Impact of Controlling Nutritional Status Score on Mortality in Elderly Patients with Idiopathic Pulmonary Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
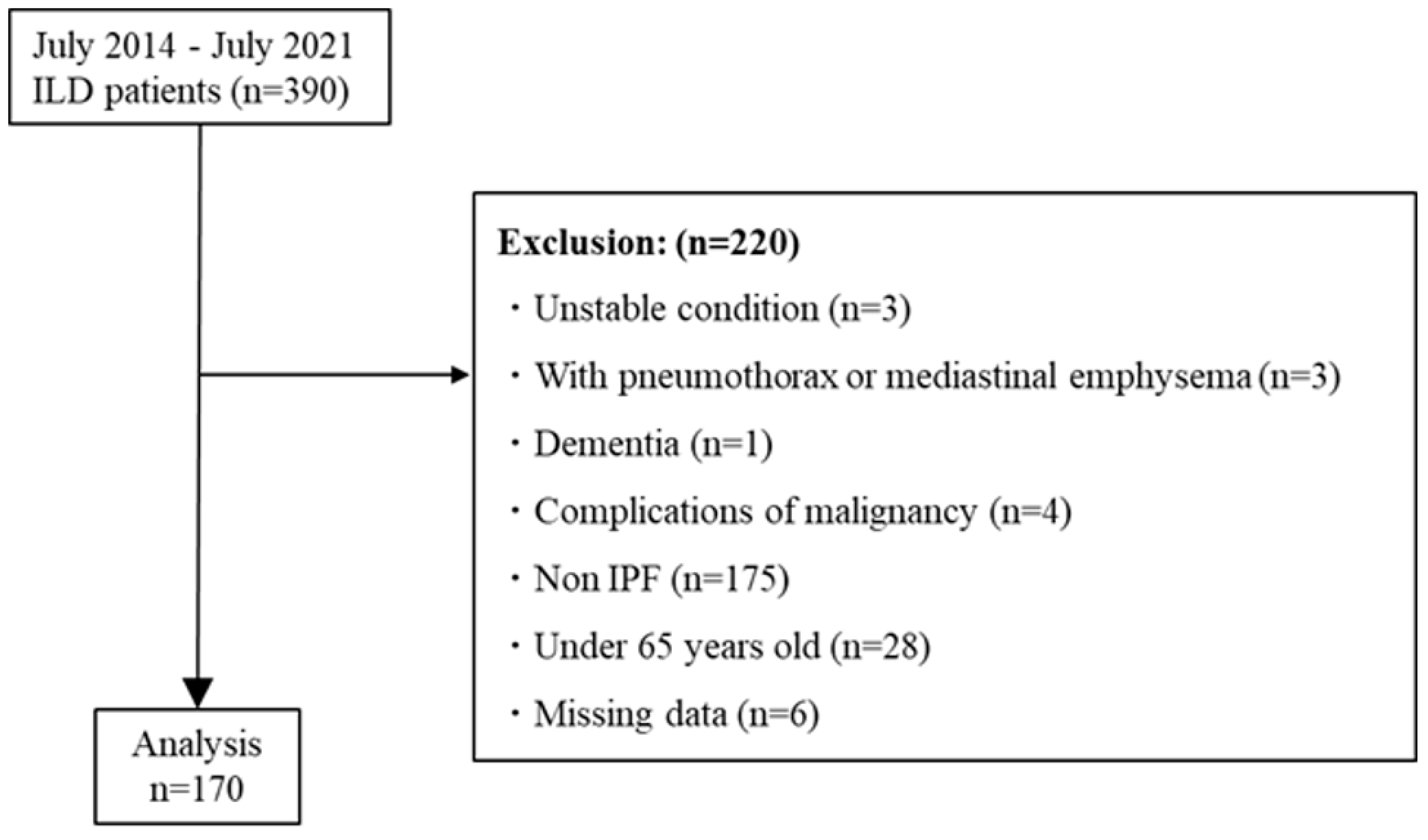
2.1. Subjects
2.2. Measurements
2.2.1. Measurements of Patient Background
2.2.2. Controlling Nutritional Status (CONUT)
2.2.3. Pulmonary Function Test
2.2.4. Severity
2.2.5. Dyspnea
2.2.6. Physical Function
2.2.7. Health-Related Quality of Life (HRQOL)
2.3. Statistical Analysis
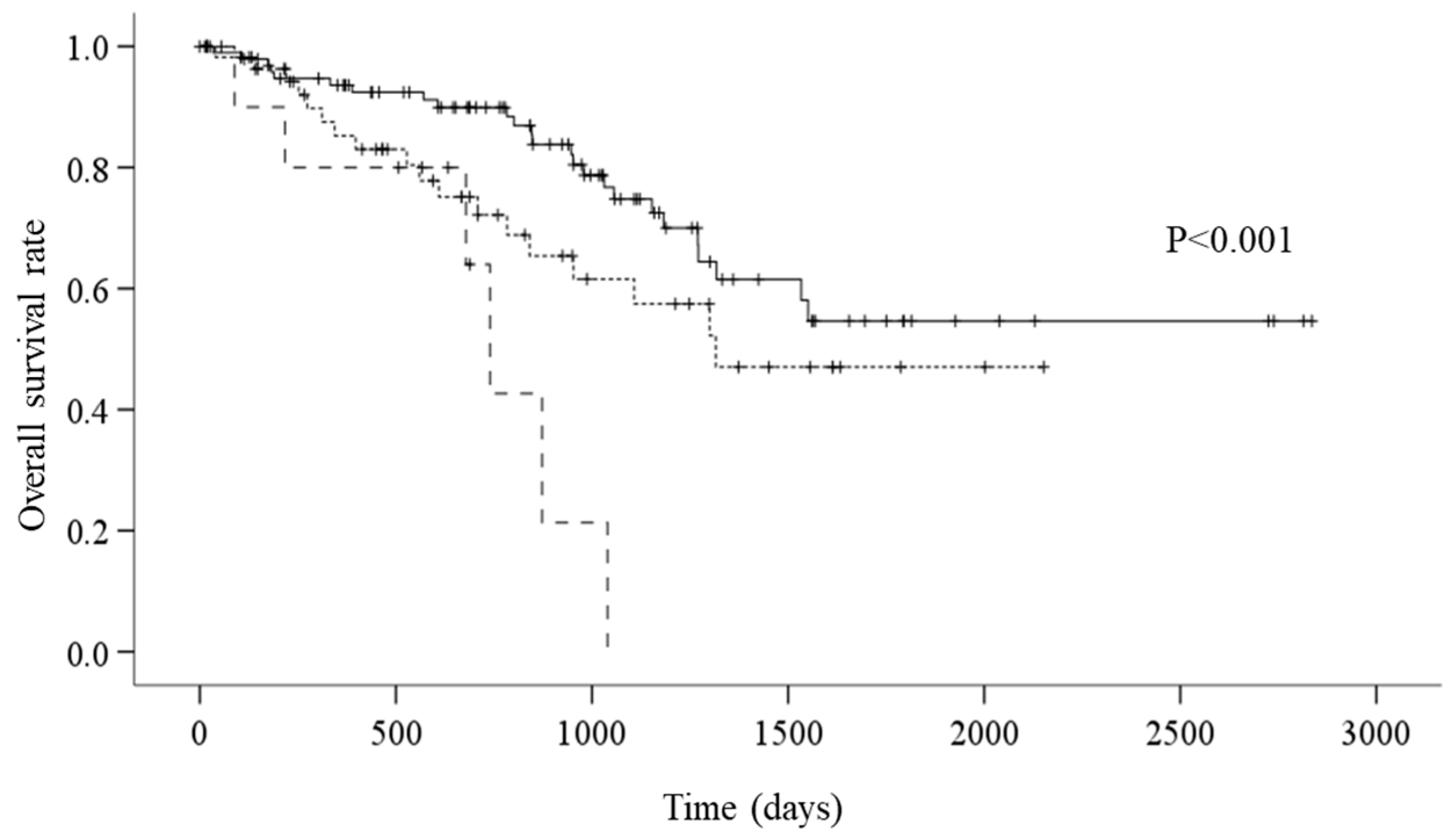
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Cuello-Garcia, C.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef]
- Anker, S.; John, M.; Pedersen, P.U.; Raguso, C.; Cicoira, M.; Dardai, E.; Laviano, A.; Ponikowski, P.; Schols, A.; Becker, H.; et al. ESPEN Guidelines on Enteral Nutrition: Cardiology and pulmonology. Clin. Nutr. 2006, 25, 311–318. [Google Scholar] [CrossRef]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.; Janssens, W.; Muscaritoli, M.; Pison, C.; Mölken, M.R.-V.; Slinde, F.; Steiner, M.; et al. Nutritional assessment and therapy in COPD: A European Respiratory Society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Seabrook, J.A.; Mura, M.; Madill, J. Fat-Free Mass Index Controlled for Age and Sex and Malnutrition Are Predictors of Survival in Interstitial Lung Disease. Respiration 2021, 100, 379–386. [Google Scholar] [CrossRef]
- Kanjrawi, A.A.; Mathers, L.; Webster, S.; Corte, T.J.; Carey, S. Nutritional status and quality of life in interstitial lung disease: A prospective cohort study. BMC Pulm. Med. 2021, 21, 51. [Google Scholar] [CrossRef]
- Alakhras, M.; Decker, P.A.; Nadrous, H.F.; Collazo-Clavell, M.; Ryu, J.H. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007, 131, 1448–1453. [Google Scholar] [CrossRef]
- Nishiyama, O.; Yamazaki, R.; Sano, H.; Iwanaga, T.; Higashimoto, Y.; Kume, H.; Tohda, Y. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 2017, 22, 480–485. [Google Scholar] [CrossRef]
- Nakatsuka, Y.; Handa, T.; Kokosi, M.; Tanizawa, K.; Puglisi, S.; Jacob, J.; Sokai, A.; Ikezoe, K.; Kanatani, K.T.; Kubo, T.; et al. The Clinical Significance of Body Weight Loss in Idiopathic Pulmonary Fibrosis Patients. Respiration 2018, 96, 338–347. [Google Scholar] [CrossRef]
