Association between Surrogate Markers of Insulin Resistance and the Incidence of Colorectal Cancer in Korea: A Nationwide Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions of Key Variables
2.3. Definitions of Covariates
2.4. Statistics and Data Analysis
2.5. Ethics Statement
3. Results
3.1. Baseline Characteristics
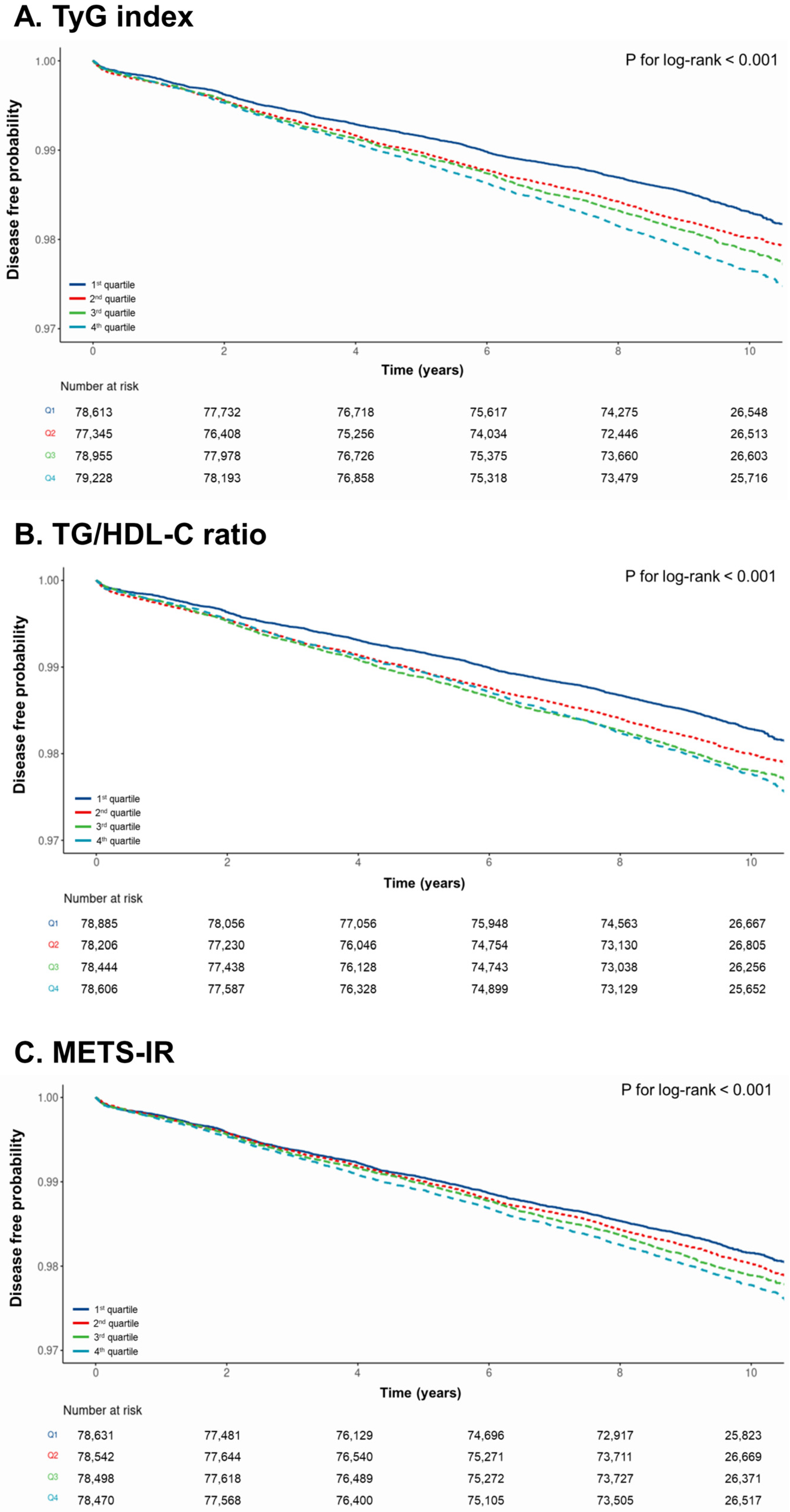
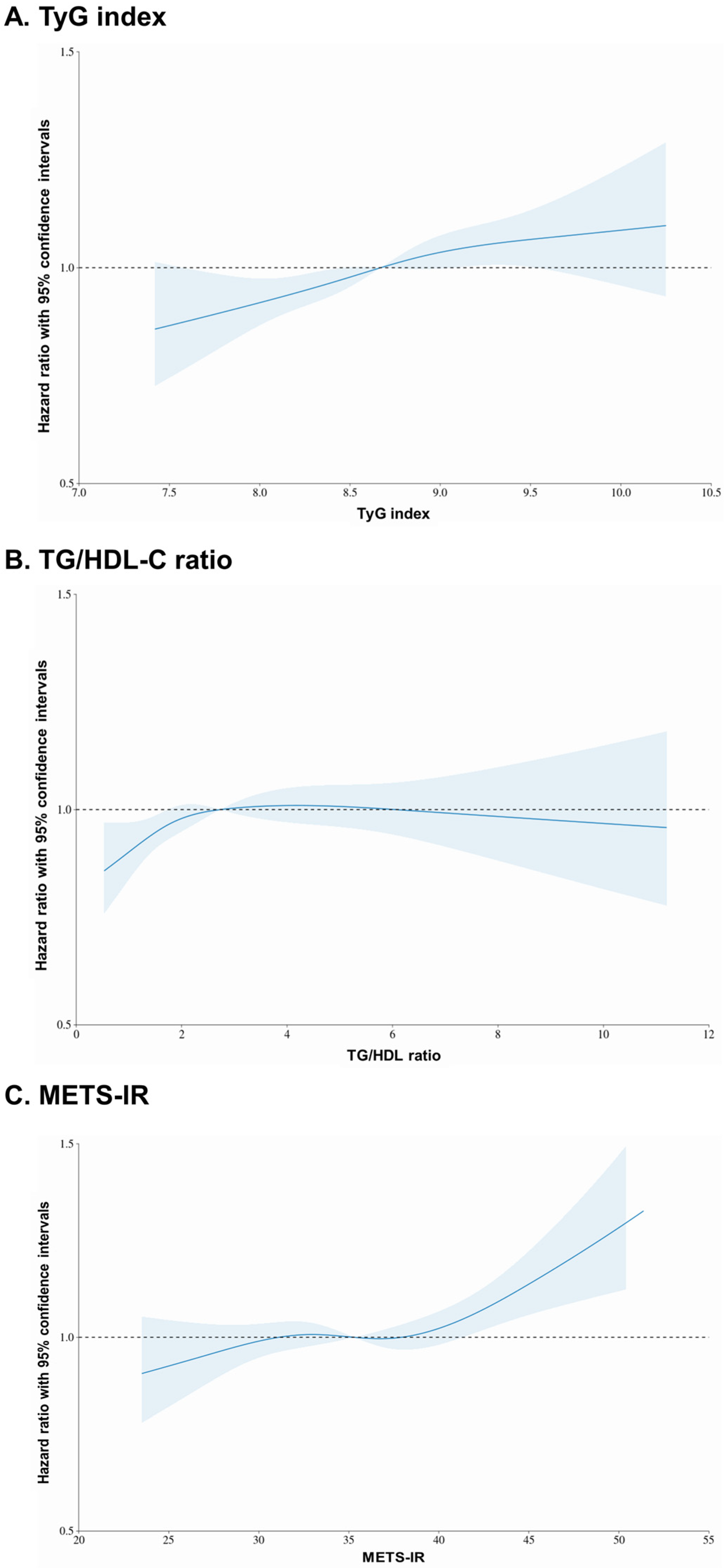
3.2. Incidence of CRC According to the TyG Index, TG/HDL-C Ratio, and METS-IR
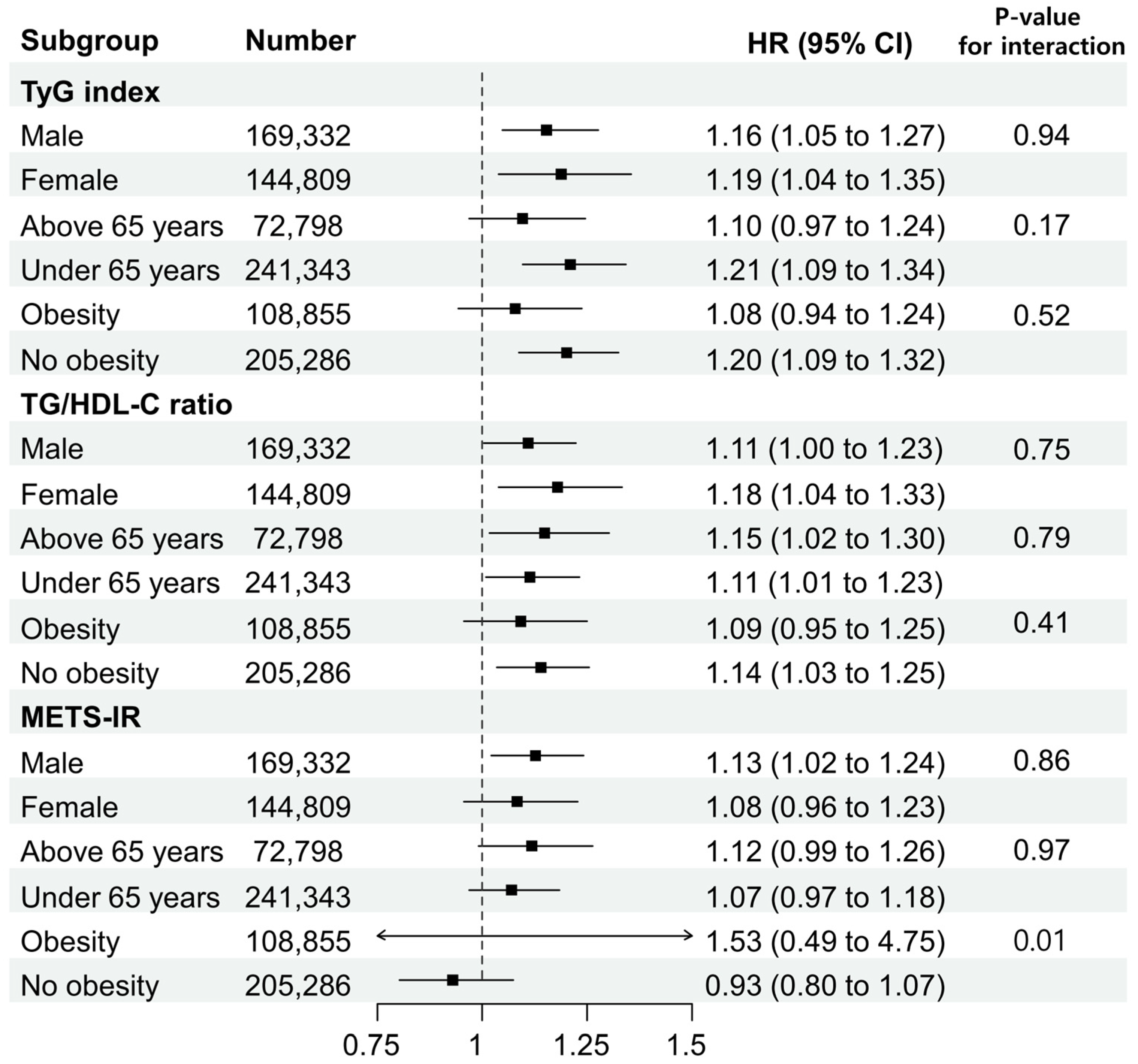
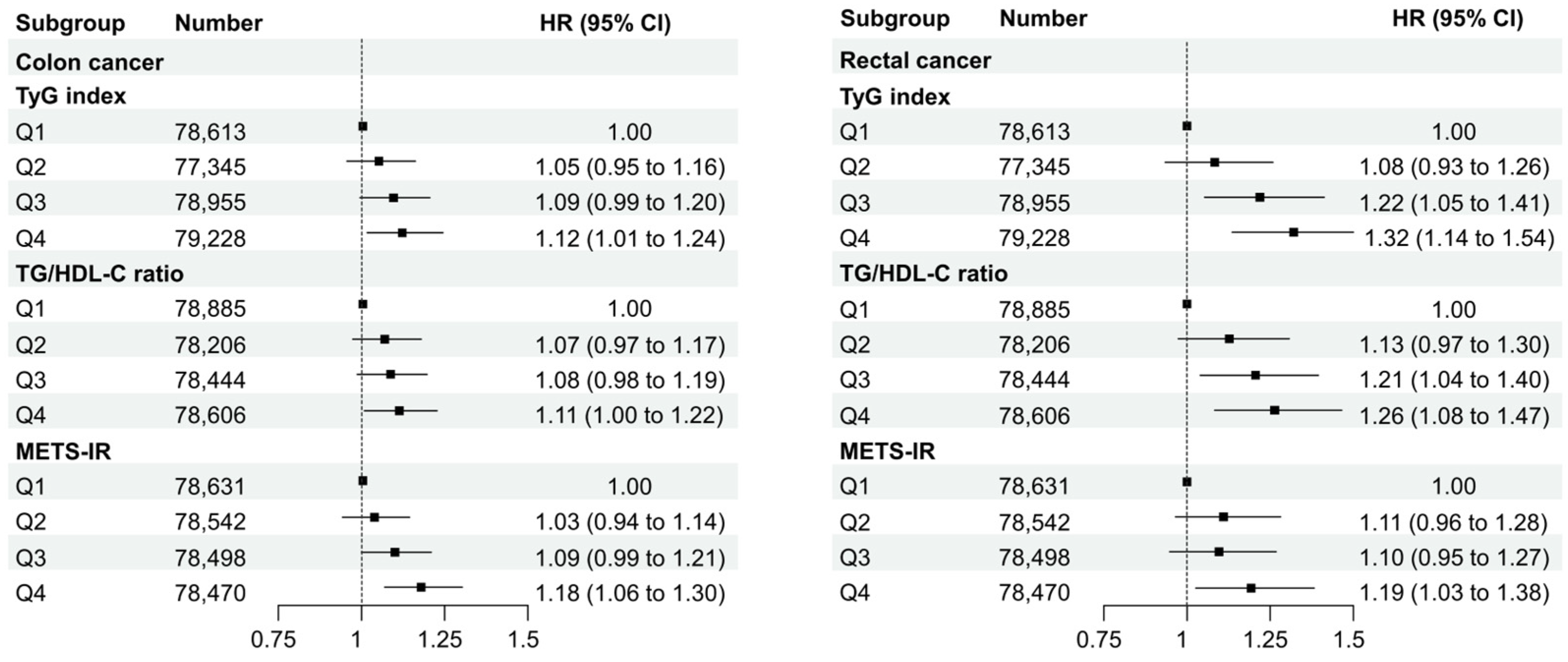
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Jenab, M.; Karimi, M.; Truong, T.; Mahamat-Saleh, Y.; Ferrari, P.; Dashti, S.G.; Kühn, T.; Cross, A.J.; Severi, G.; et al. Metabolic Syndrome and Risk of Gastrointestinal Cancers: An Investigation Using Large-scale Molecular Data. Clin. Gastroenterol. Hepatol. 2022, 20, e1338–e1352. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Gunathilake, M.; Lee, J.; Kim, J. Association between metabolic syndrome and its components and incident colorectal cancer in a prospective cohort study. Cancer 2022, 128, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 2014, 10, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Leitner, B.P.; Siebel, S.; Akingbesote, N.D.; Zhang, X.; Perry, R.J. Insulin and cancer: A tangled web. Biochem. J. 2022, 479, 583–607. [Google Scholar] [CrossRef]
- Giovannucci, E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am. J. Clin. Nutr. 2007, 86, 836S–842S. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Howard, B.V.; Wylie-Rosett, J.; Anderson, G.L.; Ho, G.Y.; et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008, 68, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Reaven, G.; Abbasi, F.; Lamendola, C.; Saad, M.; Waters, D.; Simon, J.; Krauss, R.M. Is There a Simple Way to Identify Insulin-Resistant Individuals at Increased Risk of Cardiovascular Disease? Am. J. Cardiol. 