Are Surgeons Going to Be Left Holding the Bag? Incisional Hernia Repair and Intra-Peritoneal Non-Absorbable Mesh Implant Complications
Abstract
:1. Introduction
2. Surgical Mesh
2.1. Mesh Classifications
2.2. Biological Meshes
2.3. Anatomic Review of Mesh Placement
2.3.1. Intra-Peritoneal Placement of Mesh
2.3.2. Intra-Peritoneal Onlay Mesh (IPOM) Placement
2.4. Complications of Mesh Placement
2.4.1. Management of Minor Complications of Incisional Hernia Repair with Mesh
Autoimmune Complications Have Not Been Validated
2.4.2. Major Complications of Incisional Hernia Repair with Mesh
Complications of Subsequent Surgery
Mesh Infection
2.4.3. Salvage of Infected Mesh
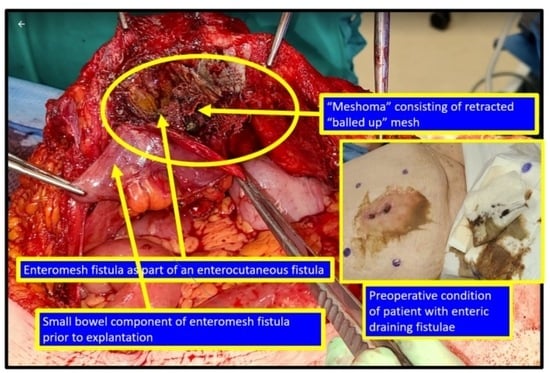
2.4.4. Mesh Shrinkage, “Meshomas”, and Bowel Obstruction after Incisional Hernia Repair with Mesh
2.4.5. Enteroprosthetic and Enterocutaneous Fistula after Incisional Hernia Repair with Mesh
2.4.6. Comparative Evidence Supporting the Use of Intra-Peritoneal Mesh for Incisional Hernia Repair
3. Discussion of the Gaps
3.1. The Surgeon–Patient Relationship and Implantable Devices
3.2. Not Better, Not Even Safe, Just “Substantially Equivalent” (To What?)
3.3. A Global Medicolegal Risk to a Hernia Surgeons
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano-Aroca, A.; Pous-Serrano, S. Prosthetic meshes for hernia repair: State of art, classification, biomaterials, antimicrobial approaches, and fabrication methods. J. Biomed. Mater. Res. A 2021, 109, 2695–2719. [Google Scholar] [CrossRef] [PubMed]
- Novitsky, Y.W.; Harrell, A.G.; Cristiano, J.A.; Paton, B.L.; Norton, H.J.; Peindl, R.D.; Kercher, K.W.; Heniford, B.T. Comparative evaluation of adhesion formation, strength of ingrowth, and textile properties of prosthetic meshes after long-term intra-abdominal implantation in a rabbit. J. Surg. Res. 2007, 140, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Soare, A.M.; Cartu, D.; Nechita, S.L.; Andronic, O.; Surlin, V. Complications of Intraperitoneal Mesh Techniques for Incisional Hernia—A Systematic Review. Chirurgia 2021, 116 (Suppl. S6), S36–S42. [Google Scholar] [PubMed]
- Halm, J.A.; de Wall, L.L.; Steyerberg, E.W.; Jeekel, J.; Lange, J.F. Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery. World J. Surg. 2007, 31, 423–429, discussion 430. [Google Scholar] [CrossRef]
- Aiolfi, A.; Bona, D.; Gambero, F.; Sozzi, A.; Bonitta, G.; Rausa, E.; Bruni, P.G.; Cavalli, M.; Campanelli, G. What is the ideal mesh location for incisional hernia prevention during elective laparotomy? A network meta-analysis of randomized trials. Int. J. Surg. 2023, 109, 1373–1381. [Google Scholar] [CrossRef]
- Millas, S.G.; Mesar, T.; Patel, R.J. Chronic abdominal pain after ventral hernia due to mesh migration and erosion into the sigmoid colon from a distant site: A case report and review of literature. Hernia 2015, 19, 849–852. [Google Scholar] [CrossRef]
- Howard, R.; Thumma, J.; Ehlers, A.; Englesbe, M.; Dimick, J.; Telem, D. Reoperation for Recurrence Up to 10 Years After Hernia Repair. JAMA 2022, 327, 872–874. [Google Scholar] [CrossRef]
- Sanders, D.L.; Pawlak, M.M.; Simons, M.P.; Aufenacker, T.; Balla, A.; Berger, C.; Berrevoet, F.; de Beaux, A.C.; East, B.; Henriksen, N.A.; et al. Midline incisional hernia guidelines: The European Hernia Society. Br. J. Surg. 2023, 110, 1732–1768. [Google Scholar] [CrossRef]
- Campanile, F.C.; Podda, M.; Pecchini, F.; Inama, M.; Molfino, S.; Bonino, M.A.; Ortenzi, M.; Silecchia, G.; Agresta, F.; Cinquini, M.; et al. Laparoscopic treatment of ventral hernias: The Italian national guidelines. Updates Surg. 2023, 75, 1305–1336. [Google Scholar] [CrossRef]
