Benefits of Robot-Assisted Upper-Limb Rehabilitation from the Subacute Stage after a Stroke of Varying Severity: A Multicenter Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Standard Operating Protocol (SOP) Development
2.2.1. Conventional Occupational Therapy Protocol
2.2.2. InMotion ARM TM Protocol
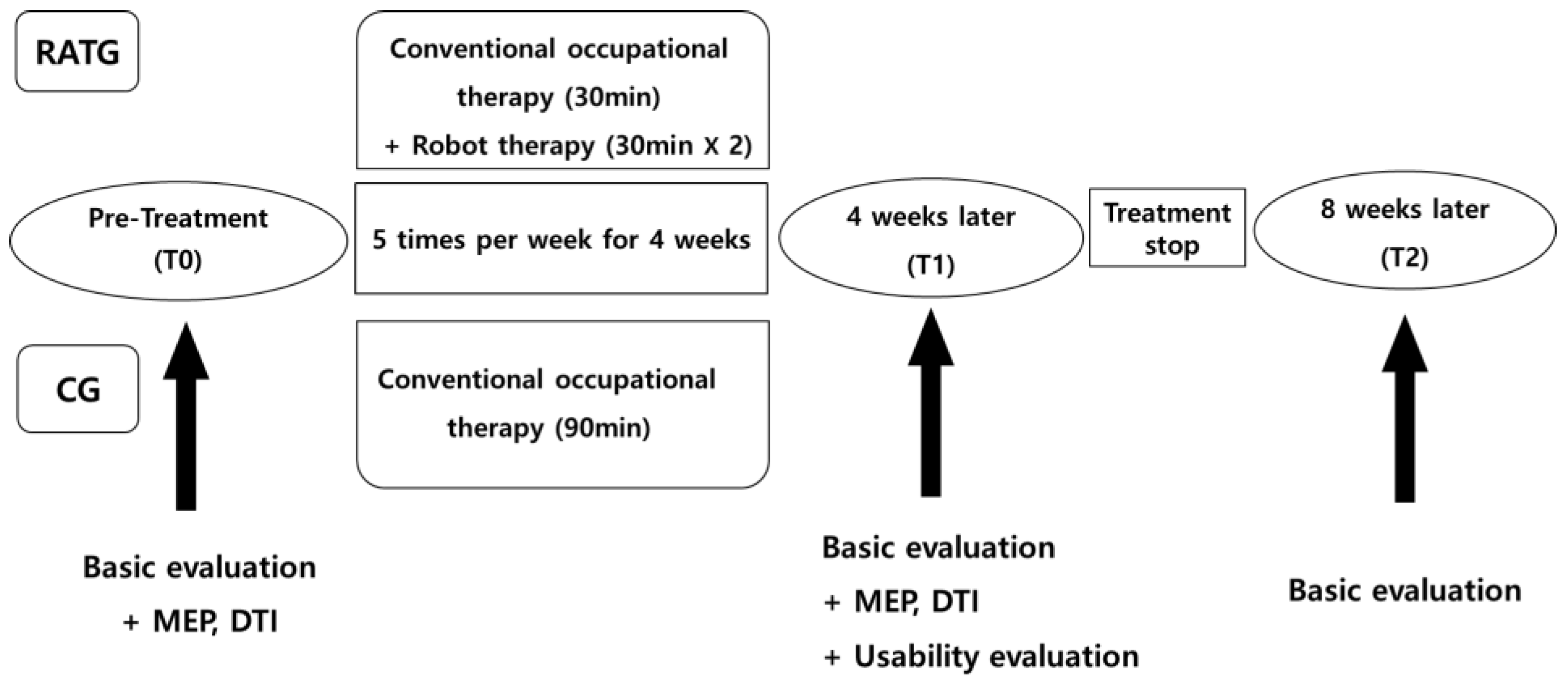
2.3. Study Design (and Procedure)
2.4. Interventions or Robot-Assisted Training
2.5. Assessment
2.5.1. Primary Outcome
2.5.2. Secondary Outcomes
2.5.3. InMotion Robot Built-in Function Evaluation (Robot Kinematic Measure)
2.5.4. Assessment of Utility
2.5.5. Motor-Evoked Potential (MEP)
2.5.6. Diffusion Tensor Imaging (DTI)
2.6. Statistical Analyses
3. Results
3.1. Participants’ Baseline Characteristics
3.2. Comparisons of Robot-Assisted Therapy with Conventional Therapy (T0–T1)
3.3. Comparisons of Robot-Assisted Therapy with Conventional Therapy (T0–T2)
3.4. Subgroup Analysis Depending on the Severity
3.5. Subgroup Analysis between Subacute and Chronic Patients
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodgers, H.; Shaw, L.; Bosomworth, H.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; Dawson, J.; Eyre, J.; Finch, T.; et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): Study protocol for a randomised controlled trial. Trials 2017, 18, 340. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of robot-assisted therapy on upper limb recovery after stroke: A systematic review. Neurorehabilit. Neural Repair 2008, 22, 111–121. [Google Scholar] [CrossRef]
- Chien, W.T.; Chong, Y.Y.; Tse, M.K.; Chien, C.W.; Cheng, H.Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef]
- Zeiler, S.R.; Krakauer, J.K. The interaction between training and plasticity in the poststroke brain. Curr. Opin. Neurol. 2013, 26, 609–616. [Google Scholar] [CrossRef]
- Hara, Y. Brain plasticity and rehabilitation in stroke patients. J. Nippon. Med. Sch. 2015, 82, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Melegari, C.; de Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Ferreira, F.M.R.M.; Chaves, M.E.A.; Oliveira, V.C.; van Petten, A.M.V.N.; Vimieiro, C.B.S. Effectiveness of robot therapy on body function and structure in people with limited upper limb function: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0200330. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; van Wegen, E.E.; Meskers, C.G.; Kwakkel, G. Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil. Neural. Repair. 