Transcaval versus Supra-Aortic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Outcomes
2.3. Statistical Analyses
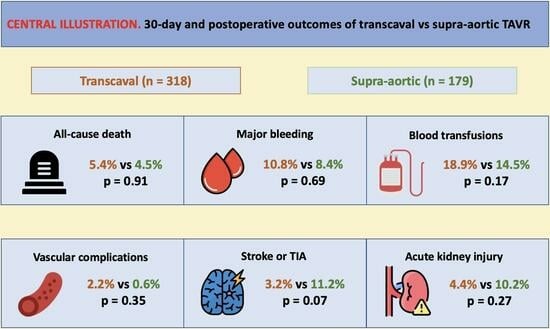
3. Results
3.1. Study Selection
3.2. Patients’ Baseline Characteristics
3.3. Perioperative Characteristics
3.4. In-Hospital or 30-Day All-Cause Mortality
3.5. Major Bleeding and Blood Transfusions
3.6. Major Vascular Complications
3.7. Stroke or Transient Ischemic Attack
3.8. Acute Kidney Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal Trends in Transcatheter Aortic Valve Replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; O’Neill, W.W.; Paone, G.; Guerrero, M.E.; Wyman, J.F.; Cooper, R.L.; Lederman, R.J. Caval-aortic access to allow transcatheter aortic valve replacement in otherwise ineligible patients: Initial human experience. J. Am. Coll. Cardiol. 2014, 63, 2795–2804. [Google Scholar] [CrossRef]
- Modine, T.; Lemesle, G.; Azzaoui, R.; Sudre, A. Aortic valve implantation with the CoreValve ReValving System via left carotid artery access: First case report. J. Thorac. Cardiovasc. Surg. 2010, 140, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Ruge, H.; Lange, R.; Bleiziffer, S.; Hutter, A.; Mazzitelli, D.; Will, A.; Schreiber, C.; Laborde, J.C.; Bauernschmitt, R. First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: A case report. Heart Surg. Forum 2008, 11, 323–324. [Google Scholar] [CrossRef]
- De Robertis, F.; Asgar, A.; Davies, S.; Delahunty, N.; Kelleher, A.; Trimlett, R.; Mullen, M.; Moat, N. The left axillary artery--a new approach for transcatheter aortic valve implantation. Eur. J. Cardiothorac. Surg. 2009, 36, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Overtchouk, P.; Modine, T. Alternate Access for TAVI: Stay Clear of the Chest. Interv. Cardiol. Rev. 2018, 13, 145–150. [Google Scholar] [CrossRef]
- Lu, H.; Monney, P.; Fournier, S.; Pavon, A.G.; Roguelov, C.; Eeckhout, E.; Muller, O.; Kirsch, M. Transcervical approach versus transfemoral approach for transcatheter aortic valve replacement. Int. J. Cardiol. 2021, 327, 58–62. [Google Scholar] [CrossRef]
- Lederman, R.J.; Babaliaros, V.C.; Lisko, J.C.; Rogers, T.; Mahoney, P.; Foerst, J.R.; Depta, J.P.; Muhammad, K.I.; McCabe, J.M.; Pop, A.; et al. Transcaval Versus Transaxillary TAVR in Contemporary Practice: A Propensity-Weighted Analysis. JACC Cardiovasc. Interv. 2022, 15, 965–975. [Google Scholar] [CrossRef]
- Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. May 2023. Available online: https://www.icmje.org/icmje-recommendations.pdf (accessed on 27 June 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardiothorac. Surg. 2012, 42, S45–S60. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.K.L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Paone, G.; Eng, M.; Kabbani, L.S.; Borgi, J.; Peterson, E.; Novitsky, B.; Burroughs, B.; Wang, D.D.; O’Neill, W.W.; Greenbaum, A.B. Transcatheter Aortic Valve Replacement: Comparing Transfemoral, Transcarotid, and Transcaval Access. Ann. Thorac. Surg. 2018, 106, 1105–1112. [Google Scholar] [CrossRef]
