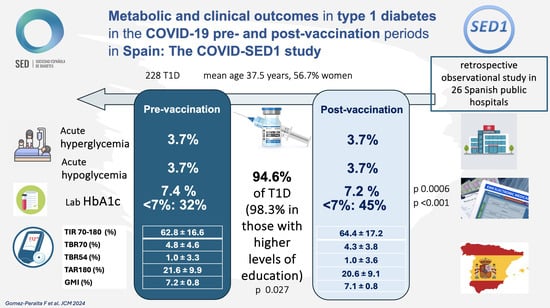
Metabolic and Clinical Outcomes in Type 1 Diabetes in the COVID-19 Pre- and Post-Vaccination Periods in Spain: The COVID-SED1 Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Endpoints
2.3. Evaluated Variables
2.4. Statistical Analysis
3. Results
3.1. Population
3.2. COVID-19 Vaccination
3.3. COVID-19-Related Events
3.4. Comorbidity and Complications Associated with T1D
3.5. HbA1c before and after Vaccination
3.6. Glucometrics and CGM-Derived Variables before and after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
SED1 Study Investigators
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Gomber, A.; Ward, Z.J.; Ross, C.; Owais, M.; Mita, C.; Yeh, J.M.; Reddy, C.L.; Atun, R. Variation in the incidence of type 1 diabetes mellitus in children and adolescents by world region and country income group: A scoping review. PLoS Glob. Public Health 2022, 2, e0001099. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Bellido, V.; Pérez, A. COVID-19 and Diabetes. J. Clin. Med. 2021, 10, 5341. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bae, J.H.; Kwon, H.-S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Fernández, E.; Cortazar, A.; Bellido, V. Impact of COVID-19 lockdown on glycemic control in patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2020, 166, 108348. [Google Scholar] [CrossRef]
- Garofolo, M.; Aragona, M.; Rodia, C.; Falcetta, P.; Bertolotto, A.; Campi, F.; Del Prato, S.; Penno, G. Glycaemic control during the lockdown for COVID-19 in adults with type 1 diabetes: A meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2021, 180, 109066. [Google Scholar] [CrossRef]
- Moreno-Domínguez, Ó.; González-Pérez de Villar, N.; Barquiel, B.; Hillman-Gadea, N.; Gaspar-Lafuente, R.; Arévalo-Gómez, M.; Herranz, L. Factors Related to Improvement of Glycemic Control Among Adults with Type 1 Diabetes During Lockdown Due to COVID-19. Diabetes Technol. Ther. 2020, 23, 399–400. [Google Scholar] [CrossRef]
- Chatterjee, T.; Ravichandran, N.; Nair, N.; Gracia-Ramos, A.E.; Barman, B.; Sen, P.; Joshi, M.; Saha, S.; Nune, A.; Pande, A.K.R.; et al. Type 1 diabetes, COVID-19 vaccines and short-term safety: Subgroup analysis from the global COVAD study. J. Diabetes Investig. 2024, 15, 131–138. [Google Scholar] [CrossRef]
- Ganakumar, V.; Jethwani, P.; Roy, A.; Shukla, R.; Mittal, M.; Garg, M.K. Diabetic ketoacidosis (DKA) in type 1 diabetes mellitus (T1DM) temporally related to COVID-19 vaccination. Diabetes Metab. Syndr. 2022, 16, 102371. [Google Scholar] [CrossRef]
- Edwards, A.E.; Vathenen, R.; Henson, S.M.; Finer, S.; Gunganah, K. Acute hyperglycaemic crisis after vaccination against COVID-19: A case series. Diabet. Med. J. Br. Diabet. Assoc. 2021, 38, e14631. [Google Scholar] [CrossRef] [PubMed]