2017, 31, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Baroni, A.; Mele, S.; Craighero, L.; Manfredini, F.; Lamberti, N.; Maietti, E.; Basaglia, N. Effects of a robot-assisted arm training plus hand functional electrical stimulation on recovery after stroke: A randomized clinical trial. Arch. Phys. Med. Rehabil. 2020, 101, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, I.; Lencioni, T.; Bowman, T.; Bertoni, R.; Turolla, A.; Ferrarin, M.; Jonsdottir, J. Effects of robot therapy on upper body kinematics and arm function in persons post stroke: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 10. [Google Scholar] [CrossRef]
- Taveggia, G.; Borboni, A.; Salvi, L.; Mulé, C.; Fogliaresi, S.; Villafañe, J.H.; Casale, R. Efficacy of robot-assisted rehabilitation for the functional recovery of the upper limb in post-stroke patients: A randomized controlled study. Eur. J. Phys. Rehabil. Med. 2016, 52, 767–773. [Google Scholar] [PubMed]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pang, R.; Chen, S.; Chen, H.; Xie, Y.; Chen, D.; Wu, K.; Liang, J.; Yan, K.; Hao, Z. Near-infrared spectroscopy as a promising tool in stroke: Current applications and future perspectives. J. Innov. Opt. Health Sci. 2021, 14, 2130006. [Google Scholar] [CrossRef]
- Morris, D.M.; Uswatte, G.; Crago, J.E.; Cook, E.W., 3rd; Taub, E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, E.; Ammenwerth, E. A Digital Box and Block Test for Hand Dexterity Measurement: Instrument Validation Study. JMIR Rehabil. Assist. Technol. 2023, 10, e50474. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Tatjana, P.S.; Martina, G.S.; Martin, P.; Othmar, S.; Gerda, V.; Christian, M.; Fialka-Moser, V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J. Rehabil. Med. 2008, 40, 665–671. [Google Scholar]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor evaluation in vascular hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Hong, I.; Lim, Y.; Han, H.; Hay, C.C.; Woo, H.S. Application of the Korean Version of the Modified Barthel Index: Development of a keyform for use in Clinical Practice. Hong Kong J. Occup. Ther. 2017, 29, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Bode, R.K.; Min Lai, S.; Perera, S. Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Arch. Phys. Med. Rehabil. 2003, 84, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jeon, S.S.; Lee, J.Y.; Cho, A.R.; Park, J.H. Korean Version of the Mini-Mental State Examination Using Smartphone: A Validation Study. Telemed. J. E Health 2017, 23, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.N.; Cho, M.J. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J. Psychosom. Res. 2004, 57, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Rossini, P.M.; Rossi, S. Clinical applications of motor evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H. A review of diffusion tensor imaging studies on motor recovery mechanisms in stroke patients. NeuroRehabilitation 2011, 28, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; Waller, S.M. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce. Int. J. Stroke 2017, 12, 444–450. [Google Scholar] [CrossRef]
- Jiang, S.; You, H.; Zhao, W.; Zhang, M. Effects of short-term upper limb robot-assisted therapy on the rehabilitation of sub-acute stroke patients. Technol. Health Care 2021, 29, 295–303. [Google Scholar] [CrossRef]
- Prange, G.B.; Jannink, M.J.; Groothuis-Oudshoorn, C.G.; Hermens, H.J.; IJzerman, M.J. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev. 2006, 43, 171–184. [Google Scholar] [CrossRef]
- Milani, G.; Antonioni, A.; Baroni, A.; Malerba, P.; Straudi, S. Relation between EEG Measures and Upper Limb Motor Recovery in Stroke Patients: A Scoping Review. Brain Topogr. 2022, 35, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, E.A.; Hakulinen, U.; Brander, A.E.; Luoto, T.M.; Ylinen, A.; Öhman, J.E. Clinical correlates of cerebral diffusion tensor imaging findings in chronic traumatic spinal cord injury. Spinal. Cord. 2014, 52, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Nair, V.A.; Young, B.M.; Walton, L.M.; Nigogosyan, Z.; Remsik, A.; Tyler, M.E.; Farrar-Edwards, D.; Caldera, K.E.; Sattin, J.A.; et al. DTI measures track and predict motor function outcomes in stroke rehabilitation utilizing BCI technology. Front. Hum. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Zolkefley, M.K.I.; Firwana, Y.M.S.; Hatta, H.Z.M.; Rowbin, C.; Nassir, C.M.N.C.M.; Hanafi, M.H.; Abdullah, M.S.; Mustapha, M. An overview of fractional anisotropy as a reliable quantitative measurement for the corticospinal tract (CST) integrity in correlation with a Fugl-Meyer assessment in stroke rehabilitation. J. Phys. Ther. Sci. 2021, 33, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Mrachacz-Kersting, N.; Jiang, N.; Stevenson, A.J.T.; Niazi, I.K.; Kostic, V.; Pavlovic, A.; Radovanovic, S.; Djuric-Jovicic, M.; Agosta, F.; Dremstrup, K.; et al. Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J. Neurophysiol. 2016, 115, 1410–1421. [Google Scholar] [CrossRef] [PubMed]
- Volz, L.J.; Sarfeld, A.-S.; Diekhoff, S.; Rehme, A.K.; Pool, E.-M.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Motor cortex excitability and connectivity in chronic stroke: A multimodal model of functional reorganization. Brain Struct. Funct. 2015, 220, 1093–1107. [Google Scholar] [CrossRef]
