Normal-Tension Glaucoma and Potential Clinical Links to Alzheimer’s Disease
Abstract
:1. Background
2. Progressive Trends in NTG and AD Epidemiology
3. Comparing Genetic Biomarkers in NTG and AD: A Comprehensive Analysis
3.1. Insights into Aβ and Tau as Biomarkers in NTG and AD
3.2. Role of APOE4 Allele in AD and NTG: A Genetic Investigation
3.3. Optineurin in Focus: Bridging the Gap between NTG and AD
4. Vascular Dysregulation and Impaired Pressure Dynamics in NTG and AD: A Unifying Perspective
4.1. Vascular Flow and the Link to NTG in AD
4.2. Exploring the Relationship of the Translaminar Pressure Gradient within NTG and AD
4.3. Altered CSF Fluid Dynamics and Bio-Active Molecules in NTG and AD Pathology
4.4. Ocular Manifestations in the Context of NTG and AD: An Investigative Analysis
4.5. A Silent Connection: Exploring Cerebral Manifestations in NTG and AD
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NTG | Normal-tension glaucoma |
| HTG | High-tension glaucoma |
| POAG | Primary open-angle glaucoma |
| ALS | Amyotrophic lateral sclerosis |
| AD | Alzheimer’s Disease |
| IOP | Intraocular pressure |
| ICP | Intracranial pressure |
| RGC | Retinal ganglion cell |
| mRGCs | Melanopsin RGCs |
| RNFL | Retinal nerve fiber layer |
| GCC | Ganglion cell complex |
| MMSE | Mini-mental state examination |
| CVI | Choroid vascular index |
| MCI | Mild cognitive impairment |
| APOE | Apolipoprotein e4 allele |
| OPTN | Optineurin |
| TBK1 | TANK Binding Kinase 1 |
| Aβ | Amyloid β |
| Aβ42 | Aβ peptide residues 1-42 |
| Aβ40 | Aβ peptides residues 1-40 |
| APP | Amyloid precursor protein |
| IL | Interleukins |
| TNFa | Tumor necrosis factor a |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 |
| 4-HNE | 4-hydroxynonenal |
| LC3B/MAP1LC3B | Microtubule associated protein 1 light chain 3 beta |
| ET-1 | Endothelin-1 |
| TLPG | Translaminar pressure gradient |
| CSFP | Cerebrospinal fluid pressure |
| AQP | Aquaporin |
| AQP-4 | Aquaporin-4 |
| L-PGDS | Lipocalin-type prostaglandin D synthases |
| BDNF | Brain-derived neurotrophic factor |
| T-MoCA | Telephone Montreal Cognitive Assessment |
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, A.; Zou, M.; Zhang, Y.; Jin, L.; Li, Y.; Zheng, D.; Jin, G.; Congdon, N. Time trends, associations and prevalence of blindness and vision loss due to glaucoma: An analysis of observational data from the Global Burden of Disease Study 2017. BMJ Open 2022, 12, e053805. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.L.; Brigatti, L. The glaucomas. Minerva Med. 2001, 92, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, L.A.; Zack, D.J.; Quigley, H.A.; Smith, S.D.; Pease, M.E. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch. Ophthalmol. 1997, 115, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.S.; Tafreshi, A.; Dorairaj, S.K.; Weinreb, R.N. Clinical applicability of the International Classification of Disease and Related Health Problems (ICD-9) glaucoma staging codes to predict disease severity in patients with open-angle glaucoma. Eur. J. Gastroenterol. Hepatol. 2014, 23, e18–e22. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, V.V.; Chan, M.P.Y.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br. J. Ophthalmol. 2015, 100, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Esporcatte, B.L.B.; Tavares, I.M. Normal-tension glaucoma: An update. Arq. Bras. Oftalmol. 2016, 79, 270–276. [Google Scholar] [CrossRef]
- Dielemans, I.; Vingerling, J.R.; Wolfs, R.C.; Hofman, A.; Grobbee, D.E.; de Jong, P.T. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. Ophthalmology 1994, 101, 1851–1855. [Google Scholar] [CrossRef]
- Kim, K.E.; Park, K.-H. Update on the Prevalence, Etiology, Diagnosis, and Monitoring of Normal-Tension Glaucoma. Asia-Pac. J. Ophthalmol. 2016, 5, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Kee, C. Population-based glaucoma prevalence studies in Asians. Surv. Ophthalmol. 2014, 59, 434–447. [Google Scholar] [CrossRef] [PubMed]
- The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am. J. Ophthalmol. 1998, 126, 498–505. [Google Scholar]
- Caprioli, J.; Song, B. New directions in the treatment of normal tension glaucoma. Indian J. Ophthalmol. 2014, 62, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Zhang, J.; Costantino, F.; De Stefano, N.; Frezzotti, P. Diffuse brain damage in normal tension glaucoma. Hum. Brain Mapp. 2018, 39, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Boucard, C.C.; Hanekamp, S.; Ćurčić-Blake, B.; Ida, M.; Yoshida, M.; Cornelissen, F.W. Neurodegeneration beyond the primary visual pathways in a population with a high incidence of normal-pressure glaucoma. Ophthalmic Physiol. Opt. 2016, 36, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Mullany, S.; Xiao, L.; Qassim, A.; Marshall, H.; Gharahkhani, P.; MacGregor, S.; Hassall, M.M.; Siggs, O.M.; Souzeau, E.; E Craig, J. Normal-tension glaucoma is associated with cognitive impairment. Br. J. Ophthalmol. 2022, 106, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Yochim, B.P.; Mueller, A.E.; Kane, K.D.; Kahook, M.Y. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. Eur. J. Gastroenterol. Hepatol. 2012, 21, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawakami, H.; Kanamoto, T.; Kato, T.; Yokoyama, T.; Sasaki, K.; Izumi, Y.; Matsumoto, M.; Mishima, H.K. High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J. Neurol. Sci. 2006, 246, 79–83. [Google Scholar] [CrossRef]
- Bayer, A.; Ferrari, F.; Erb, C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur. Neurol. 2002, 47, 165–168. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.-H. Normal-tension glaucoma and Alzheimer’s disease: Retinal vessel signs as a possible common underlying risk factor. Med. Hypotheses 2011, 77, 466. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Utsunomiya, K.; Ota, H.; Ogura, Y.; Narabayashi, I.; Ikeda, T. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Arch. Ophthalmol. 2006, 141, 394–396. [Google Scholar] [CrossRef]
- Cui, Q.N.; Jethi, M.; Driver, T.; Porco, T.C.; Kuo, J.; Lin, S.C.; Stamper, R.L.; Han, Y.; Chiu, C.S.; Ramanathan, S.; et al. Individuals with and without normal tension glaucoma exhibit comparable performance on tests of cognitive function. Int. J. Ophthalmol. 2021, 14, 1721–1728. [Google Scholar] [CrossRef]
- Kessing, L.V.M.; Lopez, A.G.M.; Andersen, P.K.M.; Kessing, S.V.M. No increased risk of developing Alzheimer disease in patients with glaucoma. Eur. J. Gastroenterol. Hepatol. 2007, 16, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Vo, B.; Lim, A.K.; Hirabayashi, D.R.; Tanaka, G.H.; Weinreb, R.N.; Lin, S.C. The characteristics of glaucoma in Japanese Americans. JAMA Ophthalmol. 2009, 127, 167. [Google Scholar] [CrossRef]
- Brahma, M.M.; Takahashi, K.; Namekata, K.; Harada, T.; Goshima, Y.; Ohshima, T. Genetic inhibition of collapsin response mediator protein-2 phosphorylation ameliorates retinal ganglion cell death in normal-tension glaucoma models. Genes. Cells 2022, 27, 526–536. [Google Scholar] [CrossRef]
- Lam, C.Y.; Fan, B.J.; Wang, D.Y.; Tam, P.O.S.; Tham, C.C.Y.; Leung, D.Y.L.; Fan, D.S.P.; Lam, D.S.C.; Pang, C.P. Association of apolipoprotein E polymorphisms with normal tension glaucoma in a Chinese population. Eur. J. Gastroenterol. Hepatol. 2006, 15, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Lai, Y.-J.; Yen, Y.-F.; Shen, Y.-C.; Wang, C.-Y.; Liang, C.-Y.; Lin, K.-H.; Fan, L.-W. Association between normal tension glaucoma and the risk of Alzheimer’s disease: A nationwide population-based cohort study in Taiwan. BMJ Open 2018, 8, e022987. [Google Scholar] [CrossRef]
- Global Burden of Disease Dementia Forecast Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Méndez-Hernández, C.; Hoz, R.D.; Ramírez, A.I.; López-Cuenca, I.; Fernández-Albarral, J.A.; Rojas, P.; Wang, S.; García-Feijoo, J.; Gil, P.; et al. Ocular Vascular Changes in Mild Alzheimer’s Disease Patients: Foveal Avascular Zone, Choroidal Thickness, and ONH Hemoglobin Analysis. J. Pers. Med. 2020, 10, 231. [Google Scholar] [CrossRef]
- Mirzaei, N.; Shi, H.; Oviatt, M.; Doustar, J.; Rentsendorj, A.; Fuchs, D.-T.; Sheyn, J.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Alzheimer’s Retinopathy: Seeing Disease in the Eyes. Front. Neurosci. 2020, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Stojcic, M.; Hentova-Sencanic, P.; Stojcic, B.; Sencanic, I. Comparison of normal tension and high tension glaucoma patients (corrected) according to age and sex. Srp. Arh. Celok. Lek. 2012, 140, 699–703. [Google Scholar] [CrossRef]
- Mallick, J.; Devi, L.; Malik, P.; Mallick, J. Update on Normal Tension Glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Katz, B.; Rimmer, S. Ophthalmologic manifestations of Alzheimer’s disease. Surv. Ophthalmol. 1989, 34, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Sadun, A.A.; Borchert, M.; DeVita, E.; Hinton, D.R.; Bassi, J. Assessment of visual impairment in patients with Alzheimer’s disease. Am. J. Ophthalmol. 1987, 104, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Au, R.; McNulty, K.; White, R.; D’Agostino, R.B. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 1997, 49, 1498–1504. [Google Scholar] [CrossRef]
- Mielke, M.M. Sex and Gender Differences in Alzheimer’s Disease Dementia. Psychiatr. Times 2018, 35, 14–17. [Google Scholar] [PubMed]