- Kamrath, C.; Tittel, S.R.; Buchal, G.; Brämswig, S.; Preiss, E.; Göldel, J.M.; Wiegand, S.; Minden, K.; Warschburger, P.; Stahl-Pehe, A.; et al. Psychosocial Burden During the COVID-19 Pandemic in Adolescents with Type 1 Diabetes in Germany and Its Association with Metabolic Control. J. Adolesc. Health 2024. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, B.; Annuzzi, G.; Creanza, A.; Giglio, C.; De Angelis, R.; Lupoli, R.; Masulli, M.; Riccardi, G.; Rivellese, A.A.; Bozzetto, L. Blood Glucose Control During Lockdown for COVID-19: CGM Metrics in Italian Adults with Type 1 Diabetes. Diabetes Care 2020, 43, e88–e89. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Maltoni, G.; Bongiorno, C.; Tucci, L.; Di Natale, V.; Moscatiello, S.; Laffi, G.; Pession, A.; Zucchini, S.; Pagotto, U. Comparison of the effects of lockdown due to COVID-19 on glucose patterns among children, adolescents, and adults with type 1 diabetes: CGM study. BMJ Open Diabetes Res. Care 2020, 8, e001664. [Google Scholar] [CrossRef] [PubMed]
- Mesa, A.; Viñals, C.; Pueyo, I.; Roca, D.; Vidal, M.; Giménez, M.; Conget, I. The impact of strict COVID-19 lockdown in Spain on glycemic profiles in patients with type 1 Diabetes prone to hypoglycemia using standalone continuous glucose monitoring. Diabetes Res. Clin. Pract. 2020, 167, 108354. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Vitale, V.; Cosentino, C.; Cresci, B.; Pala, L.; Pieri, M.; Yannas, D.; Vannucci, M.; Zago, E.; Romani, A.; et al. Interstitial glucose monitoring, type 1 diabetes and COVID-19 vaccine: The patient-reported outcomes and vaccine-associated changes in glucose and side effects (PRO-VACS). Acta Diabetol. 2022, 59, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.H.; Stedman, M.; Horne, L.; Rea, R.; Whyte, M.; Gibson, J.M.; Livingston, M.; Anderson, S.G.; Ollier, W. Analysis of Continuous Blood Glucose Data in People with Type 1 Diabetes (T1DM) After COVID-19 Vaccination Indicates a Possible Link Between the Immune and the Metabolic Response. J. Diabetes Sci. Technol. 2021, 15, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Al-Ozairi, E.; Irshad, M.; Taghadom, E.; Varghese, A.; Sojan, L.; Alkandari, J. Effect of COVID-19 vaccine on blood glucose metrics in Arabic people with type 1 diabetes. Front. Endocrinol. 2023, 14, 1120384. [Google Scholar] [CrossRef] [PubMed]
- Zilbermint, M.; Motevalli, M.; Batty, K.; Venner-Walcott, J.; Edwards, A.; Burley, T.; Jackson, K.; Akhtar, M.; Demidowich, A.P. Effects of the COVID-19 booster vaccine on glycemia and insulin resistance in people with type 1 diabetes: A prospective pilot study. Diabetes Res. Clin. Pract. 2023, 204, 110898. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Peralta, F.; Menéndez, E.; Conde, S.; Conget, I.; Novials, A.; en nombre de la SED y de los investigadores del estudio SED1. Clinical characteristics and management of type 1 diabetes in Spain. The SED1 study. Endocrinol. Diabetes Nutr. 2021, 68, 642–653. [Google Scholar] [CrossRef]