- Mochizuka, Y.; Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Inoue, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; et al. Geriatric Nutritional Risk Index is a predictor of tolerability of antifibrotic therapy and mortality risk in patients with idiopathic pulmonary fibrosis. Respirology 2023, 28, 775–783. [Google Scholar] [CrossRef]
- Cereda, E.; Pedrolli, C. The Geriatric Nutritional Risk Index. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 1–7. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.-P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Natsuizaka, M.; Chiba, H.; Kuronuma, K.; Otsuka, M.; Kudo, K.; Mori, M.; Bando, M.; Sugiyama, Y.; Takahashi, H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014, 190, 773–779. [Google Scholar] [CrossRef]
- Guler, S.A.; Kwan, J.M.; Leung, J.M.; Khalil, N.; Wilcox, P.G.; Ryerson, C.J. Functional ageing in fibrotic interstitial lung disease: The impact of frailty on adverse health outcomes. Eur. Respir. J. 2020, 55, 1900647. [Google Scholar] [CrossRef]
- Sheth, J.S.; Xia, M.; Murray, S.; Martinez, C.H.; Meldrum, C.A.; Belloli, E.A.; Salisbury, M.L.; White, E.S.; Holtze, C.H.; Flaherty, K.R. Frailty and geriatric conditions in older patients with idiopathic pulmonary fibrosis. Respir. Med. 2019, 148, 6–12. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Kato, T.; Yaku, H.; Morimoto, T.; Inuzuka, Y.; Tamaki, Y.; Yamamoto, E.; Yoshikawa, Y.; Kitai, T.; Taniguchi, R.; Iguchi, M.; et al. Association with Controlling Nutritional Status (CONUT) Score and In-hospital Mortality and Infection in Acute Heart Failure. Sci. Rep. 2020, 10, 3320. [Google Scholar] [CrossRef]
- Hara, M.; Fujii, T.; Masuhara, H.; Kawasaki, M.; Tokuhiro, K.; Watanabe, Y. The prognostic impact of the controlling nutritional status (CONUT) score in patients undergoing cardiovascular surgery. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1142–1147. [Google Scholar] [CrossRef]
- Miura, H.; Goto, Y. Impact of the Controlling Nutritional Status (CONUT) score as a prognostic factor for all-cause mortality in older patients without cancer receiving home medical care: Hospital ward-based observational cohort study. BMJ Open 2023, 13, e066121. [Google Scholar] [CrossRef]
- Czinege, M.; Halatiu, V.-B.; Nyulas, V.; Cojocariu, L.-O.; Ion, B.; Mașca, V.; Țolescu, C.; Benedek, T. Nutritional Status and Recurrent Major Cardiovascular Events Following Acute Myocardial Infarction-A Follow-Up Study in a Primary Percutaneous Coronary Intervention Center. Nutrients 2024, 16, 1088. [Google Scholar] [CrossRef]