- Coales, I.; Tsartsalis, S.; Fancy, N.; Weinert, M.; Clode, D.; Owen, D.; Matthews, P.M. Alzheimer’s disease-related transcriptional sex differences in myeloid cells. J. Neuroinflamm. 2022, 19, 1–13. [Google Scholar] [CrossRef]
- Garner, R.; Kumari, R.; Lanyon, P.; Doherty, M.; Zhang, W. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: Systematic review and meta-analysis of observational studies. BMJ Open 2015, 5, e006389. [Google Scholar] [CrossRef]
- Henry, E.; E Newby, D.; Webb, D.J.; O’Brien, C. Peripheral endothelial dysfunction in normal pressure glaucoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1710–1714. [Google Scholar]
- Drance, S.; Douglas, G.; Wijsman, K.; Schulzer, M.; Britton, R. Response of blood flow to warm and cold in normal and low-Tension glaucoma patients. Arch. Ophthalmol. 1988, 105, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Weber, B.H.; Gramer, E. Migraine and Vasospasm in Glaucoma: Age-Related Evaluation of 2027 Patients With Glaucoma or Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7999–8007. [Google Scholar] [CrossRef]
- Owens, P.; Lyons, S.; O’brien, E. Arterial hypotension: Prevalence of low blood pressure in the general population using ambulatory blood pressure monitoring. J. Hum. Hypertens. 2000, 14, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Binggeli, T.; Schoetzau, A.; Konieczka, K. In glaucoma patients, low blood pressure is accompanied by vascular dysregulation. EPMA J. 2018, 9, 387–391. [Google Scholar] [CrossRef]
- Kaiser, H.J.; Flammer, J.; Graf, T.; Stümpfig, D. Systemic blood pressure in glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 1993, 231, 677–680. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.; Shimuzu, H.; Tomita, G.; et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, L.; George, R.; Baskaran, M.; Arvind, H.; Raju, P.; Ramesh, S.V.; Kumaramanickavel, G.; McCarty, C. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population: The chennai glaucoma study. Ophthalmology 2008, 115, 648–654.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Xu, L.; Yang, H.; Jonas, J.B. Prevalence of glaucoma in north China: The Beijing Eye study. Arch. Ophthalmol. 2010, 150, 917–924. [Google Scholar] [CrossRef]
- Kim, C.-S.; Seong, G.J.; Lee, N.-H.; Song, K.-C. Prevalence of primary open-angle glaucoma in central South Korea: The Namil study. Ophthalmology 2011, 118, 1024–1030. [Google Scholar] [CrossRef]
- Bonomi, L.; Marchini, G.; Marraffa, M.; Bernardi, P.; De Franco, I.; Perfetti, S.; Varotto, A.; Tenna, V. Prevalence of glaucoma and intraocular pressure distribution in a defined population: The Egna-Neumarkt study. Ophthalmology 1998, 105, 209–215. [Google Scholar] [CrossRef]
- Leske, M.C.; Connell, A.M.; Wu, S.Y.; Hyman, L.G.; Schachat, A.P. Risk factors for open-angle glaucoma: The Barbados Eye Study. Arch. Ophthalmol. 1995, 113, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Rotchford, A.P.; Johnson, G.J. Glaucoma in Zulus: A population-based cross-sectional survey in a rural district in South Africa. Arch. Ophthalmol. 2002, 120, 471–478. [Google Scholar] [CrossRef]
- Fan, N.; Wang, P.; Tang, L.; Liu, X. Ocular Blood Flow and Normal Tension Glaucoma. BioMed Res. Int. 2015, 2015, 308505. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, R.; De Moraes, C.G.; Teng, C.C.; Liebmann, J.M.; Greenfield, D.S.; Gardiner, S.; Ritch, R.; Krupin, T. Low-pressure glaucoma treatment study group. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am. J. Ophthalmol. 2014, 157, 945–952.e1. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; de Moraes, C.G.; Link, A.; Wells, M.T.; Harmon, G.; Peterson, J.C.; Ritch, R.; Liebmann, J.M. Nocturnal systemic hypotension increases the risk of glaucoma progression. Ophthalmology 2014, 121, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Raman, P.; Suliman, N.B.; Zahari, M.; Kook, M.; Ramli, N. Low nocturnal diastolic ocular perfusion pressure as a risk factor for NTG progression: A 5-year prospective study. Eye 2018, 32, 1183–1189. [Google Scholar] [CrossRef]
- de la Torre, J.C. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke 2002, 33, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, K.; Lee, Y.C.; Kim, S.; Won, H.-H.; Yu, T.Y.; Lee, E.-M.; Kang, J.M.; Lewis, M.; Kim, D.K.; et al. Associations between vascular risk factors and subsequent Alzheimer’s disease in older adults. Alzheimers Res. Ther. 2020, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Serot, J.M.; Zmudka, J.; Jouanny, P. A possible role for CSF turnover and choroid plexus in the pathogenesis of late onset Alzheimer’s disease. J. Alzheimers Dis. 2012, 30, 17–26. [Google Scholar] [CrossRef]
- Kim, J.; Ha, W.S.; Park, S.H.; Han, K.; Baek, M.S. Association between migraine and Alzheimer’s disease: A nationwide cohort study. Front. Aging Neurosci. 2023, 15, 1196185. [Google Scholar] [CrossRef]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.-P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kim, H.J.; Park, Y.H.; Park, T.K.; Park, E.-C.; Kim, C.Y.; Lee, S.H. Association between Open-Angle Glaucoma and the Risks of Alzheimer’s and Parkinson’s Diseases in South Korea: A 10-year Nationwide Cohort Study. Sci. Rep. 2018, 8, 11161. [Google Scholar] [CrossRef]
- Bach-Holm, D.; Kessing, S.V.; Mogensen, U.; Forman, J.L.; Andersen, P.K.; Kessing, L.V. Normal tension glaucoma and Alzheimer disease: Comorbidity? Acta Ophthalmol. 2012, 90, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Delatour, B.; Potier, M.-C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009, 118, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 1984, 122, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Drummond, E.; Pires, G.; MacMurray, C.; Askenazi, M.; Nayak, S.; Bourdon, M.; Safar, J.; Ueberheide, B.; Wisniewski, T. Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
- Pîrşcoveanu, D.F.V.; Pirici, I.; Tudorică, V.; Balseanu, T.-A.; Albu, V.C.; Bondari, S.; Bumbea, A.M.; Pîrşcoveanu, M. Tau protein in neurodegenerative diseases—A review. Rom. J. Morphol. Embryol. 2017, 58, 1141–1150. [Google Scholar]
- Orr, M.E.; Sullivan, A.C.; Frost, B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 637–648. [Google Scholar] [CrossRef]
- Schmechel, D.E.; Saunders, A.M.; Strittmatter, W.J.; Crain, B.J.; Hulette, C.M.; Joo, S.H.; Pericak-Vance, M.A.; Goldgaber, D.; Roses, A.D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 9649–9653. [Google Scholar] [CrossRef]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef]
- Liu, Y.; Allingham, R.R. Major review: Molecular genetics of primary open-angle glaucoma. Exp. Eye Res. 2017, 160, 62–84. [Google Scholar] [CrossRef]
- Alward, W.L.M.; van der Heide, C.; Khanna, C.L.; Roos, B.R.; Sivaprasad, S.; Kam, J.; Ritch, R.; Lotery, A.; Igo, R.P.; Bailey, J.N.C.; et al. Myocilin Mutations in Patients with Normal-Tension Glaucoma. JAMA Ophthalmol 2019, 137, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Weisschuh, N.; Neumann, D.; Wolf, C.; Wissinger, B.; Gramer, E. Prevalence of myocilin and optineurin sequence variants in German normal tension glaucoma patients. Mol. Vis. 2005, 11, 284–287. [Google Scholar] [PubMed]
- Rezaie, T.; Child, A.; Hitchings, R.; Brice, G.; Miller, L.; Coca-Prados, M.; Héon, E.; Krupin, T.; Ritch, R.; Kreutzer, D.; et al. Adult-Onset primary open-angle glaucoma caused by mutations in optineurin. Science 2002, 295, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Liuska, P.J.; Harju, M.; Kivelä, T.T.; Turunen, J.A. Prevalence of MYOC risk variants for glaucoma in different populations. Acta Ophthalmol. 2021, 99, E1090–E1097. [Google Scholar] [CrossRef]
- Swarup, G.; Sayyad, Z. Altered Functions and Interactions of Glaucoma-Associated Mutants of Optineurin. Front. Immunol. 2018, 9, 1287. [Google Scholar] [CrossRef]
- Sakurada, Y.; Mabuchi, F. Advances in glaucoma genetics. Prog. Brain Res. 2015, 220, 107–126. [Google Scholar]
- Challa, P. Glaucoma genetics. Int. Ophthalmol. Clin. 2008, 48, 73–94. [Google Scholar] [CrossRef]
- Fingert, J.H. Primary open-angle glaucoma genes. Eye 2011, 25, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, K.; Swarup, G. Defects in autophagy caused by glaucoma-associated mutations in optineurin. Exp. Eye Res. 2016, 144, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G.; Demicheli, F.; Giorgi, S.; Bendotti, C.; Angeretti, N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: Modulation by interleukin-1. Mol. Brain Res. 1992, 16, 128–134. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Chen, H.; Autilio-Gambetti, L.; Gambetti, P. Differential APP gene expression in rat cerebral cortex, meninges, and primary astroglial, microglial and neuronal cultures. FEBS Lett. 1991, 292, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Hung, A.; Selkoe, D. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J. Neurosci. 1991, 11, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Nunan, J.; Small, D.H. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000, 483, 6–10. [Google Scholar] [CrossRef]
- Yang, M.; Teplow, D.B. Amyloid beta-protein monomer folding: Free-energy surfaces reveal alloform-specific differences. J. Mol. Biol. 2008, 384, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Sgourakis, N.G.; Yan, Y.; McCallum, S.A.; Wang, C.; Garcia, A.E. The Alzheimer’s peptides Abeta40 and 42 adopt distinct conformations in water: A combined MD/NMR study. J. Mol. Biol. 2007, 368, 1448–1457. [Google Scholar] [CrossRef]