- Long, A.; Mahoney, P. Comparative Intermediate-Term Outcomes of Subclavian and Transcaval Access for Transcatheter Aortic Valve Replacement. J. Invasive Cardiol. 2020, 32, 463–469. [Google Scholar] [PubMed]
- Abellan, C.; Antiochos, P.; Fournier, S.; Skali, H.; Shah, P.; Maurizi, N.; Eeckhout, E.; Roguelov, C.; Monney, P.; Tzimas, G.; et al. Extrathoracic Against Intrathoracic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review with Meta-Analysis. Am. J. Cardiol. 2023, 203, 473–483. [Google Scholar] [CrossRef]
- Van der Boon, R.M.A.; Marcheix, B.; Tchetche, D.; Chieffo, A.; Van Mieghem, N.M.; Dumonteil, N.; Vahdat, O.; Maisano, F.; Serruys, P.W.; Kappetein, A.P.; et al. Transapical versus transfemoral aortic valve implantation: A multicenter collaborative study. Ann. Thorac. Surg. 2014, 97, 22–28. [Google Scholar] [CrossRef]
- Arai, T.; Romano, M.; Lefèvre, T.; Hovasse, T.; Farge, A.; Le Houerou, D.; Hayashida, K.; Watanabe, Y.; Garot, P.; Benamer, H.; et al. Direct Comparison of Feasibility and Safety of Transfemoral Versus Transaortic Versus Transapical Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. Intv. 2016, 9, 2320–2325. [Google Scholar] [CrossRef]
- Greenbaum, A.B.; Babaliaros, V.C.; Chen, M.Y.; Stine, A.M.; Rogers, T.; O’Neill, W.W.; Paone, G.; Thourani, V.H.; Muhammad, K.I.; Leonardi, R.A.; et al. Transcaval Access and Closure for Transcatheter Aortic Valve Replacement: A Prospective Investigation. J. Am. Coll. Cardiol. 2017, 69, 511–521. [Google Scholar] [CrossRef]
- Lederman, R.J.; Babaliaros, V.C.; Rogers, T.; Stine, A.M.; Chen, M.Y.; Muhammad, K.I.; Leonardi, R.A.; Paone, G.; Khan, J.M.; Leshnower, B.G.; et al. The Fate of Transcaval Access Tracts: 12-Month Results of the Prospective NHLBI Transcaval Transcatheter Aortic Valve Replacement Study. JACC Cardiovasc. Interv. 2019, 12, 448–456. [Google Scholar] [CrossRef]
- Lu, H.; Monney, P.; Hullin, R.; Fournier, S.; Roguelov, C.; Eeckhout, E.; Rubimbura, V.; Faroux, L.; Barrier, A.; Muller, O.; et al. Transcarotid Access Versus Transfemoral Access for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 687168. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.M.; Lansky, A.J.; Webb, J.; Roffi, M.; Toggweiler, S.; Reinthaler, M.; Wang, D.; Hutchinson, N.; Wendler, O.; Hildick-Smith, D.; et al. Local versus general anesthesia for transcatheter aortic valve implantation (TAVR)—Systematic review and meta-analysis. BMC Med. 2014, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, U.; Deuschl, F.; Schofer, N.; Frerker, C.; Schmidt, T.; Kuck, K.H.; Kreidel, F.; Schirmer, J.; Mizote, I.; Reichenspurner, H.; et al. Safety and efficacy of the percutaneous transaxillary access for transcatheter aortic valve implantation using various transcatheter heart valves in 100 consecutive patients. Int. J. Cardiol. 2017, 232, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Toomey, N.; Ortoleva, J.; Kawabori, M.; Weintraub, A.; Chen, F.Y. Safety and efficacy of transaxillary transcatheter aortic valve replacement using a current-generation balloon-expandable valve. J. Cardiothorac. Surg. 2020, 15, 244. [Google Scholar] [CrossRef]