- Igarashi, A.; Iwanami, Y.; Sugino, K.; Gocho, K.; Homma, S.; Ebihara, S. Using 6-Min Walk Distance Expressed as a Percentage of Reference to Evaluate the Effect of Pulmonary Rehabilitation in Elderly Patients with Interstitial Lung Disease. J. Cardiopulm. Rehabilitation Prev. 2018, 38, 342–347. [Google Scholar] [CrossRef]
- Iwanami, Y.; Ebihara, K.; Nakao, K.; Sato, N.; Miyagi, M.; Nakamura, Y.; Sakamoto, S.; Kishi, K.; Homma, S.; Ebihara, S. Benefits of Pulmonary Rehabilitation in Patients with Idiopathic Pulmonary Fibrosis Receiving Antifibrotic Drug Treatment. J. Clin. Med. 2022, 11, 5336. [Google Scholar] [CrossRef]
- Ebihara, K.; Iwanami, Y.; Yamasaki, K.; Takemura, A.; Sato, N.; Usui, Y.; Nakamura, Y.; Kishi, K.; Homma, S.; Ebihara, S. Appendicular Skeletal Muscle Mass Correlates with Patient-Reported Outcomes and Physical Performance in Patients with Idiopathic Pulmonary Fibrosis. Tohoku J. Exp. Med. 2021, 253, 61–68. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- MacIntyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.M.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Natori, H.; Kawayama, T.; Suetomo, M.; Kinoshita, T.; Matsuoka, M.; Matsunaga, K.; Okamoto, M.; Hoshino, T. Evaluation of the Modified Medical Research Council Dyspnea Scale for Predicting Hospitalization and Exacerbation in Japanese Patients with Chronic Obstructive Pulmonary Disease. Intern. Med. 2016, 55, 15–24. [Google Scholar] [CrossRef]
- Dowman, L.; McDonald, C.F.; Hill, C.J.; Lee, A.; Barker, K.; Boote, C.; Glaspole, I.; Goh, N.; Southcott, A.; Burge, A.; et al. Reliability of the hand held dynamometer in measuring muscle strength in people with interstitial lung disease. Physiotherapy 2016, 102, 249–255. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Ringbaek, T.; Martinez, G.; Lange, P. A Comparison of the Assessment of Quality of Life with CAT, CCQ, and SGRQ in COPD Patients Participating in Pulmonary Rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 12–15. [Google Scholar] [CrossRef]
- Faverio, P.; Bocchino, M.; Caminati, A.; Fumagalli, A.; Gasbarra, M.; Iovino, P.; Petruzzi, A.; Scalfi, L.; Sebastiani, A.; Stanziola, A.A.; et al. Nutrition in Patients with Idiopathic Pulmonary Fibrosis: Critical Issues Analysis and Future Research Directions. Nutrients 2020, 12, 1131. [Google Scholar] [CrossRef]