- Pike, K.E.; Savage, G.; Villemagne, V.L.; Ng, S.; Moss, S.A.; Maruff, P.; Mathis, C.A.; Klunk, W.E.; Masters, C.L.; Rowe, C.C. Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer’s disease. Brain 2007, 130 Pt 11, 2837–2844. [Google Scholar] [CrossRef]
- Martin, K.R.; Quigley, H.A.; Valenta, D.; Kielczewski, J.; Pease, M.E. Optic nerve dynein motor protein distribution changes with intraocular pressure elevation in a rat model of glaucoma. Exp. Eye Res. 2006, 83, 255–262. [Google Scholar] [CrossRef]
- Kipfer-Kauer, A.; McKinnon, S.J.; Frueh, B.E.; Goldblum, D. Distribution of amyloid precursor protein and amyloid-beta in ocular hypertensive C57BL/6 mouse eyes. Curr. Eye Res. 2010, 35, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Minckler, D.S.; Bunt, A.H.; Johanson, G.W. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Investig. Ophthalmol. Vis. Sci. 1977, 16, 426–441. [Google Scholar]
- Quigley, H.A.; Addicks, E.M.; Green, W.R.; Maumenee, A.E. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch. Ophthalmol. 1981, 99, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.S.; Luo, X.; Ribas, V.T.; Petrs-Silva, H.; Koch, J.C. The Role of Axonal Transport in Glaucoma. Int. J. Mol. Sci. 2022, 23, 3935. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Zhou, B.; Wernig, M.; Sudhof, T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell 2017, 168, 427–441.e21. [Google Scholar] [CrossRef] [PubMed]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Holm, M.L.; Liu, C.C.; Shinohara, M.; Aikawa, T.; Oue, H.; Yamazaki, Y.; Martens, Y.A.; Murray, M.E.; Sullivan, P.M.; et al. APOE4-mediated amyloid-beta pathology depends on its neuronal receptor LRP1. J. Clin. Investig. 2019, 129, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.A.; Laurent, B.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef]
- Corbo, R.M.; Scacchi, R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann. Hum. Genet. 1999, 63, 301–310. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Singh, M.; Mastana, S.S. APOE distribution in world populations with new data from India and the UK. Ann. Hum. Biol. 2006, 33, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Copin, B.; Brézin, A.P.; Valtot, F.; Dascotte, J.-C.; Béchetoille, A.; Garchon, H.-J. Apolipoprotein E–promoter single-nucleotide polymorphisms affect the phenotype of primary open-angle glaucoma and demonstrate interaction with the myocilin gene. Am. J. Hum. Genet. 2002, 70, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Saadane, A.; Petrov, A.; Mast, N.; El-Darzi, N.; Dao, T.; Alnemri, A.; Song, Y.; Dunaief, J.L.; Pikuleva, I.A. Mechanisms that minimize retinal impact of apolipoprotein E absence. J. Lipid Res. 2018, 59, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.A.; Letcher, S.M.; Igo, R.P.; Cooke Bailey, J.N.; Pasquale, L.R.; Haines, J.L.; Butovsky, O.; Wiggs, J.L. Association of APOE with Primary Open-Angle Glaucoma Suggests a Protective Effect for APOE epsilon4. Investig. Ophthalmol. Vis. Scence 2020, 61, 3. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, N.M.; Al-Dohayan, N.; Arfin, M.; Tariq, M. Apolipoprotein E polymorphisms and primary glaucoma in Saudis. Mol. Vis. 2009, 15, 912–919. [Google Scholar]
- Vickers, J.C.; E Craig, J.; Stankovich, J.; McCormack, G.H.; West, A.K.; Dickinson, J.L.; McCartney, P.J.; A Coote, M.; Healey, D.L.; A Mackey, D. The apolipoprotein epsilon4 gene is associated with elevated risk of normal tension glaucoma. Mol. Vis. 2002, 8, 389–893. [Google Scholar]
- Mabuchi, F.; Tang, S.; Ando, D.; Yamakita, M.; Wang, J.; Kashiwagi, K.; Yamagata, Z.; Iijima, H.; Tsukahara, S. The apolipoprotein E gene polymorphism is associated with open angle glaucoma in the Japanese population. Mol. Vis. 2005, 11, 609–612. [Google Scholar] [PubMed]
- Saglar, E.; Yucel, D.; Bozkurt, B.; Ozgul, R.; Irkec, M.; Ogus, A. Association of polymorphisms in APOE, p53, and p21 with primary open-angle glaucoma in Turkish patients. Mol. Vis. 2009, 15, 1270–1276. [Google Scholar] [PubMed]
- Poirier, J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci. 1994, 17, 525–530. [Google Scholar] [CrossRef]
- Rudajev, V.; Novotny, J. Cholesterol as a key player in amyloid beta-mediated toxicity in Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 937056. [Google Scholar] [CrossRef]
- Jeong, W.; Lee, H.; Cho, S.; Seo, J. ApoE4-Induced Cholesterol Dysregulation and Its Brain Cell Type-Specific Implications in the Pathogenesis of Alzheimer’s Disease. Mol. Cells 2019, 42, 739–746. [Google Scholar] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Brionne, T.C.; Tesseur, I.; Masliah, E.; Wyss-Coray, T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 2003, 40, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Sestier, M.-V.; Town, T. Innate immunity in Alzheimer’s disease: A complex affair. CNS Neurol. Disord.-Drug Targets 2013, 12, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress:4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Montine, K.S.; Reich, E.; Neely, M.D.; Sidell, K.R.; Olson, S.J.; Markesbery, W.R.; Montine, T.J. Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer disease is associated with APOE genotype. J. Neuropathol. Exp. Neurol. 1998, 57, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Mattson, M.P. Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer’s disease. Neurobiol. Dis. 2020, 138, 104795. [Google Scholar] [CrossRef] [PubMed]
- Shea, T.B.; Rogers, E.; Ashline, D.; Ortiz, D.; Sheu, M.-S. Apolipoprotein E deficiency promotes increased oxidative stress and compensatory increases in antioxidants in brain tissue. Free. Radic. Biol. Med. 2002, 33, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Dose, J.; Huebbe, P.; Nebel, A.; Rimbach, G. APOE genotype and stress response—A mini review. Lipids Health Dis. 2016, 15, 121. [Google Scholar] [CrossRef]
- Miyata, M.; Smith, J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat. Genet. 1996, 14, 55–61. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Proenca, M.T.; Nogueira, A.J.; Grazina, M.M.; Oliveira, L.M.; Fernandes, A.I.; Santiago, B.; Santana, I.; Oliveira, C.R. Influence of apolipoprotein E genotype on blood redox status of Alzheimer’s disease patients. Int. J. Mol. Med. 1999, 4, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chico, L.; Simoncini, C.; Lo Gerfo, A.; Rocchi, A.; Petrozzi, L.; Carlesi, C.; Volpi, L.; Tognoni, G.; Siciliano, G.; Bonuccelli, U. Oxidative stress and APO E polymorphisms in Alzheimer’s disease and in mild cognitive impairment. Free Radic. Res. 2013, 47, 569–576. [Google Scholar] [CrossRef]
- Siegel, S.J.; Bieschke, J.; Powers, E.T.; Kelly, J.W. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 2007, 46, 1503–1510. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Redox proteomics and amyloid beta-peptide: Insights into Alzheimer disease. J. Neurochem. 2019, 151, 459–487. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Reiman, E.M.; Chen, K.; Alexander, G.E.; Caselli, R.J.; Bandy, D.; Osborne, D.; Saunders, A.M.; Hardy, J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc. Natl. Acad. Sci. USA 2004, 101, 284–289. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’ disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Scheff, S.W.; Ansari, M.A.; Mufson, E.J. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 2016, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Coban, D.T.; Bayindir, A.; Erol, M.K.; Ellidag, H.Y.; Giray, O.; Sayrac, S.; Tekeli, S.O.; Eren, E. Higher serum lipids and oxidative stress in patients with normal tension glaucoma, but not pseudoexfoliative glaucoma. Bosn. J. Basic Med Sci. 2015, 16, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C. Pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J. Fr. Ophtalmol. 2018, 41, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.B.; Barbosa, D.S.; Hsin, C.Y.; Maranhão, R.C.; Abdalla, D.S. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis 1995, 117, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-L.; Shi, Y.-H.; Hao, G.; Li, W.; Le, G.-W. Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index. J. Clin. Biochem. Nutr. 2008, 43, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Roca-Agujetas, V.; Barbero-Camps, E.; de Dios, C.; Podlesniy, P.; Abadin, X.; Morales, A.; Marí, M.; Trullàs, R.; Colell, A. Cholesterol alters mitophagy by impairing optineurin recruitment and lysosomal clearance in Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.; Hesson, L.; Peggie, M.; Cohen, P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008, 582, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, M.L.; Radha, V.; Gupta, V.; Agarwal, N.; Balasubramanian, D.; Swarup, G. A glaucoma-associated mutant of optineurin selectively induces death of retinal ganglion cells which is inhibited by antioxidants. Investig. Opthalmol. Vis. Sci. 2007, 48, 1607–1614. [Google Scholar] [CrossRef]
- Sun, J.-H.; Yu, J.-T.; Tan, L. The role of cholesterol metabolism in Alzheimer’s disease. Mol. Neurobiol. 2015, 51, 947–965. [Google Scholar] [CrossRef]
