Impact of Brightness on Choroidal Vascularity Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Patients
2.2. Clinical and Instrumental Examination
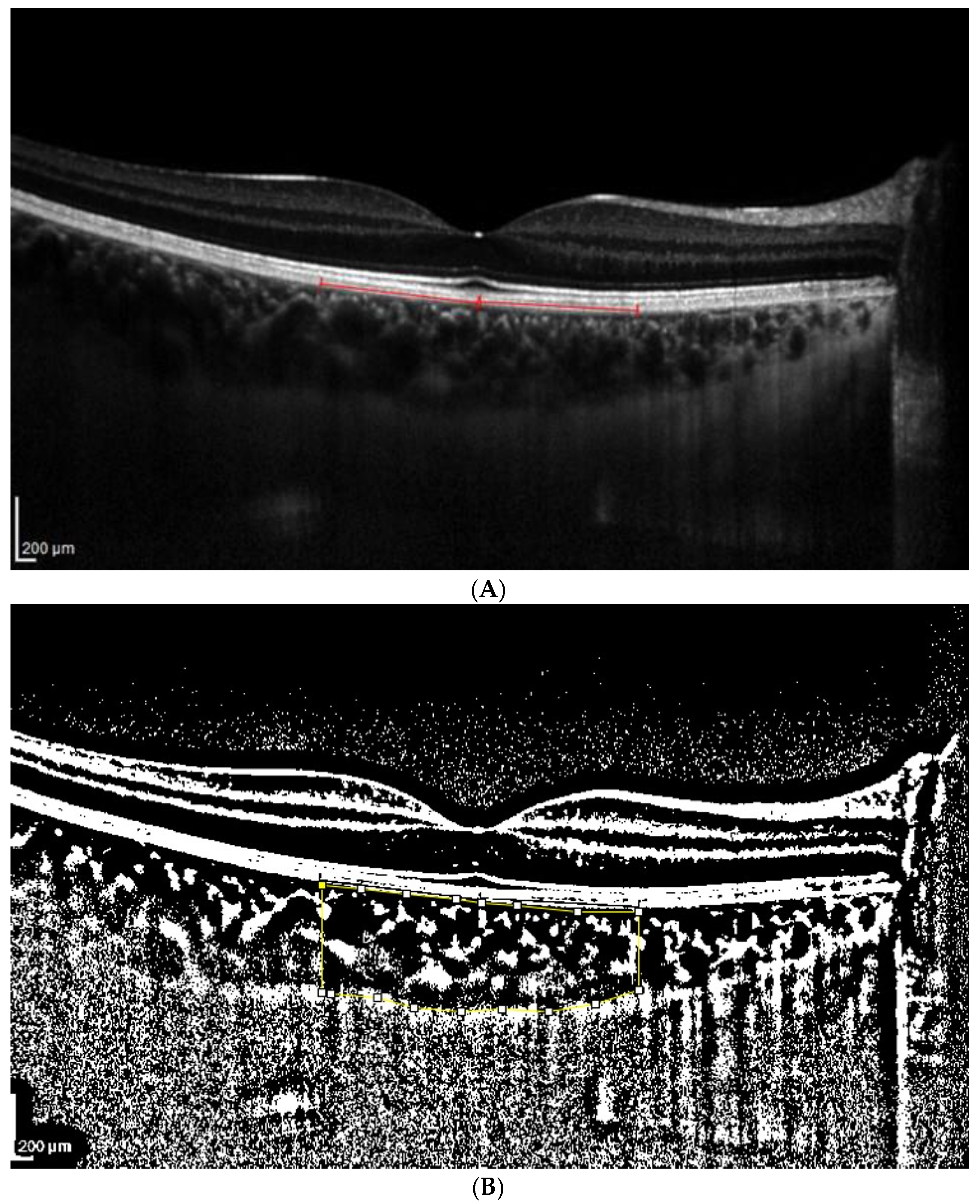
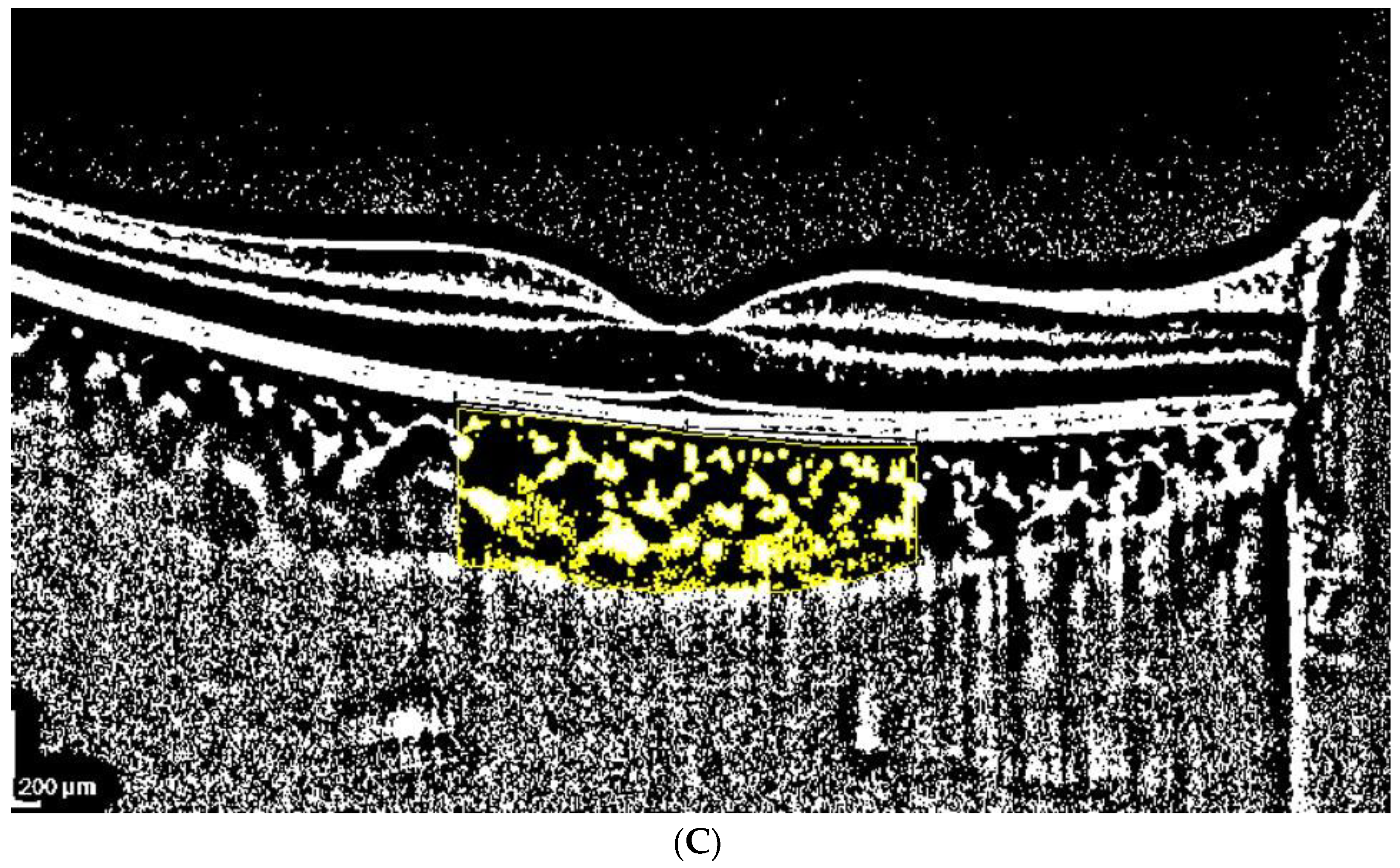