- Sagar, A.; Tapuria, N. An Evaluation of the Evidence Guiding Adult Midline Ventral Hernia Repair. Surg. J. 2022, 8, e145–e156. [Google Scholar] [CrossRef]
- Liang, M.K.; Holihan, J.L.; Itani, K.; Alawadi, Z.M.; Gonzalez, J.R.; Askenasy, E.P.; Ballecer, C.; Chong, H.S.; Goldblatt, M.I.; Greenberg, J.A.; et al. Ventral Hernia Management: Expert Consensus Guided by Systematic Review. Ann. Surg. 2017, 265, 80–89. [Google Scholar] [CrossRef]
- Birindelli, A.; Sartelli, M.; Di Saverio, S.; Coccolini, F.; Ansaloni, L.; van Ramshorst, G.H.; Campanelli, G.; Khokha, V.; Moore, E.E.; Peitzman, A.; et al. 2017 update of the WSES guidelines for emergency repair of complicated abdominal wall hernias. World J. Emerg. Surg. 2017, 12, 37. [Google Scholar] [CrossRef]
- Ventral Hernia Working, G.; Breuing, K.; Butler, C.E.; Ferzoco, S.; Franz, M.; Hultman, C.S.; Kilbridge, J.F.; Rosen, M.; Silverman, R.P.; Vargo, D. Incisional ventral hernias: Review of the literature and recommendations regarding the grading and technique of repair. Surgery 2010, 148, 544–558. [Google Scholar] [CrossRef]
- Sharma, R.; Fadaee, N.; Zarrinkhoo, E.; Towfigh, S. Why we remove mesh. Hernia 2018, 22, 953–959. [Google Scholar] [CrossRef]
- Shubinets, V.; Carney, M.J.; Colen, D.L.; Mirzabeigi, M.N.; Weissler, J.M.; Lanni, M.A.; Braslow, B.M.; Fischer, J.P.; Kovach, S.J. Management of Infected Mesh After Abdominal Hernia Repair: Systematic Review and Single-Institution Experience. Ann. Plast. Surg. 2018, 80, 145–153. [Google Scholar] [CrossRef]
- Rastegarpour, A.; Cheung, M.; Vardhan, M.; Ibrahim, M.M.; Butler, C.E.; Levinson, H. Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast. Surg. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Ansaloni, L.; Catena, F.; Coccolini, F.; Fini, M.; Gazzotti, F.; Giardino, R.; Pinna, A.D. Peritoneal adhesions to prosthetic materials: An experimental comparative study of treated and untreated polypropylene meshes placed in the abdominal cavity. J. Laparoendosc. Adv. Surg. Tech. A 2009, 19, 369–374. [Google Scholar] [CrossRef]
- Delorme, T.; Cottenet, J.; Abo-Alhassan, F.; Bernard, A.; Ortega-Deballon, P.; Quantin, C. Does intraperitoneal mesh increase the risk of bowel obstruction? A nationwide French analysis. Hernia 2023. [Google Scholar] [CrossRef] [PubMed]
- Deerenberg, E.B.; Henriksen, N.A.; Antoniou, G.A.; Antoniou, S.A.; Bramer, W.M.; Fischer, J.P.; Fortelny, R.H.; Gok, H.; Harris, H.W.; Hope, W.; et al. Updated guideline for closure of abdominal wall incisions from the European and American Hernia Societies. Br. J. Surg. 2022, 109, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, M.; Johnston, A.; Zeeshan, S.; Loughlin, P.; Bucholc, M.; Watson, A. The role of prophylactic mesh placement to prevent incisional hernia in laparotomy. Is it time to change practice? Anaesthesiol. Intensive Ther. 2019, 51, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, R.W.; Lemmen, M.H.; Hop, W.C.; Wereldsma, J.C. Incisional hernia recurrence following “vest-over-pants” or vertical Mayo repair of primary hernias of the midline. World J. Surg. 1997, 21, 62–65, discussion 66. [Google Scholar] [CrossRef]