- Barbero-Camps, E.; Roca-Agujetas, V.; Bartolessis, I.; de Dios, C.; Fernández-Checa, J.C.; Marí, M.; Morales, A.; Hartmann, T.; Colell, A. Cholesterol impairs autophagy-mediated clearance of amyloid beta while promoting its secretion. Autophagy 2018, 14, 1129–1154. [Google Scholar] [CrossRef]
- Fernandez, A.; Llacuna, L.; Fernandez-Checa, J.C.; Colell, A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J. Neurosci. 2009, 29, 6394–6405. [Google Scholar] [CrossRef] [PubMed]
- de Dios, C.; Bartolessis, I.; Roca-Agujetas, V.; Barbero-Camps, E.; Mari, M.; Morales, A.; Colell, A. Oxidative inactivation of amyloid beta-degrading proteases by cholesterol-enhanced mitochondrial stress. Redox Biol. 2019, 26, 101283. [Google Scholar] [CrossRef] [PubMed]
- Barbero-Camps, E.; Fernandez, A.; Martinez, L.; Fernandez-Checa, J.C.; Colell, A. APP/PS1 mice overexpressing SREBP-2 exhibit combined Abeta accumulation and tau pathology underlying Alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3460–3476. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Herve, V.; Ben Khedher, M.R.; Rabanel, J.M.; Ramassamy, C. Glutathione: An Old and Small Molecule with Great Functions and New Applications in the Brain and in Alzheimer’s Disease. Antioxid Redox Signal 2021, 35, 270–292. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Saharan, S.; Tripathi, M.; Murari, G. Brain Glutathione Levels–A novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol. Psychiatry 2015, 78, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Colell, A.; Fernandez, A.; Fernandez-Checa, J.C. Mitochondria, cholesterol and amyloid beta peptide: A dangerous trio in Alzheimer disease. J. Bioenerg. Biomembr. 2009, 41, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Namekata, K.; Kimura, A.; Shitara, H.; Guo, X.; Harada, C.; Mitamura, Y.; Harada, T. Differential effects of N-acetylcysteine on retinal degeneration in two mouse models of normal tension glaucoma. Cell Death Dis. 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.; Noro, T.; Kimura, A.; Guo, X.; Namekata, K.; Nakano, T.; Harada, T. Suppression of Oxidative Stress as Potential Therapeutic Approach for Normal Tension Glaucoma. Antioxidants 2020, 9, 874. [Google Scholar] [CrossRef]
- Aung, T.; Rezaie, T.; Okada, K.; Viswanathan, A.C.; Child, A.H.; Brice, G.; Bhattacharya, S.S.; Lehmann, O.J.; Sarfarazi, M.; Hitchings, R.A. Clinical features and course of patients with glaucoma with the E50K mutation in the optineurin gene. Investig. Opthalmol. Vis. Sci. 2005, 46, 2816–2822. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Z.; Liu, X.; Hou, M.; Cheng, F.; Lei, D.; Yuan, H. The E50K optineurin mutation impacts autophagy-mediated degradation of TDP-43 and leads to RGC apoptosis in vivo and in vitro. Cell Death Discov. 2021, 7, 49. [Google Scholar] [CrossRef]
- Ayala-Lugo, R.M.; Pawar, H.; Reed, D.M.; Lichter, P.R.; Moroi, S.E.; Page, M.; Eadie, J.; Azocar, V.; Maul, E.; Ntim-Amponsah, C.; et al. Variation in optineurin (OPTN) allele frequencies between and within populations. Mol. Vis. 2007, 13, 151–163. [Google Scholar]
- Umeda, T.; Matsuo, T.; Nagayama, M.; Tamura, N.; Tanabe, Y.; Ohtsuki, H. Clinical relevance of optineurin sequence alterations in Japanese glaucoma patients. Ophthalmic Genet. 2004, 25, 91–99. [Google Scholar] [CrossRef]
- Fuse, N.; Takahashi, K.; Akiyama, H.; Nakazawa, T.; Seimiya, M.; Kuwahara, S.; Tamai, M. Molecular genetic analysis of optineurin gene for primary open-angle and normal tension glaucoma in the Japanese population. Eur. J. Gastroenterol. Hepatol. 2004, 13, 299–303. [Google Scholar] [CrossRef]
- Chalasani, M.L.S.; Kumari, A.; Radha, V.; Swarup, G. E50K-OPTN-induced retinal cell death involves the rab GTPase-activating protein, TBC1D17 mediated block in autophagy. PLoS ONE 2014, 9, e95758. [Google Scholar] [CrossRef] [PubMed]
- VanderWall, K.B.; Huang, K.-C.; Pan, Y.; Lavekar, S.S.; Fligor, C.M.; Allsop, A.R.; Lentsch, K.A.; Dang, P.; Zhang, C.; Tseng, H.C.; et al. Retinal Ganglion Cells with a Glaucoma OPTN(E50K) Mutation Exhibit Neurodegenerative Phenotypes when Derived from Three-Dimensional Retinal Organoids. Stem Cell Rep. 2020, 15, 52–66. [Google Scholar] [CrossRef]
- Chi, Z.-L.; Akahori, M.; Obazawa, M.; Minami, M.; Noda, T.; Nakaya, N.; Tomarev, S.; Kawase, K.; Yamamoto, T.; Noda, S.; et al. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Hum. Mol. Genet. 2010, 19, 2606–2615. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; VanderWall, K.B.; Pan, Y.; Lu, X.; Lavekar, S.S.; Huang, K.-C.; Fligor, C.M.; Harkin, J.; Zhang, C.; Cummins, T.R.; et al. Astrocytes modulate neurodegenerative phenotypes associated with glaucoma in OPTN(E50K) human stem cell-derived retinal ganglion cells. Stem Cell Rep. 2022, 17, 1636–1649. [Google Scholar] [CrossRef]
- Minegishi, Y.; Iejima, D.; Kobayashi, H.; Chi, Z.-L.; Kawase, K.; Yamamoto, T.; Seki, T.; Yuasa, S.; Fukuda, K.; Iwata, T. Enhanced optineurin E50K–TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma. Hum. Mol. Genet. 2013, 22, 3559–3567. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Kalamida, D.; Giatromanolaki, A.; Zois, C.E.; Sivridis, E.; Pouliliou, S.; Mitrakas, A.; Gatter, K.C.; Harris, A.L. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS ONE 2015, 10, e0137675. [Google Scholar] [CrossRef] [PubMed]
- Awadalla, M.S.; Fingert, J.H.; Roos, B.E.; Chen, S.; Holmes, R.; Graham, S.L.; Chehade, M.; Galanopolous, A.; Ridge, B.; Souzeau, E.; et al. Copy number variations of TBK1 in Australian patients with primary open-angle glaucoma. Am. J. Ophthalmol. 2015, 159, 124–130.e1. [Google Scholar] [CrossRef]
- Killer, H.; Pircher, A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Demailly, P.; Cambien, F.; Plouin, P.F.; Baron, P.; Chevallier, B. Do patients with low tension glaucoma have particular cardiovascular characteristics? Ophthalmologica 1984, 188, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Tielsch, J.M.; Katz, J.; Sommer, A.; Quigley, H.A.; Javitt, J.C. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch. Ophthalmol. 1995, 113, 216–221. [Google Scholar] [CrossRef]
- Jonas, J.B.; O Naumann, G. Parapapillary retinal vessel diameter in normal and glaucoma eyes. II. Correlations. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1604–1611. [Google Scholar]
- Mitchell, P.; Leung, H.; Wang, J.J.; Rochtchina, E.; Lee, A.J.; Wong, T.Y.; Klein, R. Retinal vessel diameter and open-angle glaucoma: The Blue Mountains Eye Study. Ophthalmology 2005, 112, 245–250. [Google Scholar] [CrossRef]
- Xu, H.; Zhai, R.; Zong, Y.; Kong, X.; Jiang, C.; Sun, X.; He, Y.; Li, X. Comparison of retinal microvascular changes in eyes with high-tension glaucoma or normal-tension glaucoma: A quantitative optic coherence tomography angiographic study. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 1179–1186. [Google Scholar] [CrossRef]
- Berisha, F.; Feke, G.T.; Trempe, C.L.; McMeel, J.W.; Schepens, C.L. Retinal abnormalities in early Alzheimer’s disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2285–2289. [Google Scholar] [CrossRef]
- Kawasaki, R.; Cheung, N.; Mosley, T.; Islam, A.; Sharrett, A.R.; Klein, R.; Coker, L.; Knopman, D.; Shibata, D.; Catellier, D.; et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: The Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010, 41, 1826–1828. [Google Scholar] [CrossRef]
- Patton, N.; Pattie, A.; MacGillivray, T.; Aslam, T.; Dhillon, B.; Gow, A.; Starr, J.M.; Whalley, L.J.; Deary, I.J. The association between retinal vascular network geometry and cognitive ability in an elderly population. Investig. Opthalmol. Vis. Sci. 2007, 48, 1995–2000. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef]
- Vallon, M.; Chang, J.; Zhang, H.; Kuo, C.J. Developmental and pathological angiogenesis in the central nervous system. Cell. Mol. Life Sci. 2014, 71, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef]
- Grammas, P.; Sanchez, A.; Tripathy, D.; Luo, E.; Martinez, J. Vascular signaling abnormalities in Alzheimer disease. Cleve Clin. J. Med 2011, 78 (Suppl. S1), S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Northrup, J.M.; Keyt, B.A.; Takagi, H.; Iwamoto, M.A. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch. Ophthalmol. 1995, 113, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, Z. Neovascular Age-Related Macular Degeneration and its Association with Alzheimer’s Disease. Curr. Aging Sci. 2020, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Bae, D.G.; Kang, H.J.; Gwag, B.J.; Gho, Y.S.; Chae, C.B. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol. Aging 2004, 25, 283–290. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Alvarez, I.; Aleixandre, M.; Linares, C.; Muresanu, D.; Winter, S.; Moessler, H. Severity-Related Increase and Cognitive Correlates of Serum VEGF Levels in Alzheimer’s Disease ApoE4 Carriers. J. Alzheimers Dis. 2018, 63, 1003–1013. [Google Scholar] [CrossRef]
- Ali, M.; Bracko, O. VEGF Paradoxically Reduces Cerebral Blood Flow in Alzheimer’s Disease Mice. Neurosci. Insights 2022, 17, 26331055221109254. [Google Scholar] [CrossRef]
- Harris, R.; Miners, J.S.; Allen, S.; Love, S. VEGFR1 and VEGFR2 in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 741–752. [Google Scholar] [CrossRef]
