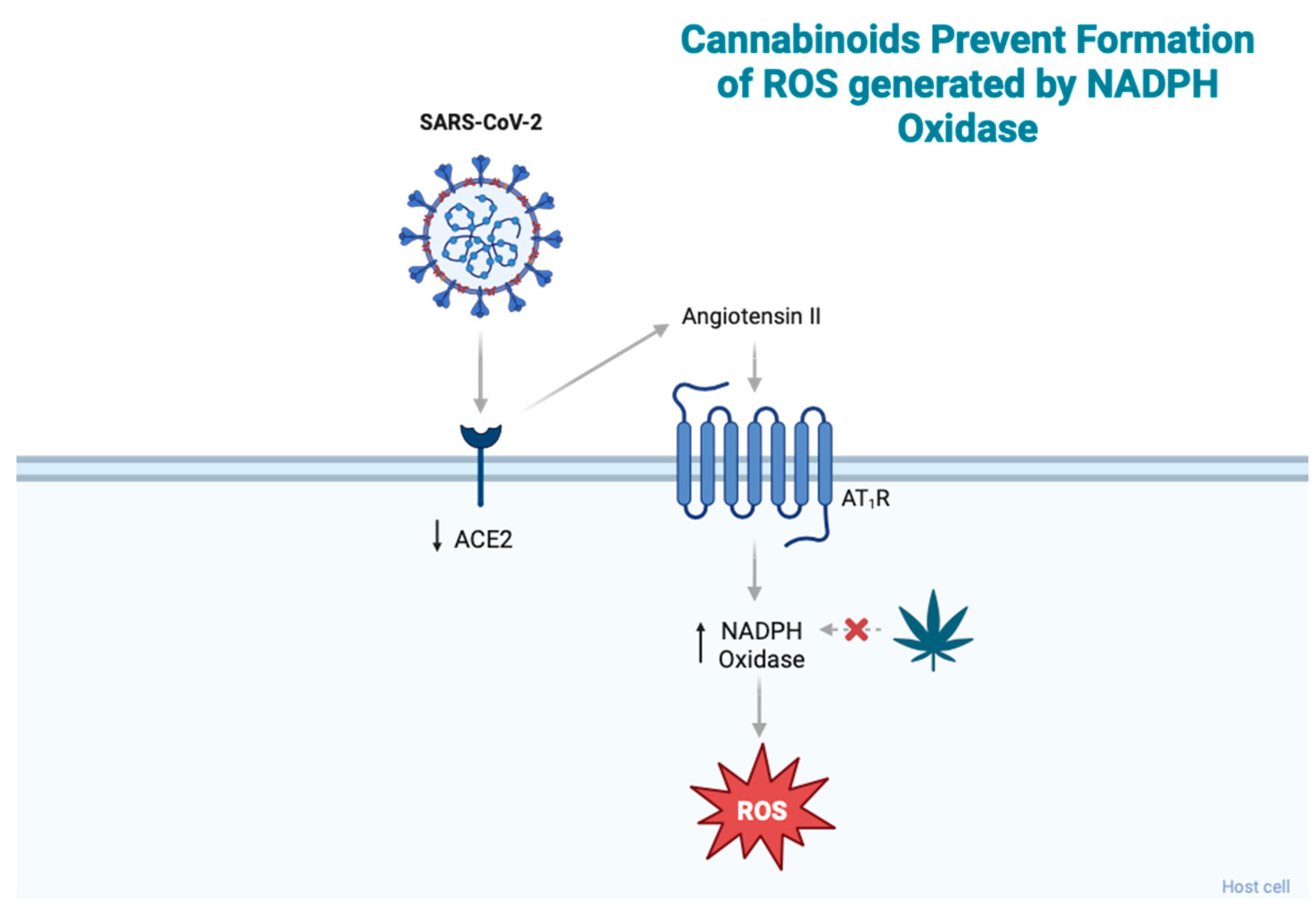
2.2. Cannabinoids and Oxidative Stress
2.3. Cannabinoids and the Cytokine Storm
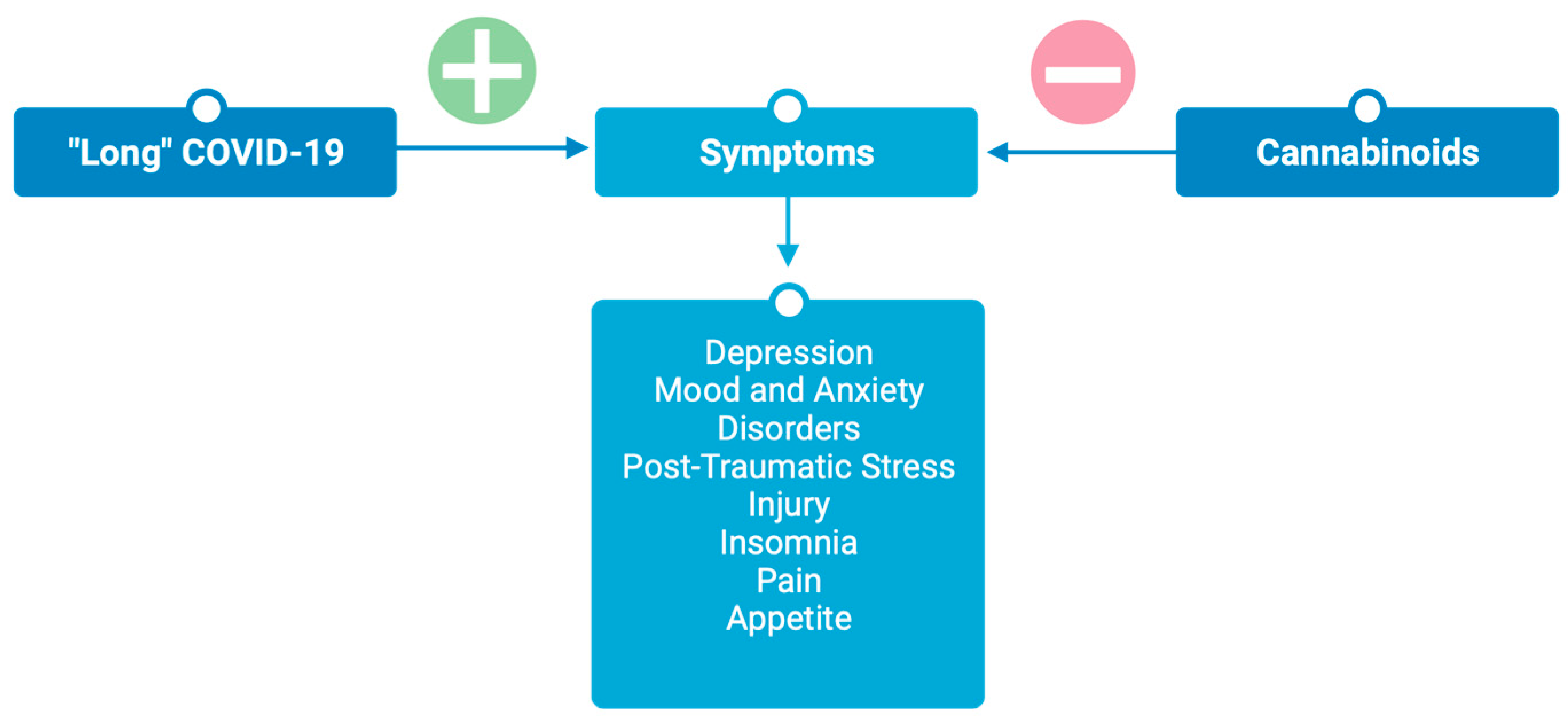
3. Cannabinoids for Post-Acute “Long” COVID-19
3.1. Neuropsychiatric Sequela
3.1.1. Depression
3.1.2. Mood and Anxiety Disorders
3.1.3. Post-Traumatic Stress Injury
3.1.4. Insomnia
3.2. Pain
3.3. Appetite
4. Therapeutic Applications
4.1. Routes of Administration
4.2. Limitations and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 2 July 2023).
- Public Health Agency of Canada. COVID-19 Vaccination in Canada; Public Health Agency of Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Anmar, A.-T. Tixagevimab and Cilgavimab: Can We See More Recommendations for Monoclonal Antibodies beyond COVID-19 Vaccination. Disaster Med. Public Health Prep. 2022, 16, 2254–2255. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2020. [Google Scholar]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B.; Meyerholz, D.K.; Perlman, S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Hussman, J.P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Reiss, C.S. Cannabinoids and viral infections. Pharmaceuticals 2010, 3, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Csete, J.; Kamarulzaman, A.; Kazatchkine, M.; Altice, F.; Balicki, M.; Buxton, J.; Cepeda, J.; Comfort, M.; Goosby, E.; Goulão, J.; et al. Public health and international drug policy. Lancet 2016, 387, 1427–1480. [Google Scholar] [CrossRef] [PubMed]
- Rabi, F.A.; Al Zoubi, M.S.; Al-Nasser, A.D.; Kasasbeh, G.A.; Salameh, D.M. SARS-CoV-2 and coronavirus disease 2019: What we know so far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N.; Bates, T.A.; Weinstein, J.B.; Leier, H.C.; Farley, S.; Tafesse, F.G. Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants. J. Nat. Prod. 2022, 85, 176–184. [Google Scholar] [CrossRef]
- Pattnaik, G.P.; Chakraborty, H. Entry Inhibitors: Efficient Means to Block Viral Infection. J. Membr. Biol. 2020, 253, 425–444. [Google Scholar] [CrossRef]
- Foster, T.L.; Pickering, S.; Neil, S.J.D. Inhibiting the Ins and Outs of HIV replication: Cell-intrinsic antiretroviral restrictions at the plasma membrane. Front. Immunol. 2018, 8, 1853. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, Q.; Caffrey, M.; Rong, L.; Du, R. Small molecule inhibitors of influenza virus entry. Pharmaceuticals 2021, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.M.; Monreal, I.A.; Ruvalcaba, M.; Ortega, V.; Aguilar, H.C. Antivirals targeting paramyxovirus membrane fusion. Curr. Opin. Virol. 2021, 51, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox biology of respiratory viral infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, P.; Skaperda, Z.; Tekos, F.; Kouretas, D. ROS and COVID. Antioxidants 2022, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Duca, L.; Ottolenghi, S.; Coppola, S.; Rinaldo, R.; Dei Cas, M.; Rubino, F.M.; Paroni, R.; Samaja, M.; Chiumello, D.A.; Motta, I. Differential redox state and iron regulation in chronic obstructive pulmonary disease, acute respiratory distress syndrome and coronavirus disease 2019. Antioxidants 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front. Microbiol. 2023, 13, 1111930. [Google Scholar] [CrossRef]
