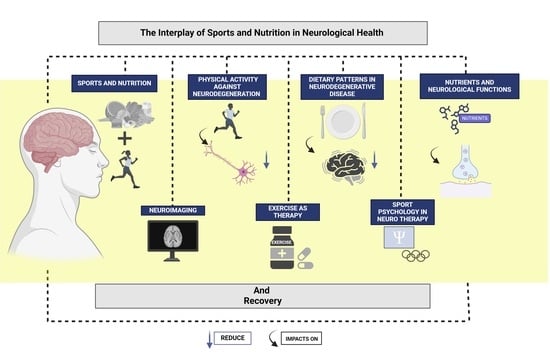
The Interplay of Sports and Nutrition in Neurological Health and Recovery
Abstract
:1. Introduction
Methodology of Search
2. The Role of Sports and Nutrition in Neurological Health
3. Interconnections between Physical Activity and Neurodegenerative Disorders
4. Dietary Patterns and Their Influence on the Progression of Neurodegenerative Diseases
5. Insights from Neuroimaging on Exercise and Nutrition’s Impact
6. Therapeutic Roles of Exercise and Physical Activity in Neurological Rehabilitation
7. Dietary Interventions for Neuroplasticity Enhancement and Recovery in Neurological Rehabilitation
8. Advancements in Technology for Evaluating the Impact of Sports and Nutrition on Neurological Health
9. Clinical Trials Assessing Exercise Efficacy in Neurological Disorder Management
10. Role of Specific Nutrients in Neurological Function and Recovery
10.1. Type of Diets
10.2. Nutritional Supplements Focused on Recovery
11. Guidelines for Integrating Physical Activity into Neurological Treatment Plans
12. Sports Psychology and Its Role in Neurological Rehabilitation
13. Case Studies and Community-Based Programs of Exercise and Nutrition Interventions in Neurology
14. Practical Applications
15. Methodological Limitations and Potential Biases
15.1. Generalizability and Applicability
15.2. Conflicting Evidence
15.3. Future Research Directions
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 8848. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Tornero-Aguilera, J.F.; Ruisoto, P.; Mielgo-Ayuso, J. Inflammation in COVID-19 and the Effects of Non-Pharmacological Interventions during the Pandemic: A Review. Int. J. Mol. Sci. 2022, 23, 15584. [Google Scholar] [CrossRef]
- Lonser, R.R.; Zipfel, G.J.; Antonio Chiocca, E. National Institute of Neurological Disorders and Stroke: Current Funding Status, Opportunities, Challenges, Emerging Scientific Advances, and Recommendations for Neurosurgery. J. Neurosurg. 2020, 133, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Scheitz, J.F.; Stengl, H.; Nolte, C.H.; Landmesser, U.; Endres, M. Neurological Update: Use of Cardiac Troponin in Patients with Stroke. J. Neurol. 2021, 268, 2284–2292. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Tamborska, A.; Wood, G.K.; Ellul, M.; Thomas, R.H.; Galea, I.; Pett, S.; Singh, B.; Solomon, T.; Pollak, T.A.; et al. Considerations for Causality Assessment of Neurological and Neuropsychiatric Complications of SARS-CoV-2 Vaccines: From Cerebral Venous Sinus Thrombosis to Functional Neurological Disorder. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Stave, U. 1157 A standardized neurological examination for infants: Optimality scores for a high risk group. Pediatr. Res. 1978, 12, 556. [Google Scholar] [CrossRef]
- Harapan, B.N.; Yoo, H.J. Neurological Symptoms, Manifestations, and Complications Associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease 19 (COVID-19). J. Neurol. 2021, 268, 3059–3071. [Google Scholar] [CrossRef]
- Ebrahim, S. Metabolomics, Nutrition and Why Epidemiology Matters. Int. J. Epidemiol. 2016, 45, 1307–1310. [Google Scholar] [CrossRef]
- Benavente, L.; Morís, G. Neurologic Disorders Associated with Inflammatory Bowel Disease. Eur. J. Neurol. 2010, 18, 138–143. [Google Scholar] [CrossRef]
- Umbrello, G.; Esposito, S. Microbiota and Neurologic Diseases: Potential Effects of Probiotics. J. Transl. Med. 2016, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Weiner, H.L. Microbiota Signaling Pathways That Influence Neurologic Disease. Neurotherapeutics 2018, 15, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Çoban, E.; Soysal, A. The Profile of a Neurology Clinic and Malnutrition Awareness. Turk. Noroloji. Derg. 2021, 27, 128–132. [Google Scholar] [CrossRef]
- Diamanti, A.; Capriati, T.; Mosca, A.; Trovato, C.M.; Laureti, F.; Mazzoli, B.; Bolasco, G.; Caldaro, T.; De Peppo, F.; Staccioli, S.; et al. Neurological Impairment and Malnutrition in Children: The Role of Home Enteral Nutrition in Real Life. Front. Nutr. 2023, 10, 1087603. [Google Scholar] [CrossRef] [PubMed]