2005, 96, 399–404. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, H.T.; Shim, J.Y.; Lee, H.R. The association between the triglyceride to high-density lipoprotein cholesterol ratio with insulin resistance (HOMA-IR) in the general Korean population: Based on the National Health and Nutrition Examination Survey in 2007–2009. Diabetes Res. Clin. Pract. 2012, 97, 132–138. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Almeda-Valdes, P.; Gomez-Velasco, D.; Viveros-Ruiz, T.; Cruz-Bautista, I.; Romo-Romo, A.; Sánchez-Lázaro, D.; Meza-Oviedo, D.; Vargas-Vázquez, A.; Campos, O.A.; et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur. J. Endocrinol. 2018, 178, 533–544. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, H.; Zhang, Y.; Liu, L.; Zhang, W.; Xiong, W.; Lu, F.; Peng, J.; Yang, J. The impact of the metabolic score for insulin resistance on cardiovascular disease: A 10-year follow-up cohort study. J. Endocrinol. Investig. 2022, 46, 523–533. [Google Scholar] [CrossRef]
- Paublini, H.; González, A.A.L.; Busquets-Cortés, C.; Tomas-Gil, P.; Riutord-Sbert, P.; Ramírez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad and Scales That Assess Insulin Resistance. Nutrients 2023, 15, 2105. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, Z.; Zhang, Y.; Zhao, G.; Xu, B. The Association of Surrogates of Insulin Resistance with Hyperuricemia among Middle-Aged and Older Individuals: A Population-Based Nationwide Cohort Study. Nutrients 2023, 15, 3139. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Bjørge, T.; Nagel, G.; Manjer, J.; Engeland, A.; Häggström, C.; Concin, H.; Teleka, S.; Tretli, S.; Gylling, B.; et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int. J. Epidemiol. 2020, 49, 193–204. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Triglyceride–glucose index (TyG index) is a predictor of incident colorectal cancer: A population-based longitudinal study. BMC Endocr. Disord. 