- Alam, M.S.; Czajkowsky, D.M. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022, 63, 44–57. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol.–Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol attenuates cisplatin-Lnduced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.A.; Karimi, J.; Talebi, S.S.; Piri, H. The Association of COVID-19 and Reactive Oxygen Species Modulator 1 (ROMO1) with Oxidative Stress. Chonnam Med. J. 2022, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Arif, M.; Varga, Z.V.; Mátyás, C.; Paloczi, J.; Lehocki, A.; Haskó, G.; Pacher, P. Cannabinoid receptor 2 activation alleviates diabetes-induced cardiac dysfunction, inflammation, oxidative stress, and fibrosis. GeroScience 2022, 44, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.H.; Sankaranarayanan, C. β-Caryophyllene, a natural sesquiterpene lactone attenuates hyperglycemia mediated oxidative and inflammatory stress in experimental diabetic rats. Chem. Biol. Interact. 2016, 245, 50–58. [Google Scholar] [CrossRef]
- Chung, Y.C.; Bok, E.; Huh, S.H.; Park, J.-Y.; Yoon, S.-H.; Kim, S.R.; Kim, Y.-S.; Maeng, S.; Hyun Park, S.; Jin, B.K. Cannabinoid Receptor Type 1 Protects Nigrostriatal Dopaminergic Neurons against MPTP Neurotoxicity by Inhibiting Microglial Activation. J. Immunol. 2011, 187, 6508–6517. [Google Scholar] [CrossRef]
- Jia, J.; Ma, L.; Wu, M.; Zhang, L.; Zhang, X.; Zhai, Q.; Jiang, T.; Wang, Q.; Xiong, L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid. Med. Cell. Longev. 2014, 2014, 893516. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.F.K.; Miranda, K.; Wilson, K.; Singh, N.P.; Cai, G.; Putluri, N.; Nagarkatti, P.; Nagarkatti, M. Δ9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int. J. Mol. Sci. 2020, 21, 6244. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Lippi, G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. J. Crit. Care 2020, 58, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Chibane, F.; Costigliola, V.; Yu, J.C.; Vaibhav, K.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol Modulates Cytokine Storm in Acute Respiratory Distress Syndrome Induced by Simulated Viral Infection Using Synthetic RNA. Cannabis Cannabinoid Res. 2020, 5, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors. Brain. Behav. Immun. 2022, 89, 594–600. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Klein, R.S. Neuroinflammation and COVID-19. Curr. Opin. Neurobiol. 2022, 76, 102608. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Cooray, R.; Gupta, V.; Suphioglu, C. Current Aspects of the Endocannabinoid System and Targeted THC and CBD Phytocannabinoids as Potential Therapeutics for Parkinson’s and Alzheimer’s Diseases: A Review. Mol. Neurobiol. 2020, 57, 4878–4890. [Google Scholar] [CrossRef]