- Faroux, L.; Junquera, L.; Mohammadi, S.; Del Val, D.; Muntané-Carol, G.; Alperi, A.; Kalavrouziotis, D.; Dumont, E.; Paradis, J.M.; Delarochellière, R.; et al. Femoral Versus Nonfemoral Subclavian/Carotid Arterial Access Route for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e017460. [Google Scholar] [CrossRef]
- Lederman, R.J.; Greenbaum, A.B.; Khan, J.M.; Bruce, C.G.; Babaliaros, V.C.; Rogers, T. Transcaval Access and Closure Best Practices. JACC Cardiovasc. Interv. 2023, 16, 371–395. [Google Scholar] [CrossRef]
- Lu, H.; Fournier, S.; Namasivayam, J.; Roguelov, C.; Ferrari, E.; Eeckhout, E.; Monney, P.; Tozzi, P.; Marcucci, C.; Muller, O.; et al. Transapical approach versus transcervical approach for transcatheter aortic valve replacement: A retrospective monocentric study. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 781–788. [Google Scholar] [CrossRef]



| Comorbidities | Echocardiographic Parameters | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, Year | Study Arm | Sample Size (n) | Age (Years) | Male Gender | STS Score | NYHA Class III or IV | HTA | Diabetes | MI | CABG | AFF | PAD | Previous Stroke/TIA | CLD/COPD | ESRD | LVEF | AV Mean Gradient (mmHg) |
| Paone et al. 2018 [15] | TCv | 58 | 79.6 ± 9.6 | 44.8 | 8.0 ± 5.2 | 81.3 | 90.0 | 44.8 | - | - | - | 75.9 | 43.1 | 43.1 | 5.2 | 53.3 ± 15.5 | 32.0 ± 13.1 |
| TC | 32 | 79.0 ± 9.6 | 50.0 | 6.9 ± 4.4 | 93.1 | 93.8 | 34.4 | - | - | - | 78.1 | 40.6 | 62.5 | 6.3 | 56.2 ± 11.9 | 36.5 ± 15.2 | |
| Long et al. 2020 [16] | TCv | 22 | 80.7 ± 3.9 | 36.4 | 9.0 ± 1.9 | - | - | 31.8 | 27.3 | 13.6 | 22.7 | 36.4 | 18.1 | 22.7 | 9.0 | 42.3 ± 4.2 | 38.2 ± 4.8 |
| TSc | 41 | 83.2 ± 3.7 | 41.5 | 10.4 ± 2.6 | - | - | 43.9 | 12.2 | 9.8 | 26.8 | 29.3 | 14.6 | 24.3 | 7.3 | 38.3 ± 5.9 | 34.8 ± 4.7 | |
| Lederman et al. 2022 [9] | TCv | 238 | 76.4 ± 9.1 | 43.7 | 5.0 (3.2, 8.4) | 60.1 | 94.1 | 43.9 | 24.4 | 26.9 | 34.6 | 55.3 | 19.8 | 40.3 | 8.8 | 58.0 (43.0, 60.0) | 40.1 ± 13.5 |
| TAx | 106 | 77.2 ± 8.8 | 56.6 | 5.6 (4.0, 8.3) | 85.5 | 92.5 | 31.8 | 25.7 | 19.8 | 34.0 | 0 | 28.3 | 34.0 | 5.7 | 57.0 (43.0, 63.0) | 41.1 ± 13.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antiochos, P.; Kirsch, M.; Monney, P.; Tzimas, G.; Meier, D.; Fournier, S.; Ferlay, C.; Nowacka, A.; Rancati, V.; Abellan, C.; et al. Transcaval versus Supra-Aortic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 455. https://doi.org/10.3390/jcm13020455
Antiochos P, Kirsch M, Monney P, Tzimas G, Meier D, Fournier S, Ferlay C, Nowacka A, Rancati V, Abellan C, et al. Transcaval versus Supra-Aortic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2024; 13(2):455. https://doi.org/10.3390/jcm13020455
Chicago/Turabian StyleAntiochos, Panagiotis, Matthias Kirsch, Pierre Monney, Georgios Tzimas, David Meier, Stephane Fournier, Clémence Ferlay, Anna Nowacka, Valentina Rancati, Christophe Abellan, and et al. 2024. "Transcaval versus Supra-Aortic Vascular Accesses for Transcatheter Aortic Valve Replacement: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 13, no. 2: 455. https://doi.org/10.3390/jcm13020455