- Saiding, Q.; Chen, Y.; Wang, J.; Pereira, C.L.; Sarmento, B.; Cui, W.; Chen, X. Abdominal wall hernia repair: From prosthetic meshes to smart materials. Mater. Today Bio 2023, 21, 100691. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Subramanian, A.; Hwang, C.S.; Chang, S.; Awad, S.S. Comparison of infectious complications with synthetic mesh in ventral hernia repair. Am. J. Surg. 2013, 205, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Van Hoef, S.; Tollens, T. Primary non-complicated midline ventral hernia: Is laparoscopic IPOM still a reasonable approach? Hernia 2019, 23, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, R.W.; Hop, W.C.; van den Tol, M.P.; de Lange, D.C.; Braaksma, M.M.; IJzermans, J.N.M.; Boelhouwer, R.U.; de Vries, B.C.; Salu, M.K.; Wereldsma, J.C.; et al. A comparison of suture repair with mesh repair for incisional hernia. N. Engl. J. Med. 2000, 343, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.W.; Luijendijk, R.W.; Hop, W.C.; Halm, J.A.; Verdaasdonk, E.G.; Jeekel, J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann. Surg. 2004, 240, 578–583, discussion 583–585. [Google Scholar] [CrossRef]
- Voisard, G.; Feldman, L.S. An unusual cause of chronic anemia and abdominal pain caused by transmural mesh migration in the small bowel after laparoscopic incisional hernia repair. Hernia 2013, 17, 673–677. [Google Scholar] [CrossRef]
- Cobb, W.S.; Carbonell, A.M.; Kalbaugh, C.L.; Jones, Y.; Lokey, J.S. Infection risk of open placement of intraperitoneal composite mesh. Am. Surg. 2009, 75, 762–767, discussion 767–768. [Google Scholar] [CrossRef]
- Prasad, P.; Tantia, O.; Patle, N.M.; Khanna, S.; Sen, B. Laparoscopic ventral hernia repair: A comparative study of transabdominal preperitoneal versus intraperitoneal onlay mesh repair. J. Laparoendosc. Adv. Surg. Tech. A 2011, 21, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Paton, B.L.; Novitsky, Y.W.; Zerey, M.; Sing, R.F.; Kercher, K.W.; Heniford, B.T. Management of infections of polytetrafluoroethylene-based mesh. Surg. Infect. 2007, 8, 337–341. [Google Scholar] [CrossRef]
- Amid, P.K. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1997, 1, 15–21. [Google Scholar] [CrossRef]
- Sajid, M.S.; Leaver, C.; Baig, M.K.; Sains, P. Systematic review and meta-analysis of the use of lightweight versus heavyweight mesh in open inguinal hernia repair. Br. J. Surg. 2012, 99, 29–37. [Google Scholar] [CrossRef] [PubMed]
- See, C.W.; Kim, T.; Zhu, D. Hernia mesh and hernia repair: A review. Eng. Regen. 2020, 1, 19–33. [Google Scholar] [CrossRef]
- Costa, A.; Adamo, S.; Gossetti, F.; D’Amore, L.; Ceci, F.; Negro, P.; Bruzzone, P. Biological Scaffolds for Abdominal Wall Repair: Future in Clinical Application? Materials 2019, 12, 2375. [Google Scholar] [CrossRef] [PubMed]
- King, K.S.; Albino, F.P.; Bhanot, P. Biologic mesh for abdominal wall reconstruction. Chronic Wound Care Manag. Res. 2014, 1, 57–65. [Google Scholar] [CrossRef]
- Rosen, M.J.; Krpata, D.M.; Petro, C.C.; Carbonell, A.; Warren, J.; Poulose, B.K.; Costanzo, A.; Tu, C.; Blatnik, J.; Prabhu, A.S. Biologic vs Synthetic Mesh for Single-stage Repair of Contaminated Ventral Hernias: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 293–301. [Google Scholar] [CrossRef]
- Parker, S.G.; Halligan, S.; Liang, M.K.; Muysoms, F.E.; Adrales, G.L.; Boutall, A.; de Beaux, A.C.; Dietz, U.A.; Divino, C.M.; Hawn, M.T.; et al. International classification of abdominal wall planes (ICAP) to describe mesh insertion for ventral hernia repair. Br. J. Surg. 2020, 107, 209–217. [Google Scholar] [CrossRef]
- Sorour, M.A. Interposition of the omentum and/or the peritoneum in the emergency repair of large ventral hernias with polypropylene mesh. Int. J. Surg. 2014, 12, 578–586. [Google Scholar] [CrossRef]