- Feingold, D.; Weinstein, A. Cannabis and Depression. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1264. [Google Scholar]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Ettman, C.K.; Abdalla, S.M.; Cohen, G.H.; Sampson, L.; Vivier, P.M.; Galea, S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw. Open 2020, 3, e2019686. [Google Scholar] [CrossRef]
- Yankelevitch-yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 17, e52587. [Google Scholar] [CrossRef]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sartim, A.G.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effect of cannabidiol injection into the ventral medial prefrontal cortex—Possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 2016, 303, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Silote, G.P.; Gatto, M.C.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S.R.L. Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol. Pharmaceuticals 2021, 14, 1269. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.L.; Strickland, J.C.; Schlienz, N.J.; Munson, J.; Jackson, H.; Bonn-miller, M.O.; Vandrey, R. Antidepressant and Anxiolytic Effects of Medicinal Cannabis Use in an Observational Trial. Front. Psychiatry 2021, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Mangoo, S.; Erridge, S.; Holvey, C.; Coomber, R.; Barros, D.A.R.; Bhoskar, U.; Mwimba, G.; Praveen, K.; Symeon, C.; Sachdeva-Mohan, S.; et al. Assessment of clinical outcomes of medicinal cannabis therapy for depression: Analysis from the UK Medical Cannabis Registry. Expert Rev. Neurother. 2022, 22, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Empower Research Inc. Exploring Medically Perceived Benefits, Use and Interest in Psychedelics and Cannabinoids; NCT04965740; Empower Research Inc.: New York, NY, USA, 2021. [Google Scholar]
- Scherma, M.; Lisa Muntoni, A.; Riedel, G.; Fratta, W.; Fadda, P. Cannabinoids and their therapeutic applications in mental disorders. Dialogues Clin. Neurosci. 2020, 22, 271–279. [Google Scholar] [CrossRef]
- Bovasso, G.B. Cannabis Abuse as a Risk Factor for Depressive Symptoms. Am. J. Psychiatry 2001, 158, 2033–2037. [Google Scholar] [CrossRef]
- Lee, K.S.K.; Clough, A.R.; Jaragba, M.J.; Conigrave, K.M.; Patton, G.C. Heavy cannabis use and depressive symptoms in three Aboriginal communities in Arnhem Land, Northern Territory. Med. J. Aust. 2008, 188, 605–608. [Google Scholar] [CrossRef]
