CARDS, a Novel Prognostic Index for Risk Stratification and In-Hospital Monitoring
Abstract
:1. Introduction
2. Materials and Methods
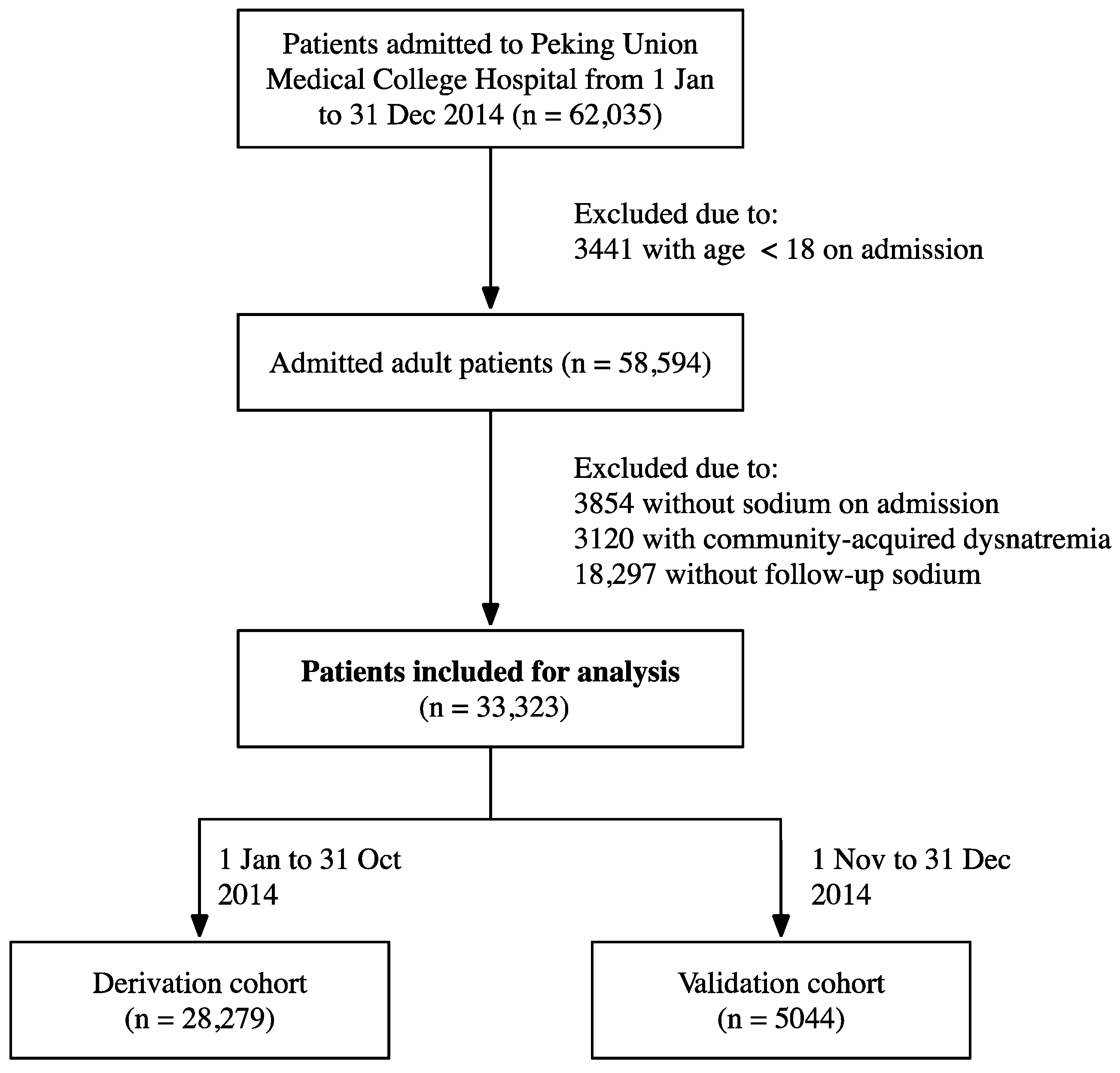
2.1. Study Population and Definition
2.2. Data Extraction and Outcome
2.3. Model Development
2.4. Model Validation
2.5. Statistics
3. Results
3.1. Patient Characteristics
3.2. Logistic Regression
3.3. Risk Stratification with CARDS
3.4. Simplified Risk Stratification with CARDS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Mawed, S.; Pankratz, V.S.; Chong, K.; Sandoval, M.; Roumelioti, M.E.; Unruh, M. Low serum sodium levels at hospital admission: Outcomes among 2.3 million hospitalized patients. PLoS ONE 2018, 13, e0194379. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006, 119 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Diker-Cohen, T.; Steinmetz, T.; Amitai, O.; Shimon, I. Sodium levels on admission are associated with mortality risk in hospitalized patients. Eur. J. Intern. Med. 2017, 46, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H. Disorders of plasma sodium—Causes, consequences, and correction. N. Engl. J. Med. 2015, 372, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Palevsky, P.M.; Bhagrath, R.; Greenberg, A. Hypernatremia in hospitalized patients. Ann. Intern. Med. 1996, 124, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Boscoe, A.; Paramore, C.; Verbalis, J.G. Cost of illness of hyponatremia in the United States. Cost. Eff. Resour. Alloc. 2006, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Mount, D.B.; Curhan, G.C. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am. J. Med. 2009, 122, 857–865. [Google Scholar] [CrossRef]
- Laureno, R.; Karp, B.I. Myelinolysis after correction of hyponatremia. Ann. Intern. Med. 1997, 126, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Ferraro, P.M.; Calvaruso, L.; Naticchia, A.; D’alonzo, S.; Gambaro, G. Sodium Fluctuations and Mortality in a General Hospitalized Population. Kidney Blood Press. Res. 2019, 44, 604–614. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W.; Yap, J.Q.; Qian, Q. Increased mortality risk associated with serum sodium variations and borderline hypo- and hypernatremia in hospitalized adults. Nephrol. Dial. Transplant 2020, 35, 1746–1752. [Google Scholar] [CrossRef]
- Sakr, Y.; Rother, S.; Ferreira, A.M.; Ewald, C.; Dünisch, P.; Riedemmann, N.; Reinhart, K. Fluctuations in serum sodium level are associated with an increased risk of death in surgical ICU patients. Crit. Care Med. 2013, 41, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Topjian, A.A.; Stuart, A.; Pabalan, A.A.; Clair, A.; Kilbaugh, T.J.; Abend, N.S.; Storm, P.B.; Berg, R.A.; Huh, J.W.; Friess, S.H. Greater fluctuations in serum sodium levels are associated with increased mortality in children with externalized ventriculostomy drains in a PICU. Pediatr. Crit. Care Med. 2014, 15, 846–855. [Google Scholar] [CrossRef]