- Rinaldi, S.; Balsillie, C.; Truchon, C.; Al-Mubarak, A.; Mura, M.; Madill, J. Nutrition implications of intrinsic restrictive lung disease. Nutr. Clin. Pract. 2022, 37, 239–255. [Google Scholar] [CrossRef]
- Kondoh, Y.; Taniguchi, H.; Kataoka, K.; Furukawa, T.; Ando, M.; Murotani, K.; Mishima, M.; Inoue, Y.; Ogura, T.; Bando, M.; et al. Disease severity staging system for idiopathic pulmonary fibrosis in Japan. Respirology 2017, 22, 1609–1614. [Google Scholar] [CrossRef]
- Kondoh, S.; Chiba, H.; Nishikiori, H.; Umeda, Y.; Kuronuma, K.; Otsuka, M.; Yamada, G.; Ohnishi, H.; Mori, M.; Kondoh, Y.; et al. Validation of the Japanese disease severity classification and the GAP model in Japanese patients with idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 327–333. [Google Scholar] [CrossRef]
- Nishiyama, O.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Kato, K.; Kataoka, K.; Ogawa, T.; Watanabe, F.; Arizono, S. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 36, 1067–1072. [Google Scholar] [CrossRef]
- Furukawa, T.; Taniguchi, H.; Ando, M.; Kondoh, Y.; Kataoka, K.; Nishiyama, O.; Johkoh, T.; Fukuoka, J.; Sakamoto, K.; Hasegawa, Y. The St. George’s Respiratory Questionnaire as a prognostic factor in IPF. Respir. Res. 2017, 18, 18. [Google Scholar] [CrossRef]
- Vestbo, J.; Prescott, E.; Almdal, T.; Dahl, M.; Nordestgaard, B.G.; Andersen, T.; Sørensen, T.I.A.; Lange, P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: Findings from the Copenhagen City Heart Study. Am. J. Respir. Crit. Care Med. 2006, 173, 79–83. [Google Scholar] [CrossRef]
- Shronts, E.P. Basic concepts of immunology and its application to clinical nutrition. Nutr. Clin. Pract. 1993, 8, 177–183. [Google Scholar] [CrossRef]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef]
- Morris, D.M.; Kitchin, E.M.; Clark, D.E. Strategies for optimizing nutrition and weight reduction in physical therapy practice: The evidence. Physiother. Theory Pract. 2009, 25, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Yokoyama, T.; Chida, K.; Azuma, A.; Suda, T.; Kudoh, S.; Sakamoto, N.; Okamoto, K.; Kobashi, G.; et al. Vegetable, fruit, and cereal intake and risk of idiopathic pulmonary fibrosis in Japan. Ann. Nutr. Metab. 