- Ali, M.; Falkenhain, K.; Njiru, B.N.; Murtaza-Ali, M.; Ruiz-Uribe, N.E.; Haft-Javaherian, M.; Catchers, S.; Nishimura, N.; Schaffer, C.B.; Bracko, O. VEGF signalling causes stalls in brain capillaries and reduces cerebral blood flow in Alzheimer’s mice. Brain 2022, 145, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Reeson, P.; Choi, K.; Brown, C.E. VEGF signaling regulates the fate of obstructed capillaries in mouse cortex. Elife 2018, 7, e33670. [Google Scholar] [CrossRef] [PubMed]
- Akiyode, O.; Dunkelly-Allen, N. Ranibizumab: A Review of Its Use in the Treatment of Diabetic Retinopathy in Patients with Diabetic Macular Edema. J. Pharm. Technol. 2016, 32, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sin, B.H.; Song, B.J.; Park, S.P. Aqueous vascular endothelial growth factor and endothelin-1 levels in branch retinal vein occlusion associated with normal tension glaucoma. J. Glaucoma 2013, 22, 104–109. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Li, H.; Xia, Y.; Xing, M.; Xiao, C.; Cai, W.; Bu, L.; Li, Y.; Park, T.E.; et al. Blockage of VEGF function by bevacizumab alleviates early-stage cerebrovascular dysfunction and improves cognitive function in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 2024, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.U.; Park, I.W.; Suh, W. Long-term intraocular pressure changes after intravitreal injection of bevacizumab. Cutan. Ocul. Toxicol. 2016, 35, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B.; Almukhtar, T.; Bhorade, A.; Bressler, N.M.; Glassman, A.R.; Huang, S.S.; Jampol, L.M.; Kim, J.E.; Melia, M.; Diabetic Retinopathy Clinical Research Network, I. Repeated intravitreous ranibizumab injections for diabetic macular edema and the risk of sustained elevation of intraocular pressure or the need for ocular hypotensive treatment. JAMA Ophthalmol. 2015, 133, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Pirinen, I.; Leinonen, S.; Helminen, M.; Hujanen, P.; Vaajanen, A.; Tuulonen, A.; Uusitalo-Jarvinen, H. Glaucoma progression in patients receiving intravitreal anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol. 2023, 101, 261–265. [Google Scholar] [CrossRef]
- Spini, A.; Giometto, S.; Donnini, S.; Posarelli, M.; Dotta, F.; Ziche, M.; Tosi, G.M.; Girardi, A.; Lucenteforte, E.; Gini, R.; et al. Risk of Intraocular Pressure Increase with Intravitreal Injections of Vascular Endothelial Growth Factor Inhibitors: A Cohort Study. Am. J. Ophthalmol. 2023, 248, 45–50. [Google Scholar] [CrossRef]
- Sultana, J.; Scondotto, G.; Cutroneo, P.M.; Morgante, F.; Trifiro, G. Intravitreal Anti-VEGF Drugs and Signals of Dementia and Parkinson-Like Events: Analysis of the VigiBase Database of Spontaneous Reports. Front. Pharmacol. 2020, 11, 315. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Takeda, N.; Yoshimoto, T.; Matsumoto, S. Hypertensive cerebral hemorrhage with undetectable plasma vascular endothelial growth factor levels in a patient receiving intravitreal injection of aflibercept for bilateral diabetic macular edema: A case report. J. Med. Case Rep. 2021, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Manz, S.N. Brain health assessment in macular degeneration patients undergoing intravitreal anti-vascular endothelial growth factor injections (the bham study): An interim analysis. Retina 2021, 41, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, H.A.; Aslan Bayhan, S.; Celikbilek, A.; Tanik, N.; Gurdal, C. Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin. Exp. Ophthalmol. 2015, 43, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Trebbastoni, A.; Parisi, F.; Manganiello, S.; Cruciani, F.; D’Antonio, F.; De Vico, U.; Imbriano, L.; Campanelli, A.; De Lena, C. Choroidal thinning as a new finding in Alzheimer’s disease: Evidence from enhanced depth imaging spectral domain optical coherence tomography. J. Alzheimers Dis. 2014, 40, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Trebbastoni, A.; Marcelli, M.; Mallone, F.; D’Antonio, F.; Imbriano, L.; Campanelli, A.; de Lena, C.; Gharbiya, M. Attenuation of Choroidal Thickness in Patients with Alzheimer Disease: Evidence From an Italian Prospective Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.P.; Proenca, R.; Dias-Santos, A.; Melancia, D.; Almeida, R.; Aguas, H.; Santos, B.O.; Alves, M.; Ferreira, J.; Papoila, A.L.; et al. Choroidal thinning: Alzheimer’s disease and aging. Alzheimers Dement. 2017, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Hui, V.W.K.; Shi, J.; Wong, M.O.M.; Chan, P.P.; Chan, N.; Lai, I.; Cheung, C.Y.; Tham, C.C. Characterization of macular choroid in normal-tension glaucoma: A swept-source optical coherence tomography study. Acta Ophthalmol. 2021, 99, e1421–e1429. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, X.; Wang, X.; Geng, Z.; Wang, L.; Xiao, G.; Wu, Y.; Zhou, S.; Liao, R.; Wei, L.; et al. The Retinal Vessel Density Can Reflect Cognitive Function in Patients with Alzheimer’s Disease: Evidence from Optical Coherence Tomography Angiography. J. Alzheimers Dis. 2021, 79, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R. Glaucoma, capillaries and pericytes. 1. Blood flow regulation. Ophthalmologica 1996, 210, 257–262. [Google Scholar] [CrossRef]
- Venkataraman, S.T.; Flanagan, J.G.; Hudson, C. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma—A review. Microcirculation 2010, 17, 568–581. [Google Scholar] [CrossRef]
- Drance, S.M. Some factors in the production of low tension glaucoma. Br. J. Ophthalmol. 1972, 56, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Podhajsky, P.; Zimmerman, M.B. Beta-blocker eyedrops and nocturnal arterial hypotension. Am. J. Ophthalmol. 1999, 128, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Jo, Y.H.; Song, M.K.; Won, H.J.; Kook, M.S. Nocturnal blood pressure dip and parapapillary choroidal microvasculature dropout in normal-tension glaucoma. Sci. Rep. 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Jo, Y.H.; Jeong, D.; Shon, K.; Kook, M.S. Baseline Systolic versus Diastolic Blood Pressure Dip and Subsequent Visual Field Progression in Normal-Tension Glaucoma. Ophthalmology 2019, 126, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Melgarejo, J.D.; Lee, J.H.; Petitto, M.; Yepez, J.B.; Murati, F.A.; Jin, Z.; Chavez, C.A.; Pirela, R.V.; Calmon, G.E.; Lee, W.; et al. Glaucomatous Optic Neuropathy Associated with Nocturnal Dip in Blood Pressure: Findings from the Maracaibo Aging Study. Ophthalmology 2018, 125, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tabara, Y.; Igase, M.; Yamamoto, M.; Ochi, N.; Kido, T.; Uetani, E.; Taguchi, K.; Miki, T.; Kohara, K. Abnormal nocturnal blood pressure profile is associated with mild cognitive impairment in the elderly: The J-SHIPP study. Hypertens. Res. 2010, 33, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Su, W.W.; Cheng, S.T.; Hsu, T.S.; Ho, W.J. Abnormal flow-mediated vasodilation in normal-tension glaucoma using a noninvasive determination for peripheral endothelial dysfunction. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3390–3394. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Hadoke, P.W.; Henry, E.; O’Brien, C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br. J. Ophthalmol. 2002, 86, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Galassi, F.; Giambene, B.; Varriale, R. Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4467–4471. [Google Scholar] [CrossRef]
- Sugiyama, T.; Moriya, S.; Oku, H.; Azuma, I. Association of endothelin-1 with normal tension glaucoma: Clinical and fundamental studies. Surv. Ophthalmol. 1995, 39 (Suppl. S1), S49–S56. [Google Scholar] [CrossRef]
- Cellini, M.; Possati, G.L.; Profazio, V.; Sbrocca, M.; Caramazza, N.; Caramazza, R. Color Doppler imaging and plasma levels of endothelin-1 in low-tension glaucoma. Acta Ophthalmol. Scand. Suppl. 1997, 75, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Kleniewska, P.; Kolodziejczyk, M.; Skibska, B.; Goraca, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. 2015, 63, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, S.; Mayama, C.; Tomidokoro, A.; Araie, M. Plasma endothelin-1 level in Japanese normal tension glaucoma patients. Curr. Eye Res. 2006, 31, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Barker, R.; Kehoe, P.G.; Love, S. Endothelin-1 is elevated in Alzheimer’s disease and upregulated by amyloid-beta. J. Alzheimers Dis. 2012, 29, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Meiliuniene, I.; Siesky, B.; Harris, A. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J. Ophthalmol. 2014, 2014, 937360. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Hohman, R.M.; Addicks, E.M.; Massof, R.W.; Green, W.R. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am. J. Ophthalmol. 1983, 95, 673–691. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Allingham, R.R.; Johnson, D.H. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 2008, 115, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Jonas, J.B.; Tian, G.; Zhen, Y.; Ma, K.; Li, S.; Wang, H.; Li, B.; Zhang, X.; Wang, N. Cerebrospinal fluid pressure in glaucoma: A prospective study. Ophthalmology 2010, 117, 259–266. [Google Scholar] [CrossRef]
- Berdahl, J.P.; Fautsch, M.P.; Stinnett, S.S.; Allingham, R.R. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: A case-control study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5412–5418. [Google Scholar] [CrossRef]