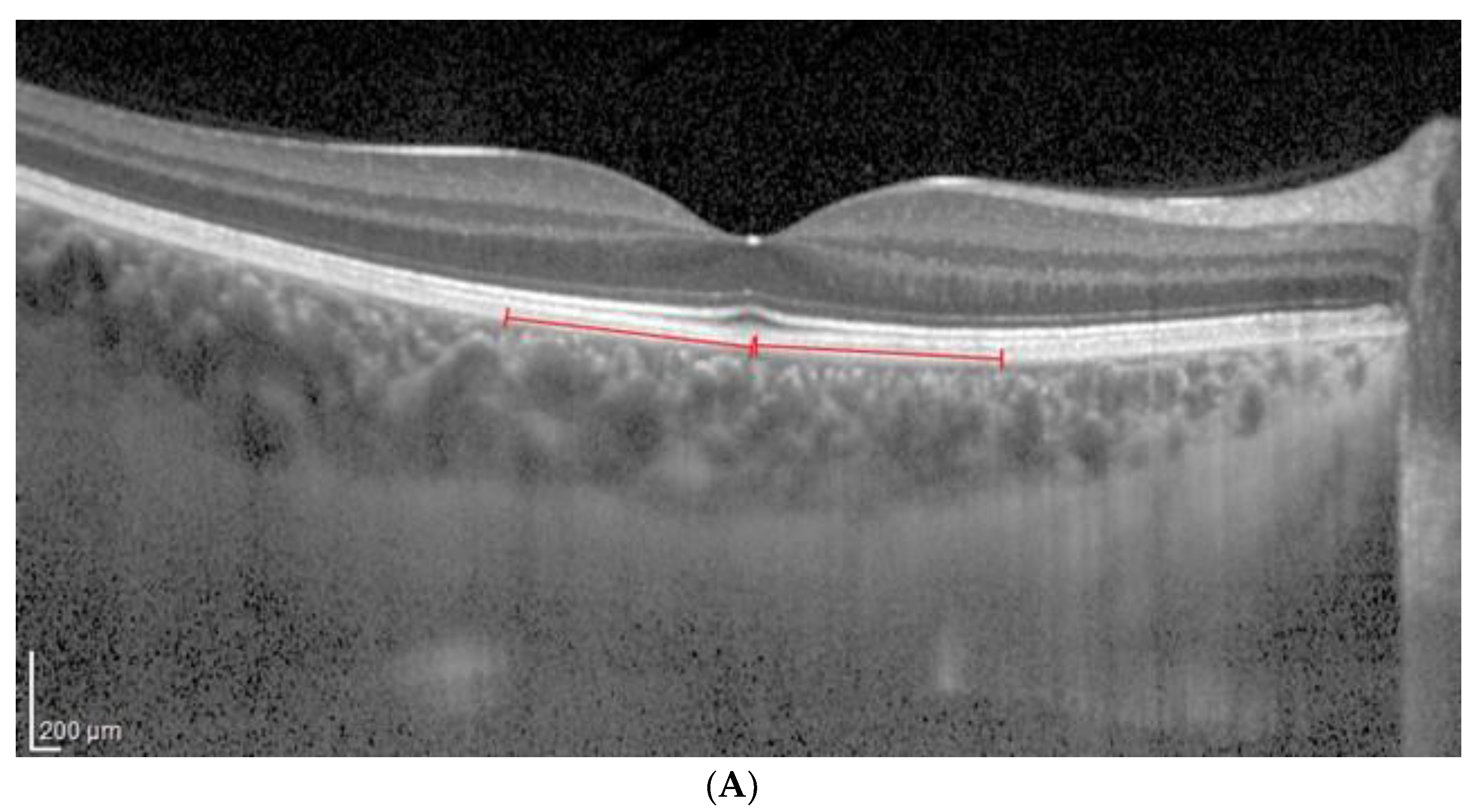
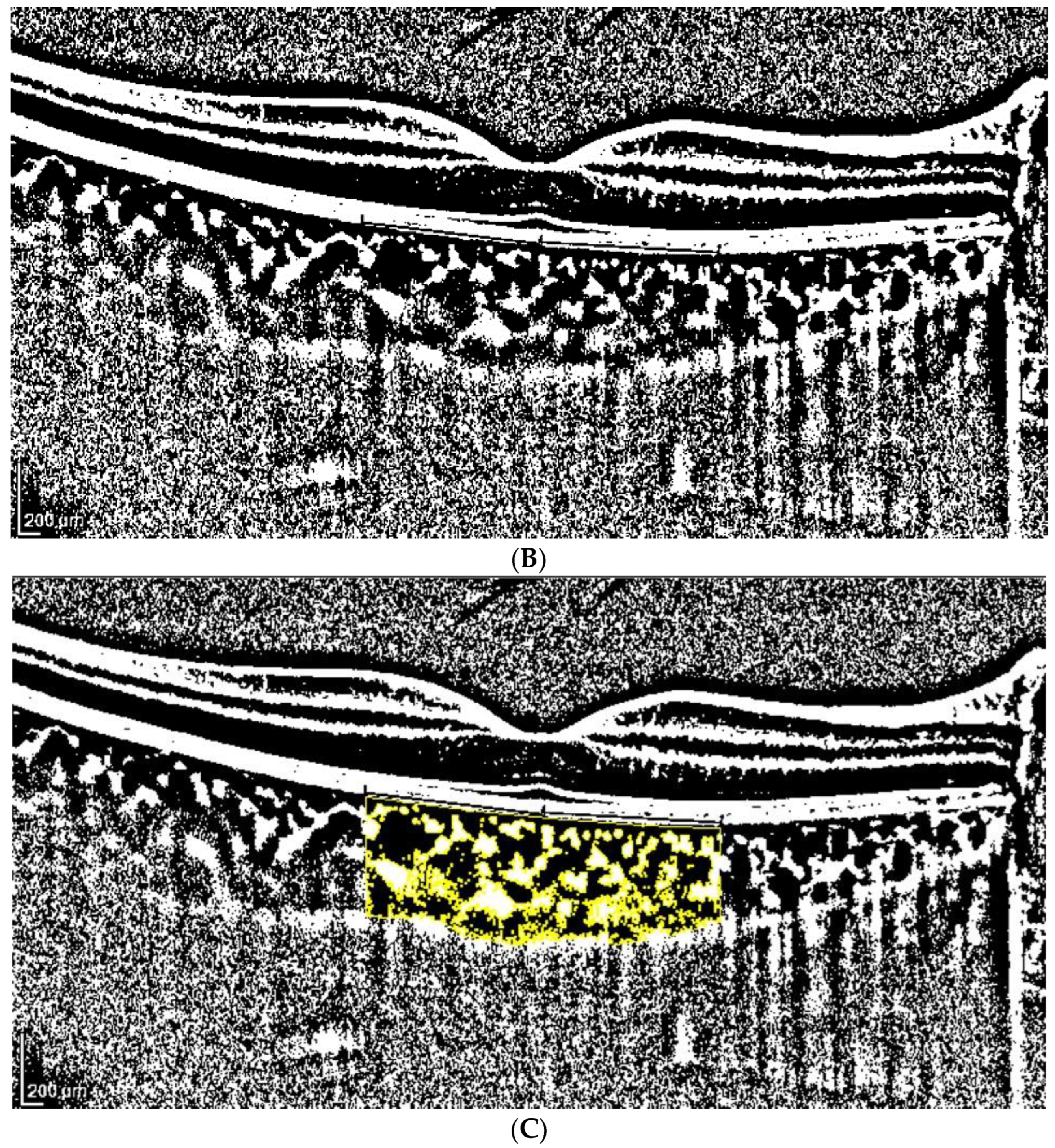
2.3. OCT Analysis
- A classic manual tracking method, manually drawing the lines to limit the choroidal area for each high and low brightness level [2].
- An alternative method, with a fixed area selection independent of the brightness level.
2.4. Statistical Analysis
3. Results
4. Discussion
- Image binarization techniques use special algorithms such as Niblack, which can isolate vascular structures. In reality, there is no certainty that the darker areas of OCT images correspond to the luminal component and the lighter areas to the stromal component, although the literature supporting this hypothesis is robust [53].