- Janowski, K.; Kurpas, D.; Kusz, J.; Mroczek, B.; Jedynak, T. Health-Related Behavior, Profile of Health Locus of Control and Acceptance of Illness in Patients Suffering from Chronic Somatic Diseases. PLoS ONE 2013, 8, e63920. [Google Scholar] [CrossRef]
- Nicholson, S.E.; Watts, L.T.; Burmeister, D.M.; Merrill, D.; Scroggins, S.; Zou, Y.; Lai, Z.; Grandhi, R.; Lewis, A.M.; Newton, L.M.; et al. Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock 2019, 52, 240–248. [Google Scholar] [CrossRef]
- Weun, C.C.; Hasnan, N.; Latif, L.A.; Majid, H.A. Nutritional Status of Post-Acute Stroke Patients during Rehabilitation Phase in Hospital. Sains Malays. 2019, 48, 129–135. [Google Scholar] [CrossRef]
- Perry, L.; McLaren, S. An Exploration of Nutrition and Eating Disabilities in Relation to Quality of Life at 6 Months Post-Stroke. Health Soc. Care Community 2004, 12, 288–297. [Google Scholar] [CrossRef]
- Pham, P.T.; Han, B.; Hoang, B.X. Nattospes as Effective and Safe Functional Supplements in Management of Stroke. J. Med. Food 2020, 23, 879–885. [Google Scholar] [CrossRef]
- Li, N.; Weng, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of Intestinal Microbiota in Cerebral Ischemic Stroke Patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, Z.; Long, S.; Li, Z.; Jiang, J.; Zhou, Q.; Huang, X.; Wu, X.; Wei, W.; Li, X. The Role of the Gut Microbiome and Its Metabolites in Cerebrovascular Diseases. Front. Microbiol. 2023, 14, 1097148. [Google Scholar] [CrossRef] [PubMed]
- Carreira Míguez, M.; Clemente Suárez, V.J. Physical activity levels affect mental health and behavior in men. J. Men’s Health 2023, 1, 12. [Google Scholar]
- Machado, J.; Caram, C.L.B.; Frank, A.A.; Soares, E.d.A.; Laks, J. Estado Nutricional Na Doença de Alzheimer. Rev. Assoc. Med. Bras. 2009, 55, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Schelke, M.W.; Hackett, K.; Chen, J.L.; Shih, C.; Shum, J.; Montgomery, M.E.; Chiang, G.C.; Berkowitz, C.; Seifan, A.; Krikorian, R.; et al. Nutritional Interventions for Alzheimer’s Prevention: A Clinical Precision Medicine Approach. Ann. N. Y. Acad. Sci. 2016, 1367, 50–56. [Google Scholar] [CrossRef]
- Dowling, L.R.; Strazzari, M.R.; Keely, S.; Kaiko, G.E. Enteric Nervous System and Intestinal Epithelial Regulation of the Gut-Brain Axis. J. Allergy Clin. Immunol. 2022, 150, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and Immune Dysfunction in Parkinson Disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Metta, V.; Leta, V.; Mrudula, K.R.; Prashanth, L.K.; Goyal, V.; Borgohain, R.; Chung-Faye, G.; Chaudhuri, K.R. Gastrointestinal Dysfunction in Parkinson’s Disease: Molecular Pathology and Implications of Gut Microbiome, Probiotics, and Fecal Microbiota Transplantation. J. Neurol. 2022, 269, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Salman, A.; Islam, S.; Saleh, M.A.; Bhinder, K.K.; Malik, Z.; Tahir, I.; Naveed, M.A. Nutritional and Biochemical Parameters Among Multiple Sclerosis Patients: A Case-Control Study. Cureus 2021, 13, r31. [Google Scholar] [CrossRef]
- Redondo Robles, L.; Pintor De La Maza, B.; Tejada García, J.; García Vieitez, J.J.; Fernández Gómez, M.J.; Barrera Mellado, I.; Ballesteros Pomar, M.D. Nutritional Profile of Multiple Sclerosis. Nutr. Hosp. 2019, 36, 340–349. [Google Scholar] [CrossRef]
- Nakamura, R.; Kurihara, M.; Ogawa, N.; Kitamura, A.; Yamakawa, I.; Bamba, S.; Sanada, M.; Sasaki, M.; Urushitani, M. Investigation of the Prognostic Predictive Value of Serum Lipid Profiles in Amyotrophic Lateral Sclerosis: Roles of Sex and Hypermetabolism. Sci. Rep. 2022, 12, 1826. [Google Scholar] [CrossRef]
- Sullivan, K.J. What Is Neurologic Physical Therapist Practice Today? J. Neurol. Phys. Ther. 2009, 33, 58–59. [Google Scholar] [CrossRef]