- Garcia, D.A.; Morris, M.; Tosunoglu, S. A review of rehabilitation strategies for stroke recovery. ASME Early Career Tech. J. 2012, 11, 148–155. [Google Scholar]

| Control (n = 89) | Treatment (n = 91) | p-Value | |
|---|---|---|---|
| Age, mean ± SD, y | 60.1 ± 13.9 | 58.4 ± 14.9 | 0.432 |
| Sex: male, No. (%) | |||
| Male | 56 (62.9) | 62 (68.1) | 0.531 |
| Female | 33 (37.1) | 29 (31.8) | |
| Side of lesion, No. (%) | |||
| Lt. | 52 (58.4) | 48 (52.7) | 0.443 |
| Rt. | 37 (41.6) | 43 (47.3) | |
| Type of stroke, No. (%) | |||
| ICH | 27 (30.3) | 30 (33.0) | 0.811 |
| SAH | 2 (2.3) | 1 (1.1) | |
| Infarction | 50 (67.4) | 60 (65.9) | |
| Onset, No. (%) | |||
| Subacute | 56 (62.9) | 64 (70.3) | 0.344 |
| Chronic | 33 (37.1) | 27 (29.7) | |
| Severity, No. (%) | |||
| Mild | 28 (31.5) | 17 (18.7) | 0.492 |
| Moderate | 11 (12.3) | 10 (11) | |
| Severe | 50 (56.2) | 64 (70.3) | |
| MMSE | 22.13 | 21.70 | 0.764 |
| Changes = Baseline–Post-Treatment | p-Value | ||
|---|---|---|---|
| Control (n = 89) | Treatment (n = 91) | ||
| Primary Outcome | |||
| WMFT | −6.00 ± 10.20 | −9.09 ± 14.60 | 0.176 |
| Secondary Outcome | |||
| F-M | −3.45 ± 12.37 | −7.77 ± 11.46 | 0.039 * |
| MI (trunk) | −6.58 ± 17.05 | −14.15 ± 20.39 | <0.001 * |
| MEP | |||
| CMAP latency (ms) | −0.43 ± 16.93 | −2.78 ± 22.54 | 0.909 |
| CMAP amplitude (mV) | 0.17 ± 18.19 | 0.05 ± 23.14 | 0.663 |
| MEP latency (ms) | −1.08 ± 10.78 | −0.92 ± 15.73 | 0.783 |
| MEP amplitude (mV) 120 | −0.07 ± 0.76 | 1.32 ± 11.63 | 0.759 |
| MEP amplitude (mV) 140 | −0.10 ± 0.88 | 1.31 ± 11.66 | 0.744 |
| MEP 140/120 | −0.42 ± 1.16 | 1.22 ± 11.69 | 0.648 |
| Diffusion Tensor Imaging | |||
| Number of tracts | 6197.44 ± 51,253.92 | −556.07 ± 8273.53 | 0.657 |
| FA mean | 0.01 ± 0.09 | −0.01 ± 0.14 | 0.754 |
| FA ratio | −0.02 ± 0.14 | −0.03 ± 0.32 | 0.630 |
| Robot Built-In Function Evaluation (Robot Kinematic Measure) | |||
| Point-to-point movement test (smoothness) | 0.00 ± 0.11 | −0.03 ± 0.09 | 0.031 * |
| Point-to-point movement test (reach error) | 0.02 ± 0.04 | 0.05 ± 0.13 | 0.075 |
| Point-to-point movement test (mean velocity) | −0.02 ± 0.10 | −0.03 ± 0.04 | 0.503 |
| Point-to-point movement test (maximal velocity) | −0.01 ± 0.11 | −0.04 ± 0.08 | <0.001 * |
| Point-to-point movement test (path error) | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.589 |
| Circle-drawing movement test (circle size) | −0.01 ± 0.08 | 0.00 ± 0.12 | 0.832 |
| Circle-drawing movement test (independence) | −0.05 ± 0.18 | −0.16 ± 0.21 | <0.001 * |