- So far, in the literature, no clear evidence exists that the blooming effect could be present in OCT images, considering the different nature of the signal from ultrasound, which uses an ultrasonic signal, while OCT uses interferometry [52].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, J.; Marziliano, P.; Baskaran, M.; Tun, T.A.; Aung, T. Automatic segmentation of the choroid in enhanced depth imaging optical coherence tomography images. Biomed. Opt. Express 2013, 4, 397–411. [Google Scholar] [CrossRef]
- Agrawal, R.; Gupta, P.; Tan, K.A.; Cheung, C.M.; Wong, T.Y.; Cheng, C.Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Kang, S.W.; Lee, J.H.; Kim, Y.T. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 2011, 118, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Gomi, F.; Tano, Y. Polypoidal choroidal vasculopathy and treatments. Curr. Opin. Ophthalmol. 2008, 19, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Forster, T.M.; Steinmetz, P.; Schlichtenbrede, F.C.; Harder, B.C. Choroidal thickness in age-related macular degeneration. Retina 2014, 34, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina 2018, 38, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Sabrosa, A.S.; Duker, J.S.; Waheed, N.K. Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: A review. Eye Vis. 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, B.; Kang, E.; Oh, J. Clustering of eyes with age-related macular degeneration or pachychoroid spectrum diseases based on choroidal thickness profile. Sci. Rep. 2021, 11, 4999. [Google Scholar] [CrossRef]
- Koizumi, H.; Yamagishi, T.; Yamazaki, T.; Kawasaki, R.; Kinoshita, S. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1123–1128. [Google Scholar] [CrossRef]
- Fujiwara, T.; Imamura, Y.; Margolis, R.; Slakter, J.S.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am. J. Ophthalmol. 2009, 148, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Gao, X.; Qian, N.; Zhuo, Y. Choroidal thickness and high myopia: A cross-sectional study and meta-analysis. BMC Ophthalmol. 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Wang, W.; Gong, X.; Ji, Y.; Guo, X.; Yuan, M.; Li, W.; Liang, X.; Huang, W.; Wen, F. Influence of high myopia on choriocapillaris perfusion and choroidal thickness in diabetic patients without diabetic retinopathy. Retina 2022, 42, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Przybyś, M.; Brydak-Godowska, J.; Kęcik, D. Subfoveal Choroidal Thickness and Its Intereye Differences in Fuchs Uveitis Syndrome Evaluated Using Optical Coherent Tomography. Ocul. Immunol. Inflamm. 2021, 29, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, R.Y.; Park, Y.H. Choroidal Vascularity Index and Choroidal Thickness in Human Leukocyte Antigen-B27-Associated Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, A.; Loza, E.; Rosario, M.P.; Espinosa, G.; de Morales, J.M.G.R.; Herrera, J.M.; Muñoz-Fernández, S.; Rodríguez-Rodríguez, L.; Cordero-Coma, M.; Spanish Society of Ocular Inflammation (SEIOC). Efficacy and safety of immunomodulatory drugs in patients with non-infectious intermediate and posterior uveitis, panuveitis and macular edema: A systematic literature review. Semin. Arthritis Rheum. 2020, 50, 1299–1306. [Google Scholar] [CrossRef]
- Montorio, D.; Giordano, M.; Concilio, M.; Cennamo, G. Structural and Vascular Changes of the Choroid in Polypoidal Choroidal Vasculopathy after Intravitreal Anti-VEGF Therapy. Ophthalmologica 2022, 245, 173–178. [Google Scholar] [CrossRef]
- Kumar, J.B.; Wai, K.M.; Ehlers, J.P.; Singh, R.P.; Rachitskaya, A.V. Subfoveal choroidal thickness as a prognostic factor in exudative age-related macular degeneration. Br. J. Ophthalmol. 2019, 103, 918–921. [Google Scholar] [CrossRef]