2004, 48, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xi, Y.; Zhang, Y.; He, P.; Su, X.; Fan, F.; Wu, M.; Kong, X.; Shi, Y. Genetic association analysis of dietary intake and idiopathic pulmonary fibrosis: A two-sample mendelian randomization study. BMC Pulm. Med. 2024, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ma, S.-F.; Ma, J.Z.; Huang, Y.; Bonham, C.A.; Oldham, J.M.; Adegunsoye, A.; Strek, M.E.; Flaherty, K.R.; Strickland, E.; et al. Associations of Plasma Omega-3 Fatty Acids with Progression and Survival in Pulmonary Fibrosis. Chest 2024, 165, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Mercader-Barceló, J.; Truyols-Vives, J.; Río, C.; López-Safont, N.; Sala-Llinàs, E.; Chaplin, A. Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 6051. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Takahashi, H.; Kasai, C.; Kiyokawa, N.; Watanabe, T.; Fujii, S.; Kashiwagura, T.; Honma, M.; Satake, M.; Shioya, T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir. Med. 2010, 104, 1883–1889. [Google Scholar] [CrossRef]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachex. Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef]
| All Patients (n = 170) | Normal Group (n = 101) | Mild Group (n = 58) | Moderate Group (n = 11) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 75.7 ± 6.3 | 74.1 ± 5.8 | 78.1 ± 6.3 # | 77.8 ± 6.3 | <0.001 a |
| Gender, male/female | 138/32 | 81/20 | 43/15 | 9/2 | 0.643 b |
| BMI (kg/m2) | 23.6 ± 3.9 | 24.4 ± 3.4 | 22.5 ± 4.2 § | 22.3 ± 5.4 | 0.005 a |
| Oxygen use, n (%) | 48 (28.2) | 23 (22.7) | 20 (34.4) | 5 (45.4) | 0.112 b |
| Smoking status (current/former/never) | 3/130/37 | 0/80/21 | 2/43/13 | 1/7/3 | 0.156 b |
| History of acute exacerbation, n (%) | 8 (4.7) | 2 (1.9) | 4 (6.8) | 2 (18.1) § | 0.034 b |
| Comorbidities, n (%) | |||||
| Diabetes | 31 (18.2) | 16 (15.8) | 15 (25.8) | 0 (0) | 0.078 b |
| Hypertension | 70 (41.1) | 41 (40.5) | 24 (41.3) | 5 (45.4) | 0.952 b |
| Dyslipidemia | 39 (22.9) | 23 (22.7) | 16 (27.5) | 0 (0) | 0.136 b |
| Cardiovascular disease | 22 (12.9) | 8 (7.9) | 12 (20.6) | 2 (18.1) | 0.06 b |
| CKD | 14 (8.2) | 7 (6.9) | 6 (10.3) | 1 (9.0) | 0.748 b |
| Medications | |||||
| Predonisolone | 35 (20.5) | 15 (14.8) | 15 (25.8) | 5 (45.4) § | 0.028 b |
| Pirfenidone | 28 (16.4) | 12 (11.8) | 13 (22.4) | 3 (27.2) | 0.137 b |
| Nintedanib | 22 (12.9) | 13 (12.8) | 8 (13.7) | 1 (9.0) | 0.913 b |
| Severity | |||||
| GAP stage | 1.5 ± 0.8 | 1.5 ± 0.7 | 1.6 ± 0.8 | 2.0 ± 0.9 | 0.284 b |