- Wang, N.; Yang, D.; Jonas, J.B. Low cerebrospinal fluid pressure in the pathogenesis of primary open-angle glaucoma: Epiphenomenon or causal relationship? The Beijing Intracranial and Intraocular Pressure (iCOP) study. J. Glaucoma 2013, 22 (Suppl. S5), S11–S12. [Google Scholar] [CrossRef]
- Silverberg, G.; Mayo, M.; Saul, T.; Fellmann, J.; McGuire, D. Elevated cerebrospinal fluid pressure in patients with Alzheimer’s disease. Cerebrospinal Fluid Res. 2006, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; Audenaert, K.; De Deyn, P.P. Alzheimer’s disease and glaucoma: Is there a causal relationship? Br. J. Ophthalmol. 2009, 93, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Killer, H.E.; Jaggi, G.P.; Flammer, J.; Miller, N.R.; Huber, A.R. The optic nerve: A new window into cerebrospinal fluid composition? Brain 2006, 129, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Omatiga, A.G.; Onakpoya, O.H.; Idowu, B.M.; Asaleye, C.M.; Adegbehingbe, B.O.; Aderibigbe, A.S. B-mode sonographic evaluation of optic nerve sheath diameter and lens thickness in Nigerian adults with glaucoma. Afr. Health Sci. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Telano, L.N.; Baker, S. Physiology, Cerebral Spinal Fluid. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Killer, H.E. Is stagnant cerebrospinal fluid involved in the pathophysiology of normal tension glaucoma. Prog. Brain Res. 2020, 256, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Gupta, N.; Ahari, A.; Zhou, X.; Hanna, J.; Yucel, Y.H. Evidence for Cerebrospinal Fluid Entry Into the Optic Nerve via a Glymphatic Pathway. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4784–4791. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; Killer, H.E. Normal-Tension Glaucoma: A Glymphopathy? Eye Brain 2023, 15, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Killer, H.E.; Miller, N.R.; Flammer, J.; Meyer, P.; Weinreb, R.N.; Remonda, L.; Jaggi, G.P. Cerebrospinal fluid exchange in the optic nerve in normal-tension glaucoma. Br. J. Ophthalmol. 2012, 96, 544–548. [Google Scholar] [CrossRef]
- Silverberg, G.D.; Mayo, M.; Saul, T.; Rubenstein, E.; McGuire, D. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: A hypothesis. Lancet Neurol. 2003, 2, 506–511. [Google Scholar] [CrossRef]
- Silverberg, G.D.; Heit, G.; Huhn, S.; Jaffe, R.A.; Chang, S.D.; Bronte-Stewart, H.; Rubenstein, E.; Possin, K.; Saul, T.A. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 2001, 57, 1763–1766. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J.; Lehman, D.M.; Kerrigan-Baumrind, L.A.; Merges, C.A.; Pease, M.E.; Kerrigan, D.F.; Ransom, N.L.; Tahzib, N.G.; Reitsamer, H.A.; Levkovitch-Verbin, H.; et al. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1077–1087. [Google Scholar] [PubMed]
- McKinnon, S.J. Glaucoma: Ocular Alzheimer’s disease? Front. Biosci. 2003, 8, s1140–s1156. [Google Scholar] [CrossRef]
- Goldblum, D.; Kipfer-Kauer, A.; Sarra, G.M.; Wolf, S.; Frueh, B.E. Distribution of amyloid precursor protein and amyloid-beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5085–5090. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Fong, J.; Ang, L.C.; Yucel, Y.H. Retinal tau pathology in human glaucomas. Can. J. Ophthalmol. 2008, 43, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bolos, M.; Perea, J.R.; Avila, J. Alzheimer’s disease as an inflammatory disease. Biomol. Concepts 2017, 8, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M. Neuroscience. Garbage truck of the brain. Science 2013, 340, 1529–1530. [Google Scholar] [CrossRef]
- Foglio, E.; Rodella, L.F. Aquaporins and neurodegenerative diseases. Curr. Neuropharmacol. 2010, 8, 112–121. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; Hu, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, S.W.; Janigro, D.; Patabendige, A. Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 2019, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Forstera, B.; Peng, S.; Shi, M.; Ladron-de-Guevara, A.; et al. An ocular glymphatic clearance system removes beta-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef] [PubMed]
- Bidot, S.; Clough, L.; Saindane, A.M.; Newman, N.J.; Biousse, V.; Bruce, B.B. The Optic Canal Size Is Associated with the Severity of Papilledema and Poor Visual Function in Idiopathic Intracranial Hypertension. J. Neuroophthalmol. 2016, 36, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Pircher, A.; Montali, M.; Berberat, J.; Remonda, L.; Killer, H.E. The Optic Canal: A Bottleneck for Cerebrospinal Fluid Dynamics in Normal-Tension Glaucoma? Front. Neurol. 2017, 8, 47. [Google Scholar] [CrossRef]
- Soldatos, T.; Karakitsos, D.; Chatzimichail, K.; Papathanasiou, M.; Gouliamos, A.; Karabinis, A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit. Care 2008, 12, R67. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, T.; Merceron, S.; Benhamou, D.; Vigue, B.; Duranteau, J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008, 34, 2062–2067. [Google Scholar] [CrossRef]
- Bachmann, G.; Achtelik, R.; Nekic, M.; Michel, O. Beta-trace protein in diagnosis of cerebrospinal fluid fistula. HNO 2000, 48, 496–500. [Google Scholar] [CrossRef]
- Hoffmann, A.; Conradt, H.S.; Gross, G.; Nimtz, M.; Lottspeich, F.; Wurster, U. Purification and chemical characterization of beta-trace protein from human cerebrospinal fluid: Its identification as prostaglandin D synthase. J. Neurochem. 1993, 61, 451–456. [Google Scholar] [CrossRef]
- Watanabe, K.; Urade, Y.; Mader, M.; Murphy, C.; Hayaishi, O. Identification of beta-trace as prostaglandin D synthase. Biochem. Biophys. Res. Commun. 1994, 203, 1110–1116. [Google Scholar] [CrossRef]
- Link, H.; Olsson, J.E. Beta-trace protein concentration in CSF in neurological disorders. Acta Neurol. Scand. 1972, 48, 57–68. [Google Scholar] [CrossRef]
- Urade, Y.; Hayaishi, O. Prostaglandin D synthase: Structure and function. Vitam. Horm. 2000, 58, 89–120. [Google Scholar] [CrossRef] [PubMed]
- Beuckmann, C.T.; Gordon, W.C.; Kanaoka, Y.; Eguchi, N.; Marcheselli, V.L.; Gerashchenko, D.Y.; Urade, Y.; Hayaishi, O.; Bazan, N.G. Lipocalin-type prostaglandin D synthase (beta-trace) is located in pigment epithelial cells of rat retina and accumulates within interphotoreceptor matrix. J. Neurosci. 1996, 16, 6119–6124. [Google Scholar] [CrossRef]
- Pircher, A.; Neutzner, A.; Montali, M.; Huber, A.; Scholl, H.P.N.; Berberat, J.; Remonda, L.; Killer, H.E. Lipocalin-type Prostaglandin D Synthase Concentration Gradients in the Cerebrospinal Fluid in Normal-tension Glaucoma Patients with Optic Nerve Sheath Compartmentation. Eye Brain 2021, 13, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kannaian, B.; Sharma, B.; Phillips, M.; Chowdhury, A.; Manimekalai, M.S.S.; Adav, S.S.; Ng, J.T.Y.; Kumar, A.; Lim, S.; Mu, Y.; et al. Abundant neuroprotective chaperone Lipocalin-type prostaglandin D synthase (L-PGDS) disassembles the Amyloid-beta fibrils. Sci. Rep. 2019, 9, 12579. [Google Scholar] [CrossRef]
- Nishida, N.; Nagata, N.; Toda, H.; Jingami, N.; Uemura, K.; Ozaki, A.; Mase, M.; Urade, Y.; Matsumoto, S.; Iwasaki, K.; et al. Association of lipocalin-type prostaglandin D synthase with disproportionately enlarged subarachnoid-space in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS 2014, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, G.P.; Harlev, M.; Ziegler, U.; Dotan, S.; Miller, N.R.; Killer, H.E. Cerebrospinal fluid segregation optic neuropathy: An experimental model and a hypothesis. Br. J. Ophthalmol. 2010, 94, 1088–1093. [Google Scholar] [CrossRef]
- Xin, X.; Huber, A.; Meyer, P.; Flammer, J.; Neutzner, A.; Miller, N.R.; Killer, H.E. L-PGDS (betatrace protein) inhibits astrocyte proliferation and mitochondrial ATP production in vitro. J. Mol. Neurosci. 2009, 39, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Maesaka, J.K.; Sodam, B.; Palaia, T.; Ragolia, L.; Batuman, V.; Miyawaki, N.; Shastry, S.; Youmans, S.; El-Sabban, M. Prostaglandin D2 synthase: Apoptotic factor in alzheimer plasma, inducer of reactive oxygen species, inflammatory cytokines and dialysis dementia. J. Nephropathol. 2013, 2, 166–180. [Google Scholar] [CrossRef]
- Guan, P.P.; Liang, Y.Y.; Cao, L.L.; Yu, X.; Wang, P. Cyclooxygenase-2 Induced the beta-Amyloid Protein Deposition and Neuronal Apoptosis Via Upregulating the Synthesis of Prostaglandin E(2) and 15-Deoxy-Delta(12,14)-prostaglandin J(2). Neurotherapeutics 2019, 16, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Peskind, E.R.; Millard, S.P.; Chi, P.; Sokal, I.; Yu, C.E.; Bekris, L.M.; Raskind, M.A.; Galasko, D.R.; Montine, T.J. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS ONE 2009, 4, e5424. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Stransky, E.; Leyhe, T.; Eschweiler, G.W.; Maetzler, W.; Wittorf, A.; Soekadar, S.; Richartz, E.; Koehler, N.; Bartels, M.; et al. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J. Psychiatr. Res. 2007, 41, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; Diniz, B.S.; Teixeira, A.L.; Radanovic, M.; Talib, L.L.; Rocha, N.P.; Gattaz, W.F. Lower Cerebrospinal Fluid Concentration of Brain-Derived Neurotrophic Factor Predicts Progression from Mild Cognitive Impairment to Alzheimer’s Disease. Neuromol. Med. 2015, 17, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Bruno, D.; Sarreal, A.S.; Hernando, R.T.; Saint-Louis, L.A.; Nierenberg, J.; Ginsberg, S.D.; Pomara, N.; Mehta, P.D.; Zetterberg, H.; et al. Plasma BDNF levels vary in relation to body weight in females. PLoS ONE 2012, 7, e39358. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takada, N.; Fujioka, A.; Himori, N.; Yokoyama, Y.; Tsuda, S.; Omodaka, K.; Kirihara, T.; Ishikawa, M.; Kunikata, H.; et al. Reduced Plasma BDNF Levels in Normal Tension Glaucoma Compared to Open Angle Glaucoma. J. Glaucoma 2023, 32, 734–737. [Google Scholar] [CrossRef]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Romano, G.L.; Eandi, C.M.; Toro, M.D.; Rejdak, R.; Di Benedetto, G.; Lazzara, F.; Bernardini, R.; Drago, F.; Cantarella, G.; et al. Brimonidine is Neuroprotective in Animal Paradigm of Retinal Ganglion Cell Damage. Front. Pharmacol. 2021, 12, 705405. [Google Scholar] [CrossRef]