- Sauerland, S.; Walgenbach, M.; Habermalz, B.; Seiler, C.M.; Miserez, M. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst. Rev. 2011, 3, CD007781. [Google Scholar] [CrossRef]
- Tofolo Pasquini, M.; Medina, P.; Arrechea Antelo, R.; Cerutti, R.; Agustín Porto, E.; Enrique Pirchi, D. Ring closure outcome for laparoscopic ventral hernia repair (IPOM plus) in medium and large defects. Long-term follow-up. Surg. Endosc. 2023, 37, 2078–2084. [Google Scholar] [CrossRef]
- Sanchez, V.M.; Abi-Haidar, Y.E.; Itani, K.M. Mesh infection in ventral incisional hernia repair: Incidence, contributing factors, and treatment. Surg. Infect. 2011, 12, 205–210. [Google Scholar] [CrossRef]
- Al Chalabi, H.; Larkin, J.; Mehigan, B.; McCormick, P. A systematic review of laparoscopic versus open abdominal incisional hernia repair, with meta-analysis of randomized controlled trials. Int. J. Surg. 2015, 20, 65–74. [Google Scholar] [CrossRef]
- Awaiz, A.; Rahman, F.; Hossain, M.B.; Yunus, R.M.; Khan, S.; Memon, B.; Memon, M.A. Meta-analysis and systematic review of laparoscopic versus open mesh repair for elective incisional hernia. Hernia 2015, 19, 449–463. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Allen, V.B. Wound drains after incisional hernia repair. Cochrane Database Syst. Rev. 2013, CD005570. [Google Scholar] [CrossRef]
- Louis, V.; Diab, S.; Villemin, A.; Brigand, C.; Manfredelli, S.; Delhorme, J.B.; Rohr, S.; Romain, B. Do surgical drains reduce surgical site occurrence and infection after incisional hernia repair with sublay mesh? A non-randomised pilot study. Hernia 2023, 27, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Mohamedahmed, A.Y.Y.; Zaman, S.; Ghassemi, N.; Ghassemi, A.; Wuheb, A.A.; Abdalla, H.E.E.; Hajibandeh, S.; Hajibandeh, S. Should routine surgical wound drainage after ventral hernia repair be avoided? A systematic review and meta-analysis. Hernia 2023, 27, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Willemin, M.; Schaffer, C.; Kefleyesus, A.; Dayer, A.; Demartines, N.; Schafer, M.; Allemann, P. Drain Versus No Drain in Open Mesh Repair for Incisional Hernia, Results of a Prospective Randomized Controlled Trial. World J. Surg. 2023, 47, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Sahm, M.; Pross, M.; Hukauf, M.; Adolf, D.; Kockerling, F.; Mantke, R. Drain versus no drain in elective open incisional hernia operations: A registry-based analysis with 39,523 patients. Hernia 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Weber, D.G.; Ruppe, E.; Bassetti, M.; Wright, B.J.; Ansaloni, L.; Catena, F.; Coccolini, F.; Abu-Zidan, F.M.; Coimbra, R.; et al. Antimicrobials: A global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J. Emerg. Surg. 2016, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Cohen Tervaert, J.W. Autoinflammatory/autoimmunity syndrome induced by adjuvants (Shoenfeld’s syndrome) in patients after a polypropylene mesh implantation. Best. Pract. Res. Clin. Rheumatol. 2018, 32, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Jisova, B.; Wolesky, J.; Strizova, Z.; de Beaux, A.; East, B. Autoimmunity and hernia mesh: Fact or fiction? Hernia 2023, 27, 741–749. [Google Scholar] [CrossRef]
- Kowalik, C.R.; Zwolsman, S.E.; Malekzadeh, A.; Roumen, R.M.H.; Zwaans, W.A.R.; Roovers, J. Are polypropylene mesh implants associated with systemic autoimmune inflammatory syndromes? A systematic review. Hernia 2022, 26, 401–410. [Google Scholar] [CrossRef]