- Bouteleux, V.; Kodjikian, L.; Mendes, M.; Agard, E.; Machkour-Bentaleb, Z.; El-Chehab, H.; Denis, P.; Mathis, T.; Dot, C. Increased choroidal thickness: A new feature to monitor age-related macular degeneration recurrence. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 699–707. [Google Scholar] [CrossRef]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Wang, W.; Zhang, J.; Chen, Y.; Bulloch, G.; Yuan, Y.; Zhang, S.; Xuan, M.; et al. Longitudinal Changes and Predictive Value of Choroidal Thickness for Myopia Control after Repeated Low-Level Red-Light Therapy. Ophthalmology 2023, 130, 286–296. [Google Scholar] [CrossRef]
- Maruko, I.; Iida, T.; Sugano, Y.; Oyamada, H.; Sekiryu, T.; Fujiwara, T.; Spaide, R.F. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina 2011, 31, 510–517. [Google Scholar] [CrossRef]
- Nishisho, R.; Kusuhara, S.; Sotani, N.; Kim, K.W.; Katsuyama-Yoshikawa, A.; Matsumiya, W.; Akashi, K.; Morinobu, A.; Nakamura, M. Changes in choroidal imaging parameters following adalimumab therapy for refractory noninfectious uveitis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Han, X.; Wu, H.; Huang, W. Choroidal thickness changes in obstructive sleep apnea syndrome: A systematic review and meta-analysis. Sleep Breath. 2016, 20, 369–378. [Google Scholar] [CrossRef]
- Wu, C.Y.; Riangwiwat, T.; Rattanawong, P.; Nesmith, B.L.W.; Deobhakta, A. Association of obstructive sleep apnea with central serous chorioretinopathy and choroidal thickness: A Systematic Review and Meta-Analysis. Retina 2018, 38, 1642–1651. [Google Scholar] [CrossRef]
- Li, S.; Lang, X.; Wang, W.; Yang, Y.; Wang, J.; Li, H.; Wang, Y.; Wang, K. Choroidal vascular changes in internal carotid artery stenosis: A retrospective cohort study in Chinese population. BMC Ophthalmol. 2019, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.E.; Kim, S.H.; Choi, B.W.; Rim, T.H.; Byeon, S.H.; Kim, S.S. Relationship between Coronary Artery Calcification and Central Chorioretinal Thickness in Patients with Subclinical Atherosclerosis. Ophthalmologica 2021, 244, 18–26. [Google Scholar] [CrossRef]
- Wei, X.; Kumar, S.; Ding, J.; Khandelwal, N.; Agarwal, M.; Agrawal, R. Choroidal Structural Changes in Smokers Measured Using Choroidal Vascularity Index. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Fryczkowski, A.W.; Sato, S.E.; Hodes, B.L. Changes in the diabetic choroidal vasculature: Scanning electron microscopy findings. Ann. Ophthalmol. 1988, 20, 299–305. [Google Scholar]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Lindner, M.; Bezatis, A.; Czauderna, J.; Becker, E.; Brinkmann, C.K.; Schmitz-Valckenberg, S.; Fimmers, R.; Holz, F.G.; Fleckenstein, M. Choroidal thickness in geographic atrophy secondary to age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 875–882. [Google Scholar] [CrossRef]
- Young, M.; Fallah, N.; Forooghian, F. Choroidal degeneration in birdshot chorioretinopathy. Retina 2015, 35, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Lee, D.H.; Joe, S.G.; Kim, J.G.; Yoon, Y.H. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Altieri, V.; Coppola, A.; Gioia, M.; Rosa, N. Choroidal evaluation in patients under alpha-lytic therapy. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Battipaglia, M.; Mascolo, F.; Iovino, C.; Capasso, L.; Ciacci, C.; Rosa, N. Choroidal structural evaluation in celiac disease. Sci. Rep. 2021, 11, 16398. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, F.E.; Altan, C.; Kesim, C.; Demircan, A.; Tunç, U.; Demir, G.; Taskapılı, M. Choroidal vascularity index as an indicator of vascular status of choroid, in eyes with nanophthalmos. Eye 2020, 34, 2336–2340. [Google Scholar] [CrossRef]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Uchino, E.; Kawano, H.; Yoshihara, N.; Terasaki, H.; Shirasawa, M.; Tomita, M.; Ishibashi, T. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am. J. Ophthalmol. 2015, 159, 1123–1131.e1. [Google Scholar] [CrossRef]