- Marshall, D.C.; Salciccioli, J.D.; Goodson, R.J.; Pimentel, M.A.; Sun, K.Y.; Celi, L.A.; Shalhoub, J. The association between sodium fluctuations and mortality in surgical patients requiring intensive care. J. Crit. Care 2017, 40, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Chen, S.; Zhang, Y.; Zhu, H.; Pan, H. Sodium fluctuation, a novel single parameter to predict hospital mortality. Eur. J. Intern. Med. 2021, 85, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Feinstein, A.R.; Holford, T.R. The risk of determining risk with multivariable models. Ann. Intern. Med. 1993, 118, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, Y.; Zhang, X.; Pang, C.; Wang, Y.; Nigwekar, S.U.; Qiu, L.; Chen, L. The prevalence and mortality of hyponatremia is seriously underestimated in Chinese general medical patients: An observational retrospective study. BMC Nephrol. 2017, 18, 328. [Google Scholar] [CrossRef]
- Spatenkova, V.; Bradac, O.; de Lacy, P.; Skrabalek, P.; Suchomel, P. Dysnatremia as a poor prognostic indicator in patients with acute subarachnoid hemorrhage. J. Neurosurg. Sci. 2017, 61, 371–379. [Google Scholar] [CrossRef]
- Shein, S.L.; Slain, K.; Martinez Schlurmann, N.; Speicher, R.; Rotta, A.T. Hyponatremia and Hypotonic Intravenous Fluids Are Associated with Unfavorable Outcomes of Bronchiolitis Admissions. Hosp. Pediatr. 2017, 7, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Chen, H.L.; Ni, S.S. Hyponatremia and short-term prognosis of patients with acute pulmonary embolism: A meta-analysis. Int. J. Cardiol. 2017, 227, 251–256. [Google Scholar] [CrossRef]
- Braconnier, P.; Delforge, M.; Garjau, M.; Wissing, K.M.; De Wit, S. Hyponatremia is a marker of disease severity in HIV-infected patients: A retrospective cohort study. BMC Infect. Dis. 2017, 17, 98. [Google Scholar] [CrossRef]
- Berardi, R.; Santoni, M.; Newsom-Davis, T.; Caramanti, M.; Rinaldi, S.; Tiberi, M.; Morgese, F.; Torniai, M.; Pistelli, M.; Onofri, A.; et al. Hyponatremia normalization as an independent prognostic factor in patients with advanced non-small cell lung cancer treated with first-line therapy. Oncotarget 2017, 8, 23871–23879. [Google Scholar] [CrossRef] [PubMed]
- Avci, B.K.; Kucuk, M.; Muderrisoglu, H.; Eren, M.; Kutlu, M.; Yılmaz, M.B.; Çavuşoğlu, Y.; Öngen, Z. Relation between serum sodium levels and clinical outcomes in Turkish patients hospitalized for heart failure: A multi-center retrospective observational study. Anatol. J. Cardiol. 