- Scherma, M.; Masia, P.; Deidda, M.; Fratta, W.; Tanda, G.; Fadda, P. New Perspectives on the Use of Cannabis in the Treatment of Psychiatric Disorders. Medicines 2018, 5, 107. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.; Cabañero, D.; Martín-García, E. The endocannabinoid system in modulating fear, anxiety, and stress. Dialogues Clin. Neurosci. 2020, 22, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Resstel, L.B.M.; Guimarães, F.S. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology 2011, 213, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Resstel, L.B.M.; Joca, S.R.L.; Moreira, F.A.; Corrêa, F.M.A.; Guimarães, F.S. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain Res. 2006, 172, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.A.; Aguiar, D.C.; Guimarães, F.S. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Green, M.R.; Martin, B.R. Pharmacological characterization of cannabinoids in the elevated plus maze. J. Pharmacol. Exp. Ther. 1990, 253, 1003–1009. [Google Scholar]
- Guimarães, F.S.; Chiaretti, T.M.; Graeff, F.G.; Zuardi, A.W. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology 1990, 100, 558–559. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimarães, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7, 82–83. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Helena, R.; Queiroz, C.; Hortes, M.; Chagas, N.; Roesler, R.; Schro, N.; Crippa, A.S.; Zuardi, A.W. Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Stevenson, C.W.; Laviolette, S.R. Could cannabidiol be a treatment for coronavirus disease-19-related anxiety disorders? Cannabis Cannabinoid Res. 2021, 6, 7–18. [Google Scholar] [CrossRef]
- Mertz Schou, T.; Joca, S.; Wegener, G.; Bay-richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Roitman, P.; Mechoulam, R.; Cooper-Kazaz, R.; Shalev, A. Preliminary, open-label, pilot study of add-on oral Δ9- tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin. Drug Investig. 2014, 34, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Watson, D.; Robinson, J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: A retrospective evaluation. J. Clin. Psychopharmacol. 2014, 34, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.A. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci. Ther. 2009, 15, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.D.M.; Foppiani, A.; Peretti, G.M.; Mangiavini, L.; Battezzati, A.; Bertoli, S.; Martinelli Boneschi, F.; Zuccotti, G.V. Long-Term Coronavirus Disease 2019 Complications in Inpatients and Outpatients: A One-Year Follow-up Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab384. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E. The role of the CB1 receptor in the regulation of sleep. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1420–1427. [Google Scholar] [CrossRef]