- De Bernardo, M.; Cione, F.; Capasso, L.; Coppola, A.; Rosa, N. A formula to improve the reliability of optical axial length measurement in IOL power calculation. Sci. Rep. 2022, 12, 18845. [Google Scholar] [CrossRef]
- Lin, Z.; Yu, H.; Shi, C.; Chen, H.; Lin, G.; Shen, M.; Wang, C. Acute hyperglycemia compromises the responses of choroidal vessels using swept-source optical coherence tomography during dark and light adaptations. Front. Endocrinol. 2023, 14, 1049326. [Google Scholar] [CrossRef]
- Di Pippo, M.; Santia, C.; Rullo, D.; Ciancimino, C.; Grassi, F.; Abdolrahimzadeh, S. The Choroidal Vascularity Index Versus Optical Coherence Tomography Angiography in the Evaluation of the Choroid with a Focus on Age-Related Macular Degeneration. Tomography 2023, 9, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.; De Bernardo, M.; Abbinante, G.; Vecchio, G.; Capasso, L.; Cione, F. Optic Nerve Drusen Evaluation: A Comparison between Ultrasound and OCT. J. Clin. Med. 2022, 11, 3715. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Optic Nerve Evaluation in Idiopathic Intracranial Hypertension. AJNR Am. J. Neuroradiol. 2019, 40, E36. [Google Scholar] [CrossRef] [PubMed]
- De Bernardo, M.; Vitiello, L.; Rosa, N. Optic nerve ultrasonography for evaluating increased intracranial pressure in severe preeclampsia. Int. J. Obstet. Anesth. 2019, 38, 147. [Google Scholar] [CrossRef]
- De Bernardo, M.; Vitiello, L.; Cornetta, P.; Rosa, N. Ocular ultrasound evaluation of optic nerve sheath diameter in military environments. Mil. Med. Res. 2019, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Cornetta, P.; Marotta, G.; De Bernardo, M.; Vitiello, L.; Rosa, N. Ultrasound and optic neuritis. Am. J. Emerg. Med. 2019, 37, 1598. [Google Scholar] [CrossRef] [PubMed]
- Ossoinig, K.C. Quantitative echography—The basis of tissue differentiation. J. Clin. Ultrasound. 1974, 2, 33–46. [Google Scholar] [CrossRef]
- Wei, X.; Sonoda, S.; Mishra, C.; Khandelwal, N.; Kim, R.; Sakamoto, T.; Agrawal, R. Comparison of choroidal vascularity markers on optical coherence tomography using two-image binarization techniques. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1206–1211. [Google Scholar] [CrossRef]
- Liu, S.; Du, L.; Zhou, Q.; Zhang, Q.; Hu, K.; Qi, J.; Liang, L.; Zhou, C.; Kijlstra, A.; Yang, P. The Choroidal Vascularity Index Decreases and Choroidal Thickness Increases in Vogt-Koyanagi-Harada Disease Patients during a Recurrent Anterior Uveitis Attack. Ocul. Immunol. Inflamm. 2018, 26, 1237–1243. [Google Scholar] [CrossRef]
- Kawano, H.; Sonoda, S.; Yamashita, T.; Maruko, I.; Iida, T.; Sakamoto, T. Relative changes in luminal and stromal areas of choroid determined by binarization of EDI-OCT images in eyes with Vogt-Koyanagi-Harada disease after treatment. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 421–426. [Google Scholar] [CrossRef]
- Agrawal, R.; Li, L.K.H.; Nakhate, V.; Khandelwal, N.; Mahendradas, P. Choroidal vascularity index in Vogt-Koyanagi-Harada disease: An EDI-OCT derived tool for monitoring disease progression. Trans. Vis. Sci. Technol. 2016, 5, 7. [Google Scholar] [CrossRef]
- Jaisankar, D.; Raman, R.; Sharma, H.R.; Khandelwal, N.; Bhende, M.; Agrawal, R.; Sridharan, S.; Biswas, J. Choroidal and Retinal Anatomical Responses Following Systemic Corticosteroid Therapy in Vogt-Koyanagi-Harada Disease Using Swept-Source Optical Coherence Tomography. Ocul. Immunol. Inflamm. 2019, 27, 235–243. [Google Scholar] [CrossRef]