2020, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Q.; Wang, Y.; Ma, X.; Zhang, Q.; Song, M.; Cao, L.; Shi, H. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer 2022, 22, 1007. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann. Intern. Med. 1995, 122, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.A.; Woodward, M.; Huxley, R. Obesity and Risk of Colorectal Cancer: A Meta-analysis of 31 Studies with 70,000 Events. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2533–2547. [Google Scholar] [CrossRef]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and colorectal cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef]
- Seo, J.Y.; Jin, E.H.; Chung, G.E.; Kim, Y.S.; Bae, J.H.; Yim, J.Y.; Han, K.D.; Yang, S.Y. The risk of colorectal cancer according to obesity status at four-year intervals: A nationwide population-based cohort study. Sci. Rep. 2023, 13, 8928. [Google Scholar] [CrossRef]
- Russo, A.; Autelitano, M.; Bisanti, L. Metabolic syndrome and cancer risk. Eur. J. Cancer 2008, 44, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Stocks, T.; Lukanova, A.; Bjørge, T.; Ulmer, H.; Manjer, J.; Almquist, M.; Concin, H.; Engeland, A.; Hallmans, G.; Nagel, G.; et al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2011, 117, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.S.; Kim, H.; Jeong, H.; Choi, M.-J.; Yoo, H.-W.; Han, T.-H.; Lee, H. The relationship between metabolic syndrome and the incidence of colorectal cancer. Environ. Health Prev. Med. 2020, 25, 6. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D. Epidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancer. Diabetes Care 2013, 36 (Suppl. 2), S233–S239. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, R.; Hallmans, G.; Rinaldi, S.; Biessy, C.; Stenling, R.; Riboli, E.; Kaaks, R. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: A prospective study in northern Sweden. Gut 2002, 50, 642. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Malkin, C.J.; Channer, K.S.; Jones, T.H. Androgens, insulin resistance and vascular disease in men. Clin. Endocrinol. 2005, 63, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Harbs, J.; Rinaldi, S.; Gicquiau, A.; Keski-Rahkonen, P.; Mori, N.; Liu, X.; Kaaks, R.; Katzke, V.; Schulze, M.B.; Agnoli, C.; et al. Circulating Sex Hormone Levels and Colon Cancer Risk in Men: A Nested Case-Control Study and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2022, 31, 793–803. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Sarma, D.K.; Kumawat, M.; Tiwari, R.; Verma, V.; Nagpal, R.; Kumar, M. Implication of Obesity and Gut Microbiome Dysbiosis in the Etiology of Colorectal Cancer. Cancers 2023, 15, 1913. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, X.; Zong, X.; Li, Z.; Li, N.; Hur, J.; Fritz, C.D.; Chapman, W., Jr.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70, 1147–1154. [Google Scholar] [CrossRef]
- Johnson, S.M.; Gulhati, P.; Rampy, B.A.; Han, Y.; Rychahou, P.G.; Doan, H.Q.; Weiss, H.L.; Evers, B.M. Novel Expression Patterns of PI3K/Akt/mTOR Signaling Pathway Components in Colorectal Cancer. J. Am. Coll. Surg. 2010, 210, 767–776. [Google Scholar] [CrossRef]
- Friedrich, N.; Thuesen, B.; Jørgensen, T.; Juul, A.; Spielhagen, C.; Wallaschofksi, H.; Linneberg, A. The association between IGF-I and insulin resistance: A general population study in Danish adults. Diabetes Care 2012, 35, 768–773. [Google Scholar] [CrossRef]
- Bogazzi, F.; Ultimieri, F.; Raggi, F.; Russo, D.; Vanacore, R.; Guida, C.; Viacava, P.; Cecchetti, D.; Acerbi, G.; Brogioni, S. PPARgamma inhibits GH synthesis and secretion and increases apoptosis of pituitary GH-secreting adenomas. Eur. J. Endocrinol. 2004, 150, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Saldeen, J.; Welsh, N. p38 MAPK inhibits JNK2 and mediates cytokine-activated iNOS induction and apoptosis independently of NF-κB translocation in insulin-producing cells. Eur. Cytokine Netw. 2004, 15, 47–52. [Google Scholar] [PubMed]