| Change = Baseline–Post-Treatment | Mean ± SD | p-Value 1 | p-Value 2 | |||
|---|---|---|---|---|---|---|
| WMFT | Mild (n = 45) | Control (n = 28) | −2.93 ± 6.05 | 0.002 * | 0.383 | |
| Treatment (n = 17) | −4.59 ± 11.72 | 0.046 * | ||||
| Moderate (n = 21) | Control (n = 11) | 42.70 ± 20.96 | 0.012 * | 0.463 | ||
| Treatment (n = 10) | −11.10 ± 13.92 | 0.045 * | ||||
| Severe (n = 114) | Control (n = 50) | −7.56 ± 11.43 | <0.001 * | 0.409 | ||
| Treatment (n = 64) | −9.97 ± 15.34 | <0.001 * | ||||
| MRC | Elbow Flexion | Mild (n = 45) | Control (n = 28) | −0.18 ± 0.61 | 0.234 | 0.637 |
| Treatment (n = 17) | −0.12 ± 0.60 | 0.688 | ||||
| Moderate (n = 21) | Control (n = 11) | −0.20 ± 0.63 | 0.625 | 0.222 | ||
| Treatment (n = 10) | −0.70 ± 0.82 | 0.063 | ||||
| Severe (n = 114) | Control (n = 50) | −0.42 ± 0.70 | <0.001 * | 0.979 | ||
| Treatment (n = 64) | −0.42 ± 0.92 | <0.001 * | ||||
| Elbow Extension | Mild (n = 45) | Control (n = 28) | −0.18 ± 0.61 | 0.234 | 0.637 | |
| Treatment (n = 17) | −0.12 ± 0.60 | 0.688 | ||||
| Moderate (n = 21) | Control (n = 11) | −0.20 ± 0.63 | 0.625 | 0.377 | ||
| Treatment (n = 10) | −0.60 ± 0.84 | 0.125 | ||||
| Severe (n = 114) | Control (n = 50) | −0.40 ± 0.78 | 0.001 * | 0.748 | ||
| Treatment (n = 64) | −0.38 ± 1.02 | <0.001 * | ||||
| Wrist Flexion | Mild (n = 45) | Control (n = 28) | −0.18 ± 0.67 | 0.273 | 0.827 | |
| Treatment (n = 17) | −0.29 ± 0.69 | 0.188 | ||||
| Moderate (n = 21) | Control (n = 11) | −0.30 ± 0.48 | 0.250 | 0.022 * | ||
| Treatment (n = 10) | −1.20 ± 0.79 | 0.008 * | ||||
| Severe (n = 114) | Control (n = 50) | −0.28 ± 0.61 | 0.003 * | 0.526 | ||
| Treatment (n = 64) | −0.20 ± 1.01 | 0.075 | ||||
| Wrist Extension | Mild (n = 45) | Control (n = 28) | −0.14 ± 0.65 | 0.398 | 0.664 | |
| Treatment (n = 17) | −0.29 ± 0.69 | 0.188 | ||||
| Moderate (n = 21) | Control (n = 11) | −0.30 ± 0.67 | 0.500 | 0.049 * | ||
| Treatment (n = 10) | −1.10 ± 0.88 | 0.016 * | ||||
| Severe (n = 114) | Control (n = 50) | −0.22 ± 0.62 | 0.023 * | 0.697 | ||
| Treatment (n = 64) | −0.27 ± 0.98 | 0.010 * | ||||
| Change = Baseline–Post-Treatment | Mean ± SD | p-Value 1 | p-Value 2 | |||
|---|---|---|---|---|---|---|
| WMFT | Subacute (n = 120) | Control (n = 56) | −8.07 ± 10.48 | <0.001 * | 0.296 | |
| Treatment (n = 64) | −11.53 ± 16.22 | <0.001 * | ||||
| Chronic (n = 60) | Control (n = 33) | −2.48 ± 8.76 | 0.039 * | 0.182 | ||
| Treatment (n = 27) | −3.30 ± 7.15 | 0.007 * | ||||
| MRC | Wrist Flexion | Subacute (n = 120) | Control (n = 56) | −0.30 ± 0.71 | 0.002 * | 0.09 |