| I/II/III | 108/33/29 | 68/19/14 | 36/11/11 | 4/3/4 | |
| GAP score | 3.3 ± 1.5 | 3.2 ± 1.4 | 3.5 ± 1.6 | 4.2 ± 1.9 | 0.091 a |
| Nutrition Status | |||||
| CONUT score | 1.5 ± 1.5 | 0.5 ± 0.5 | 2.5 ± 0.6 # | 5.7 ± 0.7 #‡ | <0.001 a |
| Pulmonary function | |||||
| FVC (%pred) | 78.3 ± 18.3 | 80.1 ± 18.6 | 76.0 ± 16.9 | 73.1 ± 22.0 | 0.281 a |
| FEV1 (%pred) | 96.5 ± 23.1 | 95.0 ± 22.4 | 99.1 ± 24.3 | 97.0 ± 25.0 | 0.590 a |
| FEV1/FVC | 84.7 ± 9.9 | 84.5 ± 10.8 | 84.9 ± 8.3 | 85.5 ± 9.5 | 0.949 a |
| TLC (%pred) | 77.0 ± 16.1 | 77.1 ± 15.5 | 75.8 ± 15.3 | 81.9 ± 25.0 | 0.559 a |
| DLco (%pred) | 62.9 ± 20.2 | 62.5 ± 19.1 | 64.4 ± 20.6 | 58.9 ± 31.1 | 0.750 a |
| PaO2, cmH2O | 82.5 ± 17.3 | 81.9 ± 14.4 | 84.2 ± 0.4 | 79.6 ± 24.6 | 0.609 a |
| PaCO2, cmH2O | 41.0 ± 5.7 | 41.1 ± 6.4 | 41.0 ± 4.4 | 39.2 ± 4.7 | 0.585 a |
| Biochemical data | |||||
| CRP, mg/dL | 0.6 ± 1.3 | 0.4 ± 1.0 | 0.7 ± 1.1 | 1.9 ± 2.8 §∫ | 0.001 a |
| Alb, g/dL | 3.7 ± 0.5 | 3.9 ± 0.3 | 3.7 ± 0.4 * | 2.7 ± 0.8 # | <0.001 a |
| T-Cho, mg/dL | 187.8 ± 39.6 | 196.5 ± 36.7 | 176.1 ± 40.5 * | 169.0 ± 42.3 | 0.002 a |
| T-Lymph | 1911.9 ± 751.1 | 2132.8 ± 665.5 | 1884.7 ± 2300.6 | 1519.2 ± 1186.5 | 0.313 a |
| TP, g/dL | 7.4 ± 0.7 | 7.5 ± 0.5 | 7.4 ± 0.8 | 6.5 ± 1.2 #∫ | <0.001 a |
| KL-6 | 997.1 ± 647.9 | 973.2 ± 588.7 | 1067.2 ± 763.0 | 821.2 ± 462.3 | 0.490 a |
| SP-D | 236.0 ± 190.0 | 223.6 ± 162.6 | 261.1 ± 237.0 | 218.0 ± 142.6 | 0.490 a |
| SP-A | 82.5 ± 65.6 | 79.3 ± 59.9 | 90.2 ± 77.7 | 70.3 ± 42.7 | 0.551 a |
| Dyspnea and functional status | |||||
| mMRC dyspnea | 1.6 ± 1.1 | 1.4 ± 1.0 | 1.8 ± 1.2 § | 2.4 ± 1.2 § | 0.004 a |
| 0/1/2/3/4 | (26/64/43/24/13) | (19/40/29/9/4) | (7/21/10/14/6) | (0/3/4/1/3) | |
| 6 min walking test | |||||
| 6MWD (m) | 369.8 ± 119.1 | 394.7 ± 106.7 | 344.5 ± 127.1 § | 257.8 ± 108.5 * | 0.001 a |
| Peripheral muscle strength | |||||
| QF (Nm/kg) | 1.24 ± 0.44 | 1.29 ± 0.42 | 1.14 ± 0.44 | 1.30 ± 0.55 | 0.116 a |
| Hand grip | 26.3 ± 7.6 | 28.3 ± 7.6 | 23.7 ± 7.0 * | 21.1 ± 3.5 § | <0.001 a |
| Health-related quality of life | |||||
| CAT | 14.5 ± 8.3 | 13.9 ± 8.0 | 15.7 ± 9.0 | 14.2 ± 6.2 | 0.531 a |
| Univariate Analysis | |||
|---|---|---|---|
| Hazard Ratio | 95%CI | p-Value | |
| Age (years) | 1.029 | 0.985–1.075 | 0.195 |
| Sex, male/female | 1.128 | 0.589–2.160 | 0.716 |