- Low, S.; Khoo, K.C.J.; Irwan, B.; Sum, C.F.; Subramaniam, T.; Lim, S.C.; Wong, T.K.M. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 143, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Íñigo, L.; Navarro-González, D.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martínez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef]
- Yan, F.; Yan, S.; Wang, J.; Cui, Y.; Chen, F.; Fang, F.; Cui, W. Association between triglyceride glucose index and risk of cerebrovascular disease: Systematic review and meta-analysis. Cardiovasc. Diabetol. 2022, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, Z.; Zheng, S.; Han, T.; Li, Y.; Liu, W.; Hu, Y. Association between Triglyceride to HDL-C Ratio (TG/HDL-C) and Insulin Resistance in Chinese Patients with Newly Diagnosed Type 2 Diabetes Mellitus. PLoS ONE 2016, 11, e0154345. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio as a Risk Marker for Metabolic Syndrome and Cardiovascular Disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef]
- Yoon, J.; Jung, D.; Lee, Y.; Park, B. The metabolic score for insulin resistance (METS-IR) as a predictor of incident ischemic heart disease: A longitudinal study among Korean without diabetes. J. Pers. Med. 2021, 11, 742. [Google Scholar] [CrossRef]
- Harriss, D.J.; Atkinson, G.; George, K.; Tim Cable, N.; Reilly, T.; Haboubi, N.; Zwahlen, M.; Egger, M.; Renehan, A.G.; the C-CLEAR group. Lifestyle factors and colorectal cancer risk (1): Systematic review and meta-analysis of associations with body mass index. Color. Dis. 2009, 11, 547–563. [Google Scholar] [CrossRef]