- Dong, Z.; Kujawa, S.A.; Wang, C.; Zhao, H. Does the use of hernia mesh in surgical inguinal hernia repairs cause male infertility? A systematic review and descriptive analysis. Reprod. Health 2018, 15, 69. [Google Scholar] [CrossRef]
- Warren, J.A.; Love, M.; Cobb, W.S.; Beffa, L.R.; Couto, F.J.; Hancock, B.H.; Morrow, D.; Ewing, J.A.; Carbonell, A.M. Factors affecting salvage rate of infected prosthetic mesh. Am. J. Surg. 2020, 220, 751–756. [Google Scholar] [CrossRef]
- Bueno-Lledo, J.; Torregrosa-Gallud, A.; Carreno-Saenz, O.; Garcia-Pastor, P.; Carbonell-Tatay, F.; Bonafe-Diana, S.; Iserte-Hernandez, J. Partial versus complete removal of the infected mesh after abdominal wall hernia repair. Am. J. Surg. 2017, 214, 47–52. [Google Scholar] [CrossRef]
- Engelsman, A.F.; van der Mei, H.C.; Ploeg, R.J.; Busscher, H.J. The phenomenon of infection with abdominal wall reconstruction. Biomaterials 2007, 28, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Leber, G.E.; Garb, J.L.; Alexander, A.I.; Reed, W.P. Long-term complications associated with prosthetic repair of incisional hernias. Arch. Surg. 1998, 133, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Houck, J.P.; Rypins, E.B.; Sarfeh, I.J.; Juler, G.L.; Shimoda, K.J. Repair of incisional hernia. Surg. Gynecol. Obstet. 1989, 169, 397–399. [Google Scholar] [PubMed]
- Ober, I.; Stulenau, T.; Ball, C.g.; Nickerson, D.; Kirkpatrick, A.W. It all doesn’t have to go: Abdominal wall reconstruction involving selective synthethic mesh explantation with bioilogic mesh salvage. Can. J. Surg. 2023, in press. [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Shao, X.; Cheng, T. The salvage of mesh infection after hernia repair with the use of negative pressure wound therapy (NPWT), a systematic review. ANZ J. Surg. 2022, 92, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Amid, P.K. Radiologic images of meshoma: A new phenomenon causing chronic pain after prosthetic repair of abdominal wall hernias. Arch. Surg. 2004, 139, 1297–1298. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B.; Muller, M.; Schumpelick, V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur. J. Surg. = Acta Chir. 1999, 165, 665–673. [Google Scholar] [CrossRef]
- Rosch, R.; Junge, K.; Schachtrupp, A.; Klinge, U.; Klosterhalfen, B.; Schumpelick, V. Mesh implants in hernia repair. Inflammatory cell response in a rat model. Eur. Surg. Res. 2003, 35, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Coccolini, F.; Kluger, Y.; Agastra, E.; Abu-Zidan, F.M.; Abbas, A.E.S.; Ansaloni, L.; Adesunkanmi, A.K.; Atanasov, B.; Augustin, G.; et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J. Emerg. Surg. 2021, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, H.; Liu, J.M.; Wang, X.; Cui, Y.F.; Lu, Z.Y. Mesh erosion into the colon following repair of parastomal hernia: A case report. World J. Gastrointest. Surg. 2023, 15, 294–302. [Google Scholar] [CrossRef] [PubMed]
- East, B.; Hill, S.; Dames, N.; Blackwell, S.; Laidlaw, L.; Gok, H.; Stabilini, C.; de Beaux, A. Patient Views Around Their Hernia Surgery: A Worldwide Online Survey Promoted Through Social Media. Front. Surg. 2021, 8, 769938. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Stokes, R.G.; Arndt, T. The Thalidomide Catastrophe: How it Hapened, Who Was Responsible and Why the Search for Justice Continues after More Than Six Decades; Onwards and Upwards Publishers: Cranbrook, UK, 2018. [Google Scholar]
- Wikepedia. Big Pharma Conspiracy Theories. Available online: https://en.wikipedia.org/wiki/Big_Pharma_conspiracy_theories (accessed on 28 August 2022).