2017, 17, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Glezerman, I.G.; Boklage, S.H.; Chiodo, J.; Tidwell, B.A.; Lamerato, L.E.; Schulman, K.L. The occurrence of hyponatremia and its importance as a prognostic factor in a cross-section of cancer patients. BMC Cancer 2016, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Chalela, R.; Gonzalez-Garcia, J.G.; Chillaron, J.J.; Valera-Hernández, L.; Montoya-Rangel, C.; Badenes, D.; Mojal, S.; Gea, J. Impact of hyponatremia on mortality and morbidity in patients with COPD exacerbations. Respir. Med. 2016, 117, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Geng, X.; Chen, R.; Zhang, P.; Lin, J.; Teng, J.; Zhang, X.; Ding, X. Dysnatremia is an independent indicator of mortality in hospitalized patients. Med. Sci. Monit. 2017, 23, 2408–2425. [Google Scholar] [CrossRef] [PubMed]
- Cluitmans, F.H.; Meinders, A.E. Management of severe hyponatremia: Rapid or slow correction? Am. J. Med. 1990, 88, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.; Funk, G.C. Hypernatremia in critically ill patients. J. Crit. Care 2013, 28, 216.e211–216.e220. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.K. Hypernatremic disorders in the intensive care unit. J. Intensive Care Med. 2013, 28, 37–45. [Google Scholar] [CrossRef]
- Peri, A. Morbidity and Mortality of Hyponatremia. Front. Horm. Res. 2019, 52, 36–48. [Google Scholar]
- Poulikakos, D.; Banerjee, D.; Malik, M. Risk of sudden cardiac death in chronic kidney disease. J. Cardiovasc. Electrophysiol. 2014, 25, 222–231. [Google Scholar] [CrossRef]
- Di Lullo, L.; Rivera, R.; Barbera, V.; Bellasi, A.; Cozzolino, M.; Russo, D.; De Pascalis, A.; Banerjee, D.; Floccari, F.; Ronco, C. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. Int. J. Cardiol. 2016, 217, 16–27. [Google Scholar] [CrossRef]
- Clancy, C.E.; Chen-Izu, Y.; Bers, D.M.; Belardinelli, L.; Boyden, P.A.; Csernoch, L.; Despa, S.; Fermini, B.; Hool, L.C.; Izu, L.; et al. Deranged sodium to sudden death. J. Physiol. 2015, 593, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Bay, J.; Kohlhaas, M.; Maack, C. Intracellular Na(+) and cardiac metabolism. J. Mol. Cell Cardiol. 2013, 61, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Eigel, B.N.; Hadley, R.W. Contribution of the Na(+) channel and Na(+)/H(+) exchanger to the anoxic rise of [Na(+)] in ventricular myocytes. Am. J. Physiol. 1999, 277, H1817–H1822. [Google Scholar] [CrossRef] [PubMed]
- Gines, P.; Guevara, M. Hyponatremia in cirrhosis: Pathogenesis, clinical significance, and management. Hepatology 2008, 48, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, E.J.; Zietse, R. Hyponatremia and mortality: Moving beyond associations. Am. J. Kidney Dis. 2013, 62, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W. Water and sodium retention in edematous disorders: Role of vasopressin and aldosterone. Am. J. Med. 2006, 119 (Suppl. S1), S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Soiza, R.L.; Cumming, K.; Clarke, J.M.; Wood, K.M.; Myint, P.K. Hyponatremia: Special Considerations in Older Patients. J. Clin. Med. 2014, 3, 944–958. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Clinical Excellence: Guidance. Acutely Ill Patients in Hospital: Recognition of and Response to Acute Illness in Adults in Hospital; National Institute for Health and Clinical Excellence: London, UK, 2007. [Google Scholar]