- Pelucchi, C.; Negri, E.; Talamini, R.; Levi, F.; Giacosa, A.; Crispo, A.; Bidoli, E.; Montella, M.; Franceschi, S.; La Vecchia, C. Metabolic syndrome is associated with colorectal cancer in men. Eur. J. Cancer 2010, 46, 1866–1872. [Google Scholar] [CrossRef]
- Chen, J.; Iverson, D. Estrogen in obesity-associated colon cancer: Friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control 2012, 23, 1767–1773. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6, 60–75. [Google Scholar] [CrossRef]

| All Subjects (n = 314,141) | TyG Index | p-Value | |||
|---|---|---|---|---|---|
| 1st Quartile, Q1 (n = 78,613) | 2nd Quartile, Q2 (n = 77,345) | 3rd Quartile, Q3 (n = 78,955) | 4th Quartile, Q4 (n = 79,228) | ||
| Demographics | |||||
| Age (years) | 57.8 (8.5) | 59.0 (8.8) | 59.4 (8.8) | 59.1 (8.7) | <0.001 |
| Sex (%) | <0.001 | ||||
| Male | 35,857 (45.6) | 39,296 (50.8) | 43,783 (55.5) | 50,396 (63.6) | |
| Female | 42,756 (54.4) | 38,049 (49.2) | 35,172 (44.5) | 28,832 (36.4) | |
| Income level (%) | <0.001 | ||||
| 1st quartile | 11,242 (14.3) | 10,919 (14.1) | 10,853 (13.7) | 10,911 (13.8) | |
| 2nd quartile | 16,832 (21.4) | 16,019 (20.7) | 15,876 (20.1) | 16,087 (20.3) | |
| 3rd quartile | 22,312 (28.4) | 22,529 (29.1) | 23,464 (29.7) | 24,290 (30.7) | |
| 4th quartile | 28,227 (35.9) | 27,878 (36.0) | 28,762 (36.4) | 27,940 (35.3) | |
| Residence (%) | <0.001 | ||||
| Urban | 51,988 (66.1) | 50,093 (64.8) | 50,240 (63.6) | 50,027 (63.1) | |
| Rural | 26,625 (33.9) | 27,252 (35.2) | 28,715 (36.4) | 29,201 (36.9) | |
| Underlying disease | |||||
| Hypertension (%) | 28,077 (35.7) | 34,020 (44.0) | 39,418 (49.9) | 44,785 (56.5) | <0.001 |
| Diabetes (%) | 3707 (4.7) | 6449 (8.3) | 10,498 (13.3) | 22,659 (28.6) | <0.001 |
| Dyslipidemia (%) | 18,337 (23.3) | 26,124 (33.8) | 34,566 (43.8) | 44,624 (56.3) | <0.001 |
| Charlson comorbidity index | <0.001 | ||||
| 0 | 40,254 (51.2) | 36,737 (47.5) | 35,754 (45.3) | 32,843 (41.5) | |
| 1 | 21,967 (27.9) | 21,470 (27.8) | 21,568 (27.3) | 21,147 (26.7) | |