- Walsh, J.H.; Maddison, K.J.; Rankin, T.; Murray, K.; McArdle, N.; Ree, M.J.; Hillman, D.R.; Eastwood, P.R. Treating insomnia symptoms with medicinal cannabis: A randomized, crossover trial of the efficacy of a cannabinoid medicine compared with placebo. Sleep 2021, 44, zsab149. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Anaya, J.; Rojas, M.; Salinas, M.L.; Rodríguez, Y.; Roa, G.; Lozano, M.; Rodríguez-Jiménez, M.; Montoya, N.; Zapata, E.; Monsalve, D.M.; et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun. Rev. 2021, 20, 102947. [Google Scholar] [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Fiala, K.; Martens, J.; Abd-Elsayed, A. Post-COVID Pain Syndromes. Curr. Pain Headache Rep. 2022, 26, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Malan, P.T.; Ibrahim, M.M.; Deng, H.; Liu, Q.; Mata, H.P.; Vanderah, T.; Porreca, F.; Makriyannis, A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 2001, 93, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Tsou, K.; Walker, J.M. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci. Lett. 1998, 242, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.M.; Rousseau, M.A.; Wanas, A.S.; Radwan, M.M.; Caldwell, S.; Sufka, K.J.; Elsohly, M.A. Role of Cannabinoids and Terpenes in Cannabis-Mediated Analgesia in Rats. Cannabis Cannabinoid Res. 2019, 4, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Gouaux, B.; Sakai, S.; Donaghe, H. Low Dose Vaporized Cannabis Significantly Improves Neuropathic Pain. J. Pain 2013, 14, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Wilsey, B.; Marcotte, T.; Deutsch, R.; Zhao, H.; Prasad, H.; Phan, A. An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain from Spinal Cord Injury and Disease. J. Pain 2016, 17, 982–1000. [Google Scholar] [CrossRef]
- Romeyke, T.; Westfal, R. Integration of Cannabis Extract Tetrahydrocannabinol:Cannabidiol in an Interdisciplinary Therapy Setting: A Case of Chronic Multilocular Pain Disorder. Med. Cannabis Cannabinoids 2022, 5, 220–225. [Google Scholar] [CrossRef]
- Nurmikko, T.J.; Serpell, M.G.; Hoggart, B.; Toomey, P.J.; Morlion, B.J.; Haines, D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007, 133, 210–220. [Google Scholar] [CrossRef]
- Pini, L.A.; Guerzoni, S.; Cainazzo, M.M.; Ferrari, A.; Sarchielli, P.; Tiraferri, I.; Ciccarese, M.; Zappaterra, M. Nabilone for the treatment of medication overuse headache: Results of a preliminary double-blind, active-controlled, randomized trial. J. Headache Pain 2012, 13, 677–684. [Google Scholar] [CrossRef]
- Pinsger, M.; Schimetta, W.; Volc, D.; Hiermann, E.; Riederer, F.; Pölz, W. 624 Benefits of an Add-On Treatment with the Synthetic Cannabinomimetic Nabilone on Patients with Chronic Pain—A Randomized Controlled Trial. Eur. J. Pain 2006, 10, S163–S164. [Google Scholar] [CrossRef]
- Ueberall, M.A.; Vila Silván, C.; Essner, U.; Mueller-Schwefe, G.H.H. Effectiveness, Safety, and Tolerability of Nabiximols Oromucosal Spray vs Typical Oral Long-Acting Opioid Analgesics in Patients with Severe Neuropathic Back Pain: Analysis of 6-Month Real-World Data from the German Pain e-Registry. Pain Med. 2022, 23, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Bayman, L.; Valestin, J.; Rizvi-Toner, A.; Hashmi, S.; Schey, R. Dronabinol increases pain threshold in patients with functional chest pain: A pilot double-blind placebo-controlled trial. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Prasad, N.; Ryan, M.; Tangri, S.; Silverberg, M.S.; Gordon, A.; Steinhart, H. Cannabis use amongst patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2011, 23, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Courtwright, A.; Lucci, M.; Korzenik, J.R.; Levine, J. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Phatak, U.P.; Rojas-Velasquez, D.; Porto, A.; Pashankar, D.S. Prevalence and Patterns of Marijuana Use in Young Adults with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Hoffenberg, E.J.; McWilliams, S.K.; Mikulich-Gilbertson, S.K.; Murphy, B.V.; Lagueux, M.; Robbins, K.; Hoffenberg, A.S.; de Zoeten, E.; Hopfer, C.J. Marijuana Use by Adolescents and Young Adults with Inflammatory Bowel Disease. J. Pediatr. 2018, 199, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.; Devlin, S.; Kaplan, G.G.; Panaccione, R.; Andrews, C.N. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 472–480. [Google Scholar] [CrossRef]
- Naftali, T.; Lev, L.B.; Yablekovitz, D.; Half, E.; Konikoff, F.M. Treatment of Crohn’s disease with Cannabis: An observational study. Isr. Med. Assoc. J. 2011, 13, 455–458. [Google Scholar]
- Naftali, T.; Bar-Lev Schleider, L.; Dotan, I.; Lansky, E.P.; Sklerovsky Benjaminov, F.; Konikoff, F.M. Cannabis induces a clinical response in patients with crohn’s disease: A prospective placebo-controlled study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280. [Google Scholar] [CrossRef]
- Yuill, M.B.; Hale, D.E.; Guindon, J.; Morgan, D.J. Anti-nociceptive interactions between opioids and a cannabinoid receptor 2 agonist in inflammatory pain. Mol. Pain 2017, 13, 1–15. [Google Scholar] [CrossRef]
- Hsieh, G.C.; Pai, M.; Chandran, P.; Hooker, B.A.; Zhu, C.Z.; Salyers, A.K.; Wensink, E.J.; Zhan, C.; Carroll, W.A.; Dart, M.J.; et al. Central and peripheral sites of action for CB 2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br. J. Pharmacol. 2011, 162, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004, 329, 253. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.R.; Robson, P.; Ho, M.; Jubb, R.W.; McCabe, C.S. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology 2006, 45, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Tariq, R.; Jena, A.; Kinzelman Vesely, E.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Ther. Adv. Vaccines 2022, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Kleckner, A.; Kleckner, I.; Kamen, C.; Tejani, M.; Janelsins, M.; Morrow, G.; Pepponee, L. Opportunities for cannabis in supportive care in cancer. Ther. Adv. Med. Oncol. 2019, 11, 1–29. [Google Scholar] [CrossRef]
- Weng, J.; Li, Y.; Li, J.; Shen, L.; Zhu, L.; Liang, Y.; Lin, X.; Jiao, N.; Cheng, S.; Huang, Y.; et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol. Hepatol. 2021, 6, 344–346. [Google Scholar] [CrossRef]