- Kamrath, C.; Mönkemöller, K.; Biester, T.; Rohrer, T.R.; Warncke, K.; Hammersen, J.; Holl, R.W. Ketoacidosis in Children and Adolescents with Newly Diagnosed Type 1 Diabetes during the COVID-19 Pandemic in Germany. JAMA 2020, 324, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Ebekozien, O.A.; Noor, N.; Gallagher, M.P.; Alonso, G.T. Type 1 Diabetes and COVID-19: Preliminary Findings from a Multicenter Surveillance Study in the U.S. Diabetes Care 2020, 43, e83–e85. [Google Scholar] [CrossRef]
- D’Onofrio, L.; Coraggio, L.; Zurru, A.; Carlone, A.; Mignogna, C.; Moretti, C.; Maddaloni, E.; Buzzetti, R. Short-term safety profile of SARS-CoV2 vaccination on glucose control: Continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res. Clin. Pract. 2021, 179, 109022. [Google Scholar] [CrossRef]
- Saseetharran, A.; Patel, S.A. COVID-19 pandemic-related healthcare interruptions and diabetes distress: A national study of US adults with diabetes. BMC Public Health 2024, 24, 493. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Peralta, F.; Choudhary, P.; Cosson, E.; Irace, C.; Rami-Merhar, B.; Seibold, A. Understanding the Clinical Implications of Differences between Glucose Management Indicator and Glycated Haemoglobin. Diabetes Obes. Metab. 2022, 24, 599–608. [Google Scholar] [CrossRef]
- Beca-Martínez, M.T.; Romay-Barja, M.; Ayala, A.; Falcon-Romero, M.; Rodríguez-Blázquez, C.; Benito, A.; Forjaz, M.J. Trends in COVID-19 Vaccine Acceptance in Spain, September 2020–May 2021. Am. J. Public Health 2022, 112, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Scoccimarro, D.; Panichi, L.; Ragghianti, B.; Silverii, A.; Mannucci, E.; Monami, M. Sars-CoV2 vaccine hesitancy in Italy: A survey on subjects with diabetes. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3243–3246. [Google Scholar] [CrossRef] [PubMed]
| n | % | |
|---|---|---|
| Number of patients | 187 | |
| Age (years) | ||
| mean ± SD | 37.5 ± 15.6 | |
| Age Groups (Years) | ||
| 0–13 | 17 | 9.1 |
| 14–17 | 7 | 3.7 |
| 18–25 | 21 | 11.2 |
| 26–49 | 102 | 54.5 |
| >49 | 40 | 21.4 |
| Gender, women | 106 | 56.7 |
| Level of education | 179 | |
| No studies | 2 | 1.1 |
| Primary education | 38 | 21.2 |
| Secondary education | 66 | 36.9 |
| University studies or similar | 67 | 37.4 |
| Student | 6 | 3.4 |
| Weight (kg) | ||
| mean ± SD | 67.6 ± 16.8 | |
| Height (cm) | 186 | |
| mean ± SD | 164.6 ± 13.1 | |
| BMI (kg/m2) | 186 | |
| mean ± SD | 24.6 ± 4.4 | |
| BMI grades (kg/m2) | 186 | |
| <18.5 | 15 | 8.1 |
| 18.5–24.9 | 94 | 50.5 |
| 25–26.9 | 29 | 15.6 |
| 27–29.9 | 24 | 12.9 |
| ≥30 | 24 | 12.9 |
| Time since diagnosis of T1DM (years) | ||
| mean ± SD | 17.8 ± 12.7 | |
| Method of Insulin Administration | ||
| Basal–bolus | 139 | 74.3 |
| Premixed insulins | 3 | 1.6 |
| Continuous subcutaneous insulin infusion (CSII) | 45 | 24.1 |
| Pre-Vaccination | Post-Vaccination | p-Value * | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Acute hyperglycemic decompensation | 6/161 | 3.7 | 7/161 | 4.3 | 1 |
| Acute hypoglycemic decompensation | 6/161 | 3.7 | 6/161 | 3.7 | 1 |