| 2 | 9434 (12.0) | 10,521 (13.6) | 11,105 (14.1) | 11,800 (14.9) | |
| ≥3 | 6958 (8.9) | 8617 (11.1) | 10,528 (13.3) | 13,438 (17.0) | |
| Health screening | |||||
| Body mass index (kg/m2) | 23.1 (2.6) | 23.8 (2.7) | 24.3 (2.7) | 24.8 (2.6) | <0.001 |
| Systolic blood pressure (mmHg) | 121.6 (14.8) | 124.5 (15.0) | 126.4 (15.0) | 128.6 (15.1) | <0.001 |
| Diastolic blood pressure (mmHg) | 75.4 (9.7) | 77.1 (9.8) | 78.2 (9.7) | 79.6 (9.9) | <0.001 |
| Fasting blood glucose (mg/dL) | 90.9 (11.4) | 95.8 (13.9) | 100.5 (17.7) | 114.2 (32.9) | <0.001 |
| Total cholesterol (mg/dL) | 189.9 (34.0) | 198.2 (35.4) | 203.6 (36.9) | 209.5 (39.2) | <0.001 |
| Triglyceride (mg/dL) | 67.7 (14.4) | 101.9 (16.3) | 140.3 (24.9) | 224.8 (67.4) | <0.001 |
| HDL cholesterol (mg/dL) | 58.7 (12.9) | 55.4 (12.6) | 52.2 (12.1) | 49.0 (15.3) | <0.001 |
| LDL cholesterol (mg/dL) | 117.4 (33.3) | 122.4 (35.9) | 123.3 (36.2) | 116.3 (40.3) | <0.001 |
| Hemoglobin (g/dL) | 13.5 (1.4) | 13.7 (1.5) | 13.9 (1.5) | 14.2 (1.5) | <0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 80.3 (31.4) | 78.7 (28.6) | 78.0 (31.5) | 77.3 (31.9) | <0.001 |
| Current smoker (%) | 9172 (11.7) | 11,452 (14.8) | 13,996 (17.7) | 18,125 (22.9) | <0.001 |
| Alcohol consumption (%) | 27,427 (34.9) | 28,735 (37.2) | 31,686 (40.1) | 37,154 (46.9) | <0.001 |
| Regular exercise (%) | 4156 (5.3) | 3683 (4.8) | 3521 (4.5) | 3207 (4.0) | <0.001 |
| TyG index | 8.0 ± 0.2 | 8.5 ± 0.1 | 8.8 ± 0.1 | 9.4 ± 0.3 | <0.001 |
| Subjects (n = 314,141) | Events | Follow-Up Duration (Person-Years) | Incidence Rate (per 1000 Person-Years) | Hazard Ratios (95% Confidence Intervals) | |||
|---|---|---|---|---|---|---|---|
| Crude | p-Value | Adjusted * | p-Value | ||||
| TyG index | |||||||
| Q1 (n = 78,613) | 1266 | 746,824 | 1.70 | 1.00 (reference) | 1.00 (reference) | ||
| Q2 (n = 77,345) | 1468 | 727,043 | 2.02 | 1.18 (1.10–1.27) | <0.001 | 1.08 (1.00–1.16) | 0.05 |