- Storm-Versloot, M.N.; Verweij, L.; Lucas, C.; Ludikhuize, J.; Goslings, J.C.; Legemate, D.A.; Vermeulen, H. Clinical relevance of routinely measured vital signs in hospitalized patients: A systematic review. J. Nurs. Scholarsh. 2014, 46, 39–49. [Google Scholar] [CrossRef]
- Zeitz, K.; McCutcheon, H. Observations and vital signs: Ritual or vital for the monitoring of postoperative patients? Appl. Nurs. Res. 2006, 19, 204–211. [Google Scholar] [CrossRef]
- Lee, A.; Bishop, G.; Hillman, K.M.; Daffurn, K. The Medical Emergency Team. Anaesth. Intensive Care 1995, 23, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Hodgetts, T.J.; Kenward, G.; Vlachonikolis, I.G.; Payne, S.; Castle, N. The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation 2002, 54, 125–131. [Google Scholar] [CrossRef]
- Gao, H.; McDonnell, A.; Harrison, D.A.; Moore, T.; Adam, S.; Daly, K.; Esmonde, L.; Goldhill, D.R.; Parry, G.J.; Rashidian, A.; et al. Systematic review and evaluation of physiological track and trigger warning systems for identifying at-risk patients on the ward. Intensive Care Med. 2007, 33, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.O.; Cuthbertson, B.H. Detecting critical illness outside the ICU: The role of track and trigger systems. Curr. Opin. Crit. Care 2010, 16, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Shiloh, A.L.; Lominadze, G.; Gong, M.N.; Savel, R.H.; Shiloh, A.L. Early Warning/Track-and-Trigger Systems to Detect Deterioration and Improve Outcomes in Hospitalized Patients. Semin. Respir. Crit. Care Med. 2016, 37, 88–95. [Google Scholar] [PubMed]
- Smith, G.B.; Prytherch, D.R.; Schmidt, P.E.; Featherstone, P.I.; Higgins, B. A review, and performance evaluation, of single-parameter “track and trigger” systems. Resuscitation 2008, 79, 11–21. [Google Scholar]
- Wuytack, F.; Meskell, P.; Conway, A.; McDaid, F.; Santesso, N.; Hickey, F.G.; Gillespie, P.; Raymakers, A.J.N.; Smith, V.; Devane, D. The effectiveness of physiologically based early warning or track and trigger systems after triage in adult patients presenting to emergency departments: A systematic review. BMC Emerg. Med. 2017, 17, 38. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Geng, X.; Zhang, X.; Xu, X.; Lin, J.; Teng, J.; Ding, X. A novel scoring system for assessing the severity of electrolyte and acid-base disorders and predicting outcomes in hospitalized patients. J. Investig. Med. 2019, 67, 750–760. [Google Scholar] [CrossRef]

| Derivation Cohort (n = 28,279) | Validation Cohort (n = 5044) | |
|---|---|---|
| Female, No. (%) | 14,567 (51.5) | 2627 (52.1) |
| Age, mean (SD), yr | 53.2 (15.4) | 53.1 (15.1) |