- Meiri, E.; Jhangiani, H.; Vredenburgh, J.J.; Barbato, L.M.; Carter, F.J.; Yang, H.M.; Baranowski, V. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr. Med. Res. Opin. 2007, 23, 533–543. [Google Scholar] [CrossRef]
- Smith, L.A.; Azariah, F.; Lavender, V.T.; Stoner, N.S.; Bettiol, S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015, 2015, CD009464. [Google Scholar] [CrossRef] [PubMed]
- Machado Rocha, F.C.; Stéfano, S.C.; De Cássia Haiek, R.; Rosa Oliveira, L.M.Q.; Da Silveira, D.X. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: Systematic review and meta-analysis. Eur. J. Cancer Care 2008, 17, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.L.B.; Campbell, M.; Hopkins, C.; Smith, B.; Kelly, C.; Deary, V. Altered smell and taste: Anosmia, parosmia and the impact of long COVID-19. PLoS ONE 2021, 16, e0256998. [Google Scholar] [CrossRef]
- Kirkham, T.C. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005, 16, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Dipatrizio, N.V.; Ja, T.U.C. Δ9-THC induced hyperphagia and tolerance assessment: Interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav. Pharmacol. 2005, 16, 373–380. [Google Scholar]
- Beal, J.E.; Olson, R.; Laubenstein, L.; Morales, J.O.; Bellman, P.; Yangco, B.; Lefkowitz, L.; Plasse, T.F.; Shepard, K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manag. 1995, 10, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, A.; Spanagel, R.; Medicine, M.C. Medical cannabinoids: A pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 2022, 20, 259. [Google Scholar] [CrossRef]
- Shiplo, S.; Asbridge, M.; Leatherdale, S.T.; Hammond, D. Medical cannabis use in Canada: Vapourization and modes of delivery. Harm Reduct. J. 2016, 13, 30. [Google Scholar] [CrossRef]
- Penzel, N.; Antonucci, L.A.; Betz, L.T.; Sanfelici, R.; Weiske, J.; Pogarell, O.; Cumming, P.; Quednow, B.B.; Howes, O.; Falkai, P.; et al. Association between age of cannabis initiation and gray matter covariance networks in recent onset psychosis. Neuropsychopharmacology 2021, 46, 1484–1493. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, C.; Hall, S.; Zhou, J.; Lehmann, C. Cannabinoids and the Endocannabinoid System in Early SARS-CoV-2 Infection and Long COVID-19—A Scoping Review. J. Clin. Med. 2024, 13, 227. https://doi.org/10.3390/jcm13010227
Scott C, Hall S, Zhou J, Lehmann C. Cannabinoids and the Endocannabinoid System in Early SARS-CoV-2 Infection and Long COVID-19—A Scoping Review. Journal of Clinical Medicine. 2024; 13(1):227. https://doi.org/10.3390/jcm13010227
Chicago/Turabian StyleScott, Cassidy, Stefan Hall, Juan Zhou, and Christian Lehmann. 2024. "Cannabinoids and the Endocannabinoid System in Early SARS-CoV-2 Infection and Long COVID-19—A Scoping Review" Journal of Clinical Medicine 13, no. 1: 227. https://doi.org/10.3390/jcm13010227