| Treatment (n = 64) | −0.61 ± 0.88 | <0.001 * | ||||
| Chronic (n = 60) | Control (n = 33) | −0.18 ± 0.39 | 0.031 * | 0.002 * | ||
| Treatment (n = 27) | 0.33 ± 0.88 | 0.063 | ||||
| Wrist Extension | Subacute (n = 120) | Control (n = 56) | −0.25 ± 0.74 | 0.022 * | 0.045 * | |
| Treatment (n = 64) | −0.59 ± 0.87 | <0.001 * | ||||
| Chronic (n = 60) | Control (n = 33) | −0.15 ± 0.36 | 0.063 | 0.021 * | ||
| Treatment (n = 27) | 0.19 ± 0.92 | 0.500 | ||||
| MI | (ARM + LEG)/2 | Subacute (n = 120) | Control (n = 56) | −11.10 ± 13.66 | <0.001 * | 0.795 |
| Treatment (n = 64) | −11.79 ± 13.77 | <0.001 * | ||||
| Chronic (n = 60) | Control (n = 33) | −3.62 ± 7.33 | 0.007 * | 0.649 | ||
| Treatment (n = 27) | −2.02 ± 4.73 | 0.006 * | ||||
| trunk | Subacute (n = 120) | Control (n = 56) | −7.09 ± 15.22 | 0.001 * | 0.002 * | |
| Treatment (n = 64) | −18.47 ± 21.74 | <0.001 * | ||||
| Chronic (n = 60) | Control (n = 33) | −5.73 ± 20.00 | 0.203 | 0.869 | ||
| Treatment (n = 27) | −3.93 ± 11.84 | 0.125 | ||||
| MEP | MEP amplitude (mV) 120 | Subacute (n = 120) | Control (n = 56) | −0.07 ± 0.90 | 0.141 | 0.700 |
| Treatment (n = 64) | −0.08 ± 0.20 | 0.001 * | ||||
| Chronic (n = 60) | Control (n = 33) | 0.05 ± 0.19 | 0.054 | 0.004 * | ||
| Treatment (n = 27) | 5.37 ± 22.92 | 0.109 | ||||
| DTI | FA mean | Subacute (n = 120) | Control (n = 56) | 0.00 ± 0.04 | 0.903 | 0.474 |
| Treatment (n = 64) | 0.01 ± 0.13 | 0.522 | ||||
| Chronic (n = 60) | Control (n = 33) | 0.02 ± 0.16 | 0.379 | 0.039 * | ||
| Treatment (n = 27) | −0.06 ± 0.13 | 0.044 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.Y.; Bok, S.-K.; Lee, J.Y.; Ryoo, H.W.; Lee, H.Y.; Park, H.J.; Oh, H.M.; Kim, T.-W. Benefits of Robot-Assisted Upper-Limb Rehabilitation from the Subacute Stage after a Stroke of Varying Severity: A Multicenter Randomized Controlled Trial. J. Clin. Med. 2024, 13, 808. https://doi.org/10.3390/jcm13030808
Ahn SY, Bok S-K, Lee JY, Ryoo HW, Lee HY, Park HJ, Oh HM, Kim T-W. Benefits of Robot-Assisted Upper-Limb Rehabilitation from the Subacute Stage after a Stroke of Varying Severity: A Multicenter Randomized Controlled Trial. Journal of Clinical Medicine. 2024; 13(3):808. https://doi.org/10.3390/jcm13030808
Chicago/Turabian StyleAhn, So Young, Soo-Kyung Bok, Ji Young Lee, Hyeon Woo Ryoo, Hoo Young Lee, Hye Jung Park, Hyun Mi Oh, and Tae-Woo Kim. 2024. "Benefits of Robot-Assisted Upper-Limb Rehabilitation from the Subacute Stage after a Stroke of Varying Severity: A Multicenter Randomized Controlled Trial" Journal of Clinical Medicine 13, no. 3: 808. https://doi.org/10.3390/jcm13030808