| Q3 (n = 78,955) | 1606 | 742,177 | 2.16 | 1.27 (1.18–1.37) | <0.001 | 1.10 (1.02–1.19) | 0.01 |
| Q4 (n = 79,228) | 1772 | 744,743 | 2.38 | 1.40 (1.31–1.51) | <0.001 | 1.16 (1.07–1.25) | <0.001 |
| TG/HDL-C ratio | |||||||
| Q1 (n = 78,885) | 1287 | 749,408 | 1.72 | 1.00 (reference) | 1.00 (reference) | ||
| Q2 (n = 78,206) | 1497 | 735,136 | 2.04 | 1.18 (1.09–1.27) | <0.001 | 1.07 (0.99–1.15) | 0.09 |
| Q3 (n = 78,444) | 1642 | 737,374 | 2.23 | 1.29 (1.20–1.39) | <0.001 | 1.11 (1.03–1.20) | 0.005 |
| Q4 (n = 78,606) | 1686 | 738,896 | 2.28 | 1.32 (1.23–1.42) | <0.001 | 1.12 (1.03–1.21) | 0.006 |
| METS-IR † | |||||||
| Q1 (n = 78,631) | 1367 | 739,131 | 1.85 | 1.00 (reference) | 1.00 (reference) | ||
| Q2 (n = 78,542) | 1485 | 746,149 | 1.99 | 1.08 (1.00–1.16) | 0.04 | 1.05 (0.97–1.13) | 0.24 |
| Q3 (n = 78,498) | 1588 | 745,731 | 2.13 | 1.15 (1.07–1.24) | <0.001 | 1.07 (0.99–1.15) | 0.07 |
| Q4 (n = 78,470) | 1672 | 745,465 | 2.24 | 1.22 (1.13–1.31) | <0.001 | 1.10 (1.02–1.18) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.; Moon, S.Y.; Koh, M.; Kang, Y.; Lee, J.Y. Association between Surrogate Markers of Insulin Resistance and the Incidence of Colorectal Cancer in Korea: A Nationwide Population-Based Study. J. Clin. Med. 2024, 13, 1628. https://doi.org/10.3390/jcm13061628
Son M, Moon SY, Koh M, Kang Y, Lee JY. Association between Surrogate Markers of Insulin Resistance and the Incidence of Colorectal Cancer in Korea: A Nationwide Population-Based Study. Journal of Clinical Medicine. 2024; 13(6):1628. https://doi.org/10.3390/jcm13061628
Chicago/Turabian StyleSon, Minkook, Sang Yi Moon, Myeongseok Koh, Yeowool Kang, and Jong Yoon Lee. 2024. "Association between Surrogate Markers of Insulin Resistance and the Incidence of Colorectal Cancer in Korea: A Nationwide Population-Based Study" Journal of Clinical Medicine 13, no. 6: 1628. https://doi.org/10.3390/jcm13061628