- Pellegrini, M.; Giannaccare, G.; Bernabei, F.; Moscardelli, F.; Schiavi, C.; Campos, E.C. Choroidal Vascular Changes in Arteritic and Nonarteritic Anterior Ischemic Optic Neuropathy. Am. J. Ophthalmol. 2019, 205, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Guduru, A.; Abdul Rasheed, M.; Goud, A.; Ashik, M.; Kumar, V.K.; Chhablani, J.; Badakere, A.; Kekunnaya, R.; Patil-Chhablani, P. Choroidal Vascularity in Non-arteritic Anterior Ischaemic Optic Neuropathy. Neuroophthalmology 2019, 43, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Agrawal, R. Author Response: Smokers’ Choroidal Changes. Investig. Ophthalmol. Vis. Sci. 2022, 63, 22. [Google Scholar] [CrossRef] [PubMed]
| TCA Low vs. High Brightness | ||||
| Low | High | |||
| TCA | TCA | Modification (%) | p | |
| Average | 3.52 | 3.54 | 0.87 | <0.681 |
| SD | 1.13 | 1.12 | 7.47 | |
| Median | 3.49 | 3.50 | −0.24 | |
| Min | 1.12 | 1.01 | −26.95 | |
| Max | 6.94 | 6.97 | 30.60 | |
| LCA Low vs. High Brightness | ||||
| Low | High | |||
| LCA | LCA | Modification (%) | p | |
| Average | 2.65 | 2.44 | −8.52 | <0.0001 * |
| SD | 0.78 | 0.69 | 5.01 | |
| Median | 2.65 | 2.43 | −8.09 | |
| Min | 0.86 | 0.78 | −39.46 | |
| Max | 4.86 | 4.49 | −0.14 | |
| SCA Low vs. High Brightness | ||||
| Low | High | |||
| SCA | SCA | Modification (%) | p | |
| Average | 0.87 | 1.10 | 28.71 | <0.0001 * |
| SD | 0.39 | 0.46 | 17.71 | |
| Median | 0.83 | 1.05 | 26.06 | |
| Min | 0.17 | 0.19 | 1.89 | |
| Max | 2.47 | 2.91 | 90.00 | |
| CVI Low vs. High Brightness | ||||
| Low | High | |||
| CVI | CVI | Modification (%) | p | |
| Average | 76.09 | 69.98 | −8.01 | <0.0001 * |
| SD | 4.49 | 4.45 | 2.82 | |
| Median | 75.52 | 69.33 | −8.01 | |
| Min | 64.43 | 56.66 | −17.13 | |
| Max | 86.57 | 83.38 | −0.38 | |
| TCA Low vs. High Brightness | ||||
| Low | High | |||
| TCA | TCA | Modification (%) | p | |
| Average | 3.52 | 3.52 | 0.0 | 0.500 |
| SD | 1.15 | 1.15 | 0.0 | |
| Median | 3.43 | 3.43 | 0.0 | |
| Min | 1.15 | 1.15 | 0.0 | |
| Max | 6.94 | 6.94 | 0.0 | |
| LCA Low vs. High Brightness | ||||
| Low | High | |||
| LCA | LCA | Modification (%) | p | |
| Average | 2.64 | 2.43 | −7.73 | <0.0001 * |
| SD | 0.78 | 0.72 | 2.82 | |
| Median | 2.60 | 2.40 | −7.91 | |
| Min | 0.90 | 0.89 | −19.49 | |
| Max | 4.86 | 4.63 | −0.79 | |
| SCA Low vs. High Brightness | ||||
| Low | High | |||
| SCA | SCA | Modification (%) | p | |
| Average | 0.88 | 1.08 | 26.32 | <0.0001 * |
| SD | 0.40 | 0.47 | 13.96 | |
| Median | 0.85 | 1.06 | 23.78 | |
| Min | 0.21 | 0.26 | 2.80 | |
| Max | 2.47 | 3.01 | 90.58 | |
| CVI Low vs. High Brightness | ||||
| Low | High | |||
| CVI | CVI | Modification (%) | p | |
| Average | 75.96 | 70.07 | −7.73 | <0.0001 * |
| SD | 4.74 | 4.61 | 2.82 | |
| Median | 75.50 | 69.42 | −7.91 | |
| Min | 64.43 | 56.65 | −19.49 | |
| Max | 88.92 | 80.41 | −0.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, N.; Gioia, M.; Orlando, R.; De Luca, M.; D’Aniello, E.; Fioretto, I.; Sannino, C.; De Bernardo, M. Impact of Brightness on Choroidal Vascularity Index. J. Clin. Med. 2024, 13, 1020. https://doi.org/10.3390/jcm13041020
Rosa N, Gioia M, Orlando R, De Luca M, D’Aniello E, Fioretto I, Sannino C, De Bernardo M. Impact of Brightness on Choroidal Vascularity Index. Journal of Clinical Medicine. 2024; 13(4):1020. https://doi.org/10.3390/jcm13041020
Chicago/Turabian StyleRosa, Nicola, Marco Gioia, Rachele Orlando, Martina De Luca, Eleonora D’Aniello, Isabella Fioretto, Ciro Sannino, and Maddalena De Bernardo. 2024. "Impact of Brightness on Choroidal Vascularity Index" Journal of Clinical Medicine 13, no. 4: 1020. https://doi.org/10.3390/jcm13041020