- Darrow, J.J.; Dhruva, S.S.; Redberg, R.F. Changing FDA Approval Standards: Ethical Implications for Patient Consent. J. Gen. Intern. Med. 2021, 36, 3212–3214. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Minor, S.; Coccolini, F. Why is there no data? Critically ill patients deserve better protection from both Regulatory Authorities and Surgeons. J. Trauma Acute Care Surg. 2023, 95, e61–e62. [Google Scholar] [CrossRef] [PubMed]
- Zargar, N.; Carr, A. The regulatory ancestral network of surgical meshes. PLoS ONE 2018, 13, e0197883. [Google Scholar] [CrossRef]
- Dietrich, E.M.; Sharfstein, J.M. Improving medical device regulation: A work in progress. JAMA Intern. Med. 2014, 174, 1779–1780. [Google Scholar] [CrossRef]
- Dubin, J.R.; Simon, S.D.; Norrell, K.; Perera, J.; Gowen, J.; Cil, A. Risk of Recall Among Medical Devices Undergoing US Food and Drug Administration 510(k) Clearance and Premarket Approval, 2008–2017. JAMA Netw Open 2021, 4, e217274. [Google Scholar] [CrossRef]
- Curfman, G.D.; Redberg, R.F. Medical devices—Balancing regulation and innovation. N. Engl. J. Med. 2011, 365, 975–977. [Google Scholar] [CrossRef]
- Ashar, B.S.; Dang, J.M.; Krause, D.; Luke, M.C. Performing clinical studies involving hernia mesh devices: What every investigator should know about the FDA investigational device exemption (IDE) process. Hernia 2011, 15, 603–605. [Google Scholar] [CrossRef]
- Shah, P.; Olavarria, O.; Dhanani, N.; Ciomperlik, H.; Mohr, C.; Bernardi, K.; Neela, N.; Coelho, R.; Ali, Z.; Prabhu, A.; et al. The Food and Drug Administration’s (FDA’s) 510(k) Process: A Systematic Review of 1000 Cases. Am. J. Med. 2023, 136, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Center for Devices and Radiological Health Food and Drug Administration. Premarket Notification 510(k). Available online: www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-notification-510k (accessed on 28 December 2023).
- Kahan, L.G.; Blatnik, J.A. Critical Under-Reporting of Hernia Mesh Properties and Development of a Novel Package Label. J. Am. Coll. Surg. 2018, 226, 117–125. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Medical Device Recalls. 2023. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?start_search=1&event_id=&productdescriptiontxt=kugel&productcode=&IVDProducts=&rootCauseText=&recallstatus=%C2%A2erclassificationtypetext=&recallnumber=&postdatefrom=&postdateto=&productshortreasontxt=&firmlegalnam=&PMA_510K_Num=&pnumber=&knumber=&sortcolumn=cda (accessed on 24 August 2023).
- Turner, T.; Miller, E.K. Kugel Patch. 2022. Available online: https://www.drugwatch.com/hernia-mesh/kugel-patch/ (accessed on 24 August 2023).