| Number of cases of acute hypoglycemic decompensation, mean ± SD | 6 | 2.3 ± 1.4 | 6 | 1.3 ± 0.8 | |
| 1 | 1 | 16.6 | 5 | 83.3 | |
| 2 | 4 | 66.7 | 0 | 0.0 | |
| 3 | 0 | 0.0 | 1 | 16.7 | |
| 5 | 1 | 16.7 | 0 | 0.0 | |
| 6 | 0 | 0.0 | 0 | 0.0 | |
| Mortality | 1/187 | 0.53 | 1/181 | 0.55 | |
| Pre-Vaccination | Post-Vaccination | Total | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Comirnaty | ||||||
| Acute hyperglycemic decompensation | 6/116 | 5.2 | 4.3 | 8/116 | 6.9 | 1.0 |
| Acute hypoglycemic decompensation | 5/116 | 4.3 | 6/116 | 5.2 | 8/116 | 6.9 |
| Spikevax | ||||||
| Acute hyperglycemic decompensation | 0/21 | 0.0 | 1/21 | 4.8 | 1/21 | 4.8 |
| Acute hypoglycemic decompensation | 0/21 | 0.0 | 0/21 | 0.0 | 0/21 | 0.0 |
| Pre-Vaccination | Post-Vaccination | p-Value * | |
|---|---|---|---|
| Average daily scans (n = 85) | 9.9 ± 5.6 | 10.3 ± 6.6 | 0.314 |
| Mean glucose (n = 113) | 158.6 ± 31.7 | 156.2 ± 32.9 | 0.200 |
| Coefficient of glycemic variation (CV) (n = 110) | 37.3 ± 6.4 | 36.3 ± 6.8 | 0.096 |
| Time in glucose range 70–180 mg/dL (TIR) (%) (n = 117) | 62.8 ± 16.6 | 64.4 ± 17.2 | 0.192 |
| Time with glucose below 70 mg/dL (TBR70) (%) (n = 114) | 4.8 ± 4.6 | 4.3 ± 3.8 | 0.247 |
| Time with glucose below range 54 mg/dL (TBR54) (%) (n = 112) | 1.0 ± 3.3 | 1.0 ± 3.6 | 0.900 |
| Number of events of glucose below 70 mg/dL (n = 83) | 8.0 ± 7.0 | 7.6 ± 7.0 | 0.549 |
| Average duration of hypoglycemic events below 70 mg/dL (min) (n = 83) | 66.8 ± 48.6 | 68.2 ± 58.5 | 0.799 |
| Time with glucose above 180 mg/dL (TAR180) (%) (n = 107) | 21.6 ± 9.9 | 20.6 ± 9.1 | 0.188 |
| Time with glucose above 250 mg/dL (TAR250) (%) (n = 107) | 10.3 ± 11.5 | 10.2 ± 11.7 | 0.970 |
| Glucose management indicator (GMI) (%) (n = 106) | 7.2 ± 0.8 | 7.1 ± 0.8 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Peralta, F.; Menéndez, E.; Conde, S.; Abellán-Galiana, P.; Brito, M.; Beléndez, M.; Pérez, A., on behalf of SED1 Study Investigators. Metabolic and Clinical Outcomes in Type 1 Diabetes in the COVID-19 Pre- and Post-Vaccination Periods in Spain: The COVID-SED1 Study. J. Clin. Med. 2024, 13, 1922. https://doi.org/10.3390/jcm13071922
Gómez-Peralta F, Menéndez E, Conde S, Abellán-Galiana P, Brito M, Beléndez M, Pérez A on behalf of SED1 Study Investigators. Metabolic and Clinical Outcomes in Type 1 Diabetes in the COVID-19 Pre- and Post-Vaccination Periods in Spain: The COVID-SED1 Study. Journal of Clinical Medicine. 2024; 13(7):1922. https://doi.org/10.3390/jcm13071922
Chicago/Turabian StyleGómez-Peralta, Fernando, Edelmiro Menéndez, Santiago Conde, Pablo Abellán-Galiana, Miguel Brito, Marina Beléndez, and Antonio Pérez on behalf of SED1 Study Investigators. 2024. "Metabolic and Clinical Outcomes in Type 1 Diabetes in the COVID-19 Pre- and Post-Vaccination Periods in Spain: The COVID-SED1 Study" Journal of Clinical Medicine 13, no. 7: 1922. https://doi.org/10.3390/jcm13071922