| CCI, median (IQR) | 2 (1–2) | 2 (1–2) |
| Sodium, mean (SD), mmol/L | ||
| Admission level | 140.05 (2.36) | 139.85 (2.25) |
| Lowest level | 136.63 (3.69) | 136.69 (3.34) |
| Highest level | 142.55 (2.94) | 142.00 (2.65) |
| Range of fluctuation, mean (SD), mmol/L | 5.92 (4.51) | 5.31 (3.98) |
| Duration of fluctuation, median (IQR), d | 9 (3–44) | 8 (3–33) |
| Length of stay, median (IQR), d | 8 (3–15) | 5 (1–12) |
| Bivariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age, per year | 1.031 (1.020–1.042) | <0.001 | 1.024 (1.013–1.035) | <0.001 |
| CCI | 1.136 (1.117–1.155) | <0.001 | 1.138 (1.116–1.160) | <0.001 |
| Range of fluctuation, per 1 mmol/L | 1.200 (1.181–1.219) | <0.001 | 1.222 (1.200–1.244) | <0.001 |
| Duration of fluctuation, per day | 0.996 (0.992–0.999) | 0.014 | 0.987 (0.983–0.991) | <0.001 |
| Risk Factor | Categories | Points |
|---|---|---|
| Age, yr | ≤65 | 0 |
| 66–75 | 2 | |
| >75 | 3 | |
| CCI | ≤2 | 0 |
| >2 | 5 | |
| Range of fluctuation, mmol/L | ≤6 | 0 |
| 7–10 | 4 | |
| >10 | 10 | |
| Duration of fluctuation, d | >3 | 0 |
| ≤3 | 3 | |
| Total score | 0–7 | Low risk |
| 8–14 | Intermediate risk | |
| 15–21 | High risk |
| Derivation Cohort | Validation Cohort | |||
|---|---|---|---|---|
| RR (95% CI) | p | RR (95%CI) | p | |
| Intermediate risk vs. low risk | 10.24 (6.42–16.91) | <0.001 | 21.92 (5.64–143.95) | <0.001 |
| High risk vs. low risk | 87.04 (55.71–141.59) | <0.001 | 187.02 (48.58–1226.27) | <0.001 |
| No. Died/No. at Risk (%) | 95%CI | No. Died/No. at Risk (%) | 95%CI | |
| Low risk | 23/21,676 (0.106) | 0.069–0.162 | 2/4079 (0.049) | 0.008–0.198 |
| Intermediate risk | 60/5575 (1.076) | 0.829–1.393 | 9/846 (1.064) | 0.520–2.086 |
| High risk | 87/1028 (8.463) | 6.868–10.376 | 10/119 (8.403) | 4.327–15.290 |
| AUC | 0.907 (0.885–0.928) | 0.932 (0.895–0.970) | ||
| Risk Factors * | Fluctuation Range | ||
|---|---|---|---|
| ≤6 | 7–10 | >10 | |
| 0 | 0 point | 4 points | 10 points |
| 1 | 2–5 points | 6–10 points | 12–15 points |
| 2 | 5–8 points | 9–12 points | 15–18 points |
| 3 | 10–11 points | 14–15 points | 20–21 points |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Chang, Q.; Zhang, Y.; Du, H.; Zhu, H.; Chen, S.; Pan, H. CARDS, a Novel Prognostic Index for Risk Stratification and In-Hospital Monitoring. J. Clin. Med. 2024, 13, 1961. https://doi.org/10.3390/jcm13071961
Liang S, Chang Q, Zhang Y, Du H, Zhu H, Chen S, Pan H. CARDS, a Novel Prognostic Index for Risk Stratification and In-Hospital Monitoring. Journal of Clinical Medicine. 2024; 13(7):1961. https://doi.org/10.3390/jcm13071961
Chicago/Turabian StyleLiang, Siyu, Qing Chang, Yuelun Zhang, Hanze Du, Huijuan Zhu, Shi Chen, and Hui Pan. 2024. "CARDS, a Novel Prognostic Index for Risk Stratification and In-Hospital Monitoring" Journal of Clinical Medicine 13, no. 7: 1961. https://doi.org/10.3390/jcm13071961