- Yukita, M.; Omodaka, K.; Machida, S.; Yasuda, M.; Sato, K.; Maruyama, K.; Nishiguchi, K.M.; Nakazawa, T. Brimonidine Enhances the Electrophysiological Response of Retinal Ganglion Cells through the Trk-MAPK/ERK and PI3K Pathways in Axotomized Eyes. Curr. Eye Res. 2017, 42, 125–133. [Google Scholar] [CrossRef]
- Shen, T.; Gupta, V.K.; Klistorner, A.; Chitranshi, N.; Graham, S.L.; You, Y. Sex-Specific Effect of BDNF Val66Met Genotypes on the Progression of Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1069–1075. [Google Scholar] [CrossRef]
- Quigley, H.A.; McKinnon, S.J.; Zack, D.J.; Pease, M.E.; Kerrigan-Baumrind, L.A.; Kerrigan, D.F.; Mitchell, R.S. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3460–3466. [Google Scholar]
- Gonzalez, H.; Elgueta, D.; Montoya, A.; Pacheco, R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J. Neuroimmunol. 2014, 274, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.; Ruscica, M.; Dozio, E.; Rizzi, E.; Beretta, G.; Maffei Facino, R. Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-alpha and NF-kappaB nuclear translocation in BV-2 microglia. Phytother. Res. 2012, 26, 1405–1409. [Google Scholar] [CrossRef]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Polans, J.; Keller, B.; Carrasco-Zevallos, O.M.; LaRocca, F.; Cole, E.; Whitson, H.E.; Lad, E.M.; Farsiu, S.; Izatt, J.A. Wide-field retinal optical coherence tomography with wavefront sensorless adaptive optics for enhanced imaging of targeted regions. Biomed. Opt. Express 2017, 8, 16–37. [Google Scholar] [CrossRef]
- Kirbas, S.; Turkyilmaz, K.; Anlar, O.; Tufekci, A.; Durmus, M. Retinal nerve fiber layer thickness in patients with Alzheimer disease. J. Neuroophthalmol. 2013, 33, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Tepelus, T.C.; Song, S.; Borrelli, E.; Nittala, M.G.; Baghdasaryan, E.; Sadda, S.R.; Chopra, V. Quantitative Analysis of Retinal and Choroidal Vascular Parameters in Patients with Low Tension Glaucoma. J. Glaucoma 2019, 28, 557–562. [Google Scholar] [CrossRef]
- Parisi, V.; Restuccia, R.; Fattapposta, F.; Mina, C.; Bucci, M.G.; Pierelli, F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin. Neurophysiol. 2001, 112, 1860–1867. [Google Scholar] [CrossRef]
- Tan, O.; Chopra, V.; Lu, A.T.; Schuman, J.S.; Ishikawa, H.; Wollstein, G.; Varma, R.; Huang, D. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology 2009, 116, 2305–2314. [Google Scholar] [CrossRef]
- Eraslan, M.; Cerman, E.; Cekic, O.; Balci, S.; Dericioglu, V.; Sahin, O.; Suer, D.; Chabou, B.; Tuncer Elmaci, E.N. Neurodegeneration in ocular and central nervous systems: Optical coherence tomography study in normal-tension glaucoma and Alzheimer disease. Turk. J. Med. Sci. 2015, 45, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.R.; Lee, E.S.; Seong, G.J.; Kim, J.H.; An, H.G.; Kim, C.Y. Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4646–4651. [Google Scholar] [CrossRef] [PubMed]
- Asanad, S.; Ross-Cisneros, F.N.; Nassisi, M.; Barron, E.; Karanjia, R.; Sadun, A.A. The Retina in Alzheimer’s Disease: Histomorphometric Analysis of an Ophthalmologic Biomarker. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Huang, E.J.; Kuo, C.N.; Wu, P.L.; Chen, C.L.; Wu, P.C.; Wu, S.H.; King, Y.C.; Lai, C.H. The relationship between age, axial length and retinal nerve fiber layer thickness in the normal elderly population in Taiwan: The Chiayi eye study in Taiwan. PLoS ONE 2018, 13, e0194116. [Google Scholar] [CrossRef]
- Parisi, V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin. Ophthalmol. 2003, 18, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Iseri, P.K.; Altinas, O.; Tokay, T.; Yuksel, N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J. Neuroophthalmol. 2006, 26, 18–24. [Google Scholar] [CrossRef]
- Kergoat, H.; Kergoat, M.J.; Justino, L.; Chertkow, H.; Robillard, A.; Bergman, H. An evaluation of the retinal nerve fiber layer thickness by scanning laser polarimetry in individuals with dementia of the Alzheimer type. Acta Ophthalmol. Scand. 2001, 79, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; Boissonnot, M.; Roger, F.; Dighiero, P.; Gil, R.; Hugon, J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2007, 420, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Markopoulos, I.; Chatziralli, I.; Rouvas, A.; Papageorgiou, S.G.; Ladas, I.; Vassilopoulos, D. Structural and functional impairment of the retina and optic nerve in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 782–788. [Google Scholar] [CrossRef]
- Marziani, E.; Pomati, S.; Ramolfo, P.; Cigada, M.; Giani, A.; Mariani, C.; Staurenghi, G. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5953–5958. [Google Scholar] [CrossRef]
- Moreno-Ramos, T.; Benito-Leon, J.; Villarejo, A.; Bermejo-Pareja, F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. J. Alzheimers Dis. 2013, 34, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Kromer, R.; Serbecic, N.; Hausner, L.; Froelich, L.; Aboul-Enein, F.; Beutelspacher, S.C. Detection of Retinal Nerve Fiber Layer Defects in Alzheimer’s Disease Using SD-OCT. Front. Psychiatry 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Wu, Y.; Wang, M.; Cao, J.; Feng, W.; Cheng, Y.; Li, C.; Shen, Y. Greater attenuation of retinal nerve fiber layer thickness in Alzheimer’s disease patients. J. Alzheimers Dis. 2014, 40, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Renzo, A.; Ziccardi, L.; Martelli, F.; Fadda, A.; Manni, G.; Barboni, P.; Pierelli, F.; Sadun, A.A.; Parisi, V. Optical Coherence Tomography in Alzheimer’s Disease: A Meta-Analysis. PLoS ONE 2015, 10, e0134750. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Li, X.; Bai, Q.; Liu, P. Abnormal retinal nerve fiber layer thickness and macula lutea in patients with mild cognitive impairment and Alzheimer’s disease. Arch. Gerontol. Geriatr. 2015, 60, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Li, Z.; Zhang, X.; Wu, Y.; Yang, H.; Min, B.; Zhang, X.; Ma, D.; Lu, Y. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- La Morgia, C.; Ross-Cisneros, F.N.; Koronyo, Y.; Hannibal, J.; Gallassi, R.; Cantalupo, G.; Sambati, L.; Pan, B.X.; Tozer, K.R.; Barboni, P.; et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 2016, 79, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.P.; Moura-Coelho, N.; Proenca, R.P.; Dias-Santos, A.; Ferreira, J.; Louro, C.; Castanheira-Dinis, A. Alzheimer’s disease: A review of its visual system neuropathology. Optical coherence tomography—A potential role as a study tool in vivo. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Doustar, J.; Torbati, T.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Optical Coherence Tomography in Alzheimer’s Disease and Other Neurodegenerative Diseases. Front. Neurol. 2017, 8, 701. [Google Scholar] [CrossRef]
- Ferrari, L.; Huang, S.C.; Magnani, G.; Ambrosi, A.; Comi, G.; Leocani, L. Optical Coherence Tomography Reveals Retinal Neuroaxonal Thinning in Frontotemporal Dementia as in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 56, 1101–1107. [Google Scholar] [CrossRef]
- Polo, V.; Rodrigo, M.J.; Garcia-Martin, E.; Otin, S.; Larrosa, J.M.; Fuertes, M.I.; Bambo, M.P.; Pablo, L.E.; Satue, M. Visual dysfunction and its correlation with retinal changes in patients with Alzheimer’s disease. Eye 2017, 31, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Yaman, A.; Erol, M.K.; Kurtulus, F.; Toslak, D.; Dogan, B.; Turgut Coban, D.; Kaya Basar, E. Choroidal Thickness in Patients with Mild Cognitive Impairment and Alzheimer’s Type Dementia. J. Ophthalmol. 2016, 2016, 7291257. [Google Scholar] [CrossRef] [PubMed]
- Janez-Escalada, L.; Janez-Garcia, L.; Salobrar-Garcia, E.; Santos-Mayo, A.; de Hoz, R.; Yubero, R.; Gil, P.; Ramirez, J.M. Spatial analysis of thickness changes in ten retinal layers of Alzheimer’s disease patients based on optical coherence tomography. Sci. Rep. 2019, 9, 13000. [Google Scholar] [CrossRef] [PubMed]
- Czako, C.; Kovacs, T.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; Csipo, T.; Lipecz, A.; Horvath, H.; Sandor, G.L.; et al. Retinal biomarkers for Alzheimer’s disease and vascular cognitive impairment and dementia (VCID): Implication for early diagnosis and prognosis. Geroscience 2020, 42, 1499–1525. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, O.M.; Koronyo-Hamaoui, M. Retinal vessel changes in cerebrovascular disease. Curr. Opin. Neurol. 2020, 33, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Vergara, A.J.; Karanjia, R.; Sadun, A.A. OCT parameters of the optic nerve head and the retina as surrogate markers of brain volume in a normal population, a pilot study. J. Neurol. Sci. 2021, 420, 117213. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Votruba, M. Can the retina be used to diagnose and plot the progression of Alzheimer’s disease? Acta Ophthalmol. 2017, 95, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.T.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef] [PubMed]