- Yasuda, A.; Yasuda, T.; Kato, H.; Iwama, M.; Shiraishi, O.; Hiraki, Y.; Tanaka, Y.; Shinkai, M.; Imano, M.; Kimura, Y.; et al. A case of incisional hernia repair using Composix mesh prosthesis after antethoracic pedicled jejunal flap reconstruction following an esophagectomy. Surg. Case Rep. 2017, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Aramaki, O.; Yoshida, N.; Mitsuka, Y.; Kawai, T.; Yamazaki, S.; Kang, W.; Nakayama, H.; Moriguchi, M.; Higaki, T.; et al. Laparoscopic-assisted modified Kugel herniorrhaphy for obturator hernia: A case report. J. Surg. Case Rep. 2022, 2022, rjac035. [Google Scholar] [CrossRef] [PubMed]
- Miller, P. Hernia Mesh Complications: Allergy to Mesh and The Bodies’ Response. 2017. Available online: https://herniameshlawsuit.ca/article/hernia-mesh-complications-allergy-mesh-bodies-response/ (accessed on 24 August 2023).
- Favaro, A.; St Phillip, E. Growing Concerns in Canada Over Surgical Mesh Usage, Recalls: CTV News. 2017. Available online: https://www.ctvnews.ca/health/growing-concerns-in-canada-over-surgical-mesh-usage-recalls-1.3372733#:~:text=Figures%20from%20Health%20Canada%20show%20that%20some%2012,serious%20injury%20and%20other%20complications%2C%20including%20three%20deaths (accessed on 24 August 2023).
- Collinson, R.; Furst, J. Hernia Mesh Implants Used ‘With No Clinical Evidence’: British Broadcasting Network. 2020. Available online: https://www.bbc.com/news/health-51024974?fbclid=IwAR3YX7TvYt30kiwsJ__QnClyYFshUZLIuzQRqZOFF1oHyiiWnHIaR7rGDeo (accessed on 24 August 2023).

| Location | Posterior Structures | Anterior Structures | Location-Pros | Location-Cons |
|---|---|---|---|---|
| Intraperitoneal | Peritoneal cavity | Peritoneum | Biomechanically strong | Adjacent to viscera Inaccessible if infected |
| Preperitoneal | Peritoneum | Transversalis fascia | Biomechanically strong | Potentially adjacent to viscera (peritoneal defect) |
| Retrorectus | Posterior Rectus Sheath | Rectus Abdominus Muscle | Biomechanically strong | Limited width of mesh (except TAR 1 uses very large mesh) |
| Inlay | Mesh inlaid between edges of hernia defect with no overlap | Subcutaneous tissue | None | Adjacent to Viscera Biochanically very weak |
| Onlay | Anterior rectus sheath and External oblique | Subcutaneous tissue | Accessible to local salvage therapies in case of infection Distant from viscera | Less biomechanically strong |
| Minor |
| Seroma |
| Hematoma |
| Recurrent pain |
| Surgical site infection |
| Not-validated |
| Autoimmune reactions |
| Male infertility |
| Major |
| Hernia recurrence |
| Complication of subsequent surgery |
| Adhesive bowel obstruction |
| Mesh contraction |
| Mesh infection |
| Enteroprosthethic fistula |
| Enterocutaneous fistula |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkpatrick, A.W.; Coccolini, F.; Tolonen, M.; Minor, S.; Catena, F.; Celotti, A.; Gois, E., Jr.; Perrone, G.; Novelli, G.; Garulli, G.; et al. Are Surgeons Going to Be Left Holding the Bag? Incisional Hernia Repair and Intra-Peritoneal Non-Absorbable Mesh Implant Complications. J. Clin. Med. 2024, 13, 1005. https://doi.org/10.3390/jcm13041005
Kirkpatrick AW, Coccolini F, Tolonen M, Minor S, Catena F, Celotti A, Gois E Jr., Perrone G, Novelli G, Garulli G, et al. Are Surgeons Going to Be Left Holding the Bag? Incisional Hernia Repair and Intra-Peritoneal Non-Absorbable Mesh Implant Complications. Journal of Clinical Medicine. 2024; 13(4):1005. https://doi.org/10.3390/jcm13041005
Chicago/Turabian StyleKirkpatrick, Andrew W., Federico Coccolini, Matti Tolonen, Samual Minor, Fausto Catena, Andrea Celotti, Emanuel Gois, Jr., Gennaro Perrone, Giuseppe Novelli, Gianluca Garulli, and et al. 2024. "Are Surgeons Going to Be Left Holding the Bag? Incisional Hernia Repair and Intra-Peritoneal Non-Absorbable Mesh Implant Complications" Journal of Clinical Medicine 13, no. 4: 1005. https://doi.org/10.3390/jcm13041005