| History of acute exacerbation | 2.012 | 0.719–5.627 | 0.180 |
| Use of AFD | 1.715 | 0.981–2.998 | 0.059 |
| GAP stage | 2.465 | 1.777–3.420 | <0.0001 |
| BMI (kg/m2) | 0.910 | 0.837–0.990 | 0.028 |
| CONUT, grade | 1.926 | 1.230–3.015 | 0.004 |
| FVC (%pred) | 0.954 | 0.937–0.972 | 0.0001 |
| FEV1 (%pred) | 0.975 | 0.960–0.989 | <0.001 |
| FEV1/FVC | 1.026 | 0.998–1.054 | 0.060 |
| TLC (%pred) | 0.960 | 0.941–0.981 | <0.001 |
| DLco (%pred) | 0.965 | 0.947–0.984 | <0.0001 |
| PaO2 (Torr) | 0.988 | 0.696–1.008 | 0.232 |
| PaCO2 (Torr) | 0.990 | 0.935–1.048 | 0.727 |
| CRP, mg/dL | 1.044 | 0.738–1.475 | 0.809 |
| Alb, g/dL | 0.592 | 0.347–1.012 | 0.050 |
| T-Cho, mg/dL | 1.000 | 0.994–1.006 | 0.999 |
| T-Lymph, /μL | 1.000 | 0.999–1.000 | 0.350 |
| TP, g/dL | 0.861 | 0.541–1.370 | 0.520 |
| KL-6, U/mL | 1.000 | 0.999–1.000 | 0.590 |
| SP-D, U/mL | 1.001 | 1.000–1.003 | 0.030 |
| SP-A, U/mL | 0.996 | 0.988–1.004 | 0.300 |
| mMRC dyspnea | 1.415 | 1.117–1.792 | 0.004 |
| 6MWD (m) | 0.994 | 0.992–0.997 | <0.0001 |
| QF (Nm/kg) | 0.922 | 0.477–1.781 | 0.809 |
| Hand grip | 0.967 | 0.93–1.004 | 0.083 |
| CAT score | 1.047 | 1.010–1.086 | 0.010 |
| Multivariate Analysis | |||
|---|---|---|---|
| Hazard Ratio | 95%CI | p-Value | |
| GAP stage | 5.972 | 2.901–12.291 | <0.001 |
| CONUT | 2.012 | 1.192–3.395 | 0.009 |
| mMRC dyspnea | 0.615 | 0.389–0.971 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanami, Y.; Ebihara, K.; Nakao, K.; Kubo, R.; Miyagi, M.; Nakamura, Y.; Sakamoto, S.; Kishi, K.; Okuni, I.; Ebihara, S. Impact of Controlling Nutritional Status Score on Mortality in Elderly Patients with Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2024, 13, 2825. https://doi.org/10.3390/jcm13102825
Iwanami Y, Ebihara K, Nakao K, Kubo R, Miyagi M, Nakamura Y, Sakamoto S, Kishi K, Okuni I, Ebihara S. Impact of Controlling Nutritional Status Score on Mortality in Elderly Patients with Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine. 2024; 13(10):2825. https://doi.org/10.3390/jcm13102825
Chicago/Turabian StyleIwanami, Yuji, Kento Ebihara, Keiko Nakao, Ryuki Kubo, Midori Miyagi, Yasuhiko Nakamura, Susumu Sakamoto, Kazuma Kishi, Ikuko Okuni, and Satoru Ebihara. 2024. "Impact of Controlling Nutritional Status Score on Mortality in Elderly Patients with Idiopathic Pulmonary Fibrosis" Journal of Clinical Medicine 13, no. 10: 2825. https://doi.org/10.3390/jcm13102825