- den Haan, J.; Verbraak, F.D.; Visser, P.J.; Bouwman, F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimers Dement. 2017, 6, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.R.; Sadun, A.A.; Blanks, J.C.; Miller, C.A. Optic-nerve degeneration in Alzheimer’s disease. N. Engl. J. Med. 1986, 315, 485–487. [Google Scholar] [CrossRef]
- Blanks, J.C.; Schmidt, S.Y.; Torigoe, Y.; Porrello, K.V.; Hinton, D.R.; Blanks, R.H. Retinal pathology in Alzheimer’s disease. II. Regional neuron loss and glial changes in GCL. Neurobiol. Aging 1996, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Blanks, J.C.; Hinton, D.R.; Sadun, A.A.; Miller, C.A. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res. 1989, 501, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Sadun, A.A.; Bassi, C.J. Optic nerve damage in Alzheimer’s disease. Ophthalmology 1990, 97, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Syed, A.B. Alzheimer’s disease and the eye. Ophthalmic Physiol. Opt. 1996, 16 (Suppl. S1), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Risacher, S.L.; WuDunn, D.; Tallman, E.F.; West, J.D.; Gao, S.; Farlow, M.R.; Brosch, J.R.; Apostolova, L.G.; Saykin, A.J. Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Commun. 2020, 2, fcaa019. [Google Scholar] [CrossRef] [PubMed]
- Trick, G.L.; Barris, M.C.; Bickler-Bluth, M. Abnormal pattern electroretinograms in patients with senile dementia of the Alzheimer type. Ann. Neurol. 1989, 26, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Tam, S.K.; Hughes, S.; Pothecary, C.A.; Jagannath, A.; Hankins, M.W.; Bannerman, D.M.; Lightman, S.L.; Vyazovskiy, V.V.; Nolan, P.M.; et al. Melanopsin Regulates Both Sleep-Promoting and Arousal-Promoting Responses to Light. PLoS Biol. 2016, 14, e1002482. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Holtzman, D.M. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2020, 45, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, L.; Moorthy, M.; Mathew, S.; Siesky, B.; Verticchio Vercellin, A.C.; Price, D.; Januleviciene, I.; Harris, A. Circadian Rhythm and Glaucoma: What do We Know? J. Glaucoma 2020, 29, 127–132. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Ding, J.; Wang, N. Changes in the circadian rhythm in patients with primary glaucoma. PLoS ONE 2013, 8, e62841. [Google Scholar] [CrossRef] [PubMed]
- Drouyer, E.; Dkhissi-Benyahya, O.; Chiquet, C.; WoldeMussie, E.; Ruiz, G.; Wheeler, L.A.; Denis, P.; Cooper, H.M. Glaucoma alters the circadian timing system. PLoS ONE 2008, 3, e3931. [Google Scholar] [CrossRef] [PubMed]
- Obara, E.A.; Hannibal, J.; Heegaard, S.; Fahrenkrug, J. Loss of Melanopsin-Expressing Retinal Ganglion Cells in Severely Staged Glaucoma Patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Lanzani, M.F.; de Zavalia, N.; Fontana, H.; Sarmiento, M.I.; Golombek, D.; Rosenstein, R.E. Alterations of locomotor activity rhythm and sleep parameters in patients with advanced glaucoma. Chronobiol. Int. 2012, 29, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.S.; Ritch, R.; Schwartz, B.; Lee, S.S.; Miller, N.R.; Chi, T.; Hsieh, F.Y. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch. Ophthalmol. 1991, 109, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.R.; Xu, L.; Yang, Y. A comparative study of optic nerve damage between primary open angle glaucoma and normal tension glaucoma. Zhonghua Yan Ke Za Zhi 2005, 41, 136–140. [Google Scholar] [PubMed]
- Frezzotti, P.; Giorgio, A.; Toto, F.; De Leucio, A.; De Stefano, N. Early changes of brain connectivity in primary open angle glaucoma. Hum. Brain Mapp. 2016, 37, 4581–4596. [Google Scholar] [CrossRef]
- Jiang, M.M.; Zhou, Q.; Liu, X.Y.; Shi, C.Z.; Chen, J.; Huang, X.H. Structural and functional brain changes in early- and mid-stage primary open-angle glaucoma using voxel-based morphometry and functional magnetic resonance imaging. Medicine 2017, 96, e6139. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Morelli, J.N.; Ai, F.; Yin, D.; Hu, C.; Xu, D.; Li, Y. Resting-state functional MRI: Functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. Hum. Brain Mapp. 2013, 34, 2455–2463. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Wang, N.; Xian, J.; He, H. Graph theoretical analysis reveals the reorganization of the brain network pattern in primary open angle glaucoma patients. Eur. Radiol. 2016, 26, 3957–3967. [Google Scholar] [CrossRef]
- Song, S.K.; Sun, S.W.; Ramsbottom, M.J.; Chang, C.; Russell, J.; Cross, A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.J.; Fisman, M.; Hachinski, V.; Blume, W.; Fox, A.; Kral, V.A.; Kirshen, A.J.; Fox, H.; Merskey, H. A new definition of Alzheimer’s disease: A hippocampal dementia. Lancet 1985, 1, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; Morris, J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 1999, 45, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Larossa, G.N.; Sheline, Y.I.; Dence, C.S.; Lee, S.Y.; Mach, R.H.; Klunk, W.E.; Mathis, C.A.; DeKosky, S.T.; Morris, J.C. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology 2006, 67, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R., Jr.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Leow, A.D.; Parikshak, N.; Lee, S.; Chiang, M.C.; Toga, A.W.; Jack, C.R., Jr.; Weiner, M.W.; Thompson, P.M.; Alzheimer’s Disease Neuroimaging, I. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: An MRI study of 676 AD, MCI, and normal subjects. Neuroimage 2008, 43, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 2011, 32, 1341–1371. [Google Scholar] [CrossRef]
- Braskie, M.N.; Jahanshad, N.; Stein, J.L.; Barysheva, M.; Johnson, K.; McMahon, K.L.; de Zubicaray, G.I.; Martin, N.G.; Wright, M.J.; Ringman, J.M.; et al. Relationship of a variant in the NTRK1 gene to white matter microstructure in young adults. J. Neurosci. 2012, 32, 5964–5972. [Google Scholar] [CrossRef] [PubMed]
- Karas, G.B.; Scheltens, P.; Rombouts, S.A.; Visser, P.J.; van Schijndel, R.A.; Fox, N.C.; Barkhof, F. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage 2004, 23, 708–716. [Google Scholar] [CrossRef]
- Grady, C.L.; Furey, M.L.; Pietrini, P.; Horwitz, B.; Rapoport, S.I. Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain 2001, 124, 739–756. [Google Scholar] [CrossRef]
- Bozzali, M.; Falini, A.; Franceschi, M.; Cercignani, M.; Zuffi, M.; Scotti, G.; Comi, G.; Filippi, M. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 2002, 72, 742–746. [Google Scholar] [CrossRef]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef] [PubMed]
- Naggara, O.; Oppenheim, C.; Rieu, D.; Raoux, N.; Rodrigo, S.; Dalla Barba, G.; Meder, J.F. Diffusion tensor imaging in early Alzheimer’s disease. Psychiatry Res. 2006, 146, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Jones, B.F.; Nolte, G.; Breakspear, M.; Scheltens, P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb. Cortex 2007, 17, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lowe, M.J.; Dzemidzic, M.; Lurito, J.T.; Mathews, V.P.; Phillips, M.D. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. NeuroImage 2000, 12, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D.; Haughton, V.M.; Arfanakis, K.; Wendt, G.J.; Turski, P.A.; Moritz, C.H.; Quigley, M.A.; Meyerand, M.E. Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am. J. Neuroradiol. 2000, 21, 1636–1644. [Google Scholar] [PubMed]
- Golden, H.L.; Agustus, J.L.; Nicholas, J.M.; Schott, J.M.; Crutch, S.J.; Mancini, L.; Warren, J.D. Functional neuroanatomy of spatial sound processing in Alzheimer’s disease. Neurobiol. Aging 2016, 39, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Akrofi, K.; Carpenter-Thompson, J.R.; Husain, F.T. Default mode, dorsal attention and auditory resting state networks exhibit differential functional connectivity in tinnitus and hearing loss. PLoS ONE 2013, 8, e76488. [Google Scholar] [CrossRef]
- Zhao, J.; Du, Y.H.; Ding, X.T.; Wang, X.H.; Men, G.Z. Alteration of functional connectivity in patients with Alzheimer’s disease revealed by resting-state functional magnetic resonance imaging. Neural Regen. Res. 2020, 15, 285–292. [Google Scholar] [CrossRef]



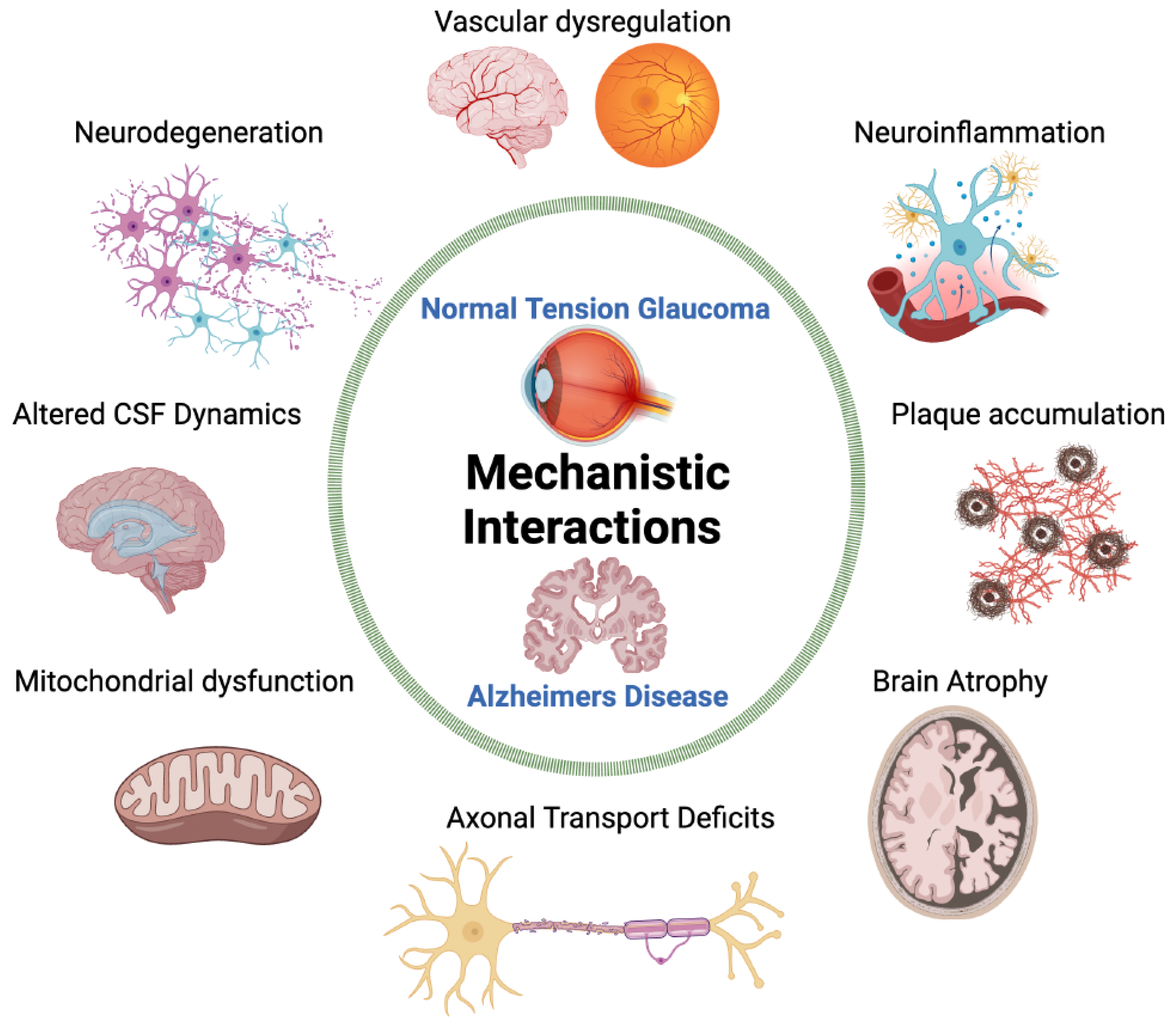
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.; Bodi, N.E.; Sharma, T.P. Normal-Tension Glaucoma and Potential Clinical Links to Alzheimer’s Disease. J. Clin. Med. 2024, 13, 1948. https://doi.org/10.3390/jcm13071948
Ho K, Bodi NE, Sharma TP. Normal-Tension Glaucoma and Potential Clinical Links to Alzheimer’s Disease. Journal of Clinical Medicine. 2024; 13(7):1948. https://doi.org/10.3390/jcm13071948
Chicago/Turabian StyleHo, Kathleen, Nicole E. Bodi, and Tasneem P. Sharma. 2024. "Normal-Tension Glaucoma and Potential Clinical Links to Alzheimer’s Disease" Journal of Clinical Medicine 13, no. 7: 1948. https://doi.org/10.3390/jcm13071948