- Rafferty, M.R.; Held Bradford, E.C.; Fritz, S.; Hutchinson, K.J.; Miczak, K.; Resnick, A.; Billinger, S.A. Health Promotion and Wellness in Neurologic Physical Therapy: Strategies to Advance Practice. J. Neurol. Phys. Ther. 2022, 46, 103–117. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Gostian-Ropotin, L.A.; Beltrán-Velasco, A.I.; Belando-Pedreño, N.; Simón, J.A.; López-Mora, C.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F.; Clemente-Suárez, V.J. Sporting Mind: The Interplay of Physical Activity and Psychological Health. Sports 2024, 12, 37. [Google Scholar] [CrossRef]
- Fritz, N.E.; Cheek, F.M.; Nichols-Larsen, D.S. Motor-Cognitive Dual-Task Training in Persons with Neurologic Disorders: A Systematic Review. J. Neurol. Phys. Ther. 2015, 39, 142–153. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Felisatti, F.; Gonneaud, J.; Palix, C.; Garnier-Crussard, A.; Mézenge, F.; Landeau, B.; Chocat, A.; Quillard, A.; Ferrand-Devouge, E.; De La Sayette, V.; et al. Role of Cardiovascular Risk Factors on the Association Between Physical Activity and Brain Integrity Markers in Older Adults. Neurology 2022, 98, e2023–e2035. [Google Scholar] [CrossRef]
- Xu, L.; Liu, R.; Qin, Y.; Wang, T. Brain Metabolism in Alzheimer’s Disease: Biological Mechanisms of Exercise. Transl. Neurodegener. 2023, 12, 33. [Google Scholar] [CrossRef]
- Nowacka-Chmielewska, M.M.; Liśkiewicz, D.; Grabowska, K.; Liśkiewicz, A.; Marczak, Ł.; Wojakowska, A.; Pondel, N.; Grabowski, M.; Barski, J.J.; Małecki, A. Effects of Simultaneous Exposure to a Western Diet and Wheel-Running Training on Brain Energy Metabolism in Female Rats. Nutrients 2021, 13, 4242. [Google Scholar] [CrossRef]
- Keawtep, P.; Wichayanrat, W.; Boripuntakul, S.; Chattipakorn, S.C.; Sungkarat, S. Cognitive Benefits of Physical Exercise, Physical–Cognitive Training, and Technology-Based Intervention in Obese Individuals with and without Postmenopausal Condition: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 13364. [Google Scholar] [CrossRef] [PubMed]
- Párraga-Montilla, J.A.; Aibar-Almazán, A.; Cabrera-Linares, J.C.; Lozano-Aguilera, E.; Huete, V.S.; Arrieta, M.D.E.; Latorre-Román, P.Á. A Randomized Controlled Trial Protocol to Test the Efficacy of a Dual-Task Multicomponent Exercise Program vs. A Simple Program on Cognitive and Fitness Performance in Elderly People. Int. J. Environ. Res. Public Health 2021, 18, 6507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Gao, X.; Na, M.; Kris-Etherton, P.; Mitchell, D.; Jensen, G. Dietary Pattern Score, Diet Quality, and Major Neurodegenerative Diseases: A Meta-Analysis of Observational Cohort Studies (OR33-07-19). Curr. Dev. Nutr. 2019, 3, nzz039-OR33. [Google Scholar] [CrossRef]
- Vizzi, L.; Padua, E.; D’Amico, A.G.; Tancredi, V.; D’Arcangelo, G.; Cariati, I.; Scimeca, M.; Maugeri, G.; D’Agata, V.; Montorsi, M. Beneficial Effects of Physical Activity on Subjects with Neurodegenerative Disease. J. Funct. Morphol. Kinesiol. 2020, 5, 94. [Google Scholar] [CrossRef]
- Farì, G.; Lunetti, P.; Pignatelli, G.; Raele, M.V.; Cera, A.; Mintrone, G.; Ranieri, M.; Megna, M.; Capobianco, L. The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. Int. J. Mol. Sci. 2021, 22, 11632. [Google Scholar] [CrossRef]
- Marques-Aleixo, I.; Beleza, J.; Sampaio, A.; Stevanović, J.; Coxito, P.; Gonçalves, I.; Ascensão, A.; Magalhães, J. Preventive and Therapeutic Potential of Physical Exercise in Neurodegenerative Diseases. Antioxid. Redox Signal. 2021, 34, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.A.; Coleman, J.J.; Amara, A.W. Effects of Exercise on Sleep in Neurodegenerative Disease. Neurobiol. Dis. 2020, 140, 104859. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Kumar, V.B. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Santiago, J.A.; Quinn, J.P.; Potashkin, J.A. Physical Activity Rewires the Human Brain against Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 6223. [Google Scholar] [CrossRef]
- Ruiz-González, D.; Hernández-Martínez, A.; Valenzuela, P.L.; Morales, J.S.; Soriano-Maldonado, A. Effects of Physical Exercise on Plasma Brain-Derived Neurotrophic Factor in Neurodegenerative Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Neurosci. Biobehav. Rev. 2021, 128, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.; Zauli, G.; Maiorano, P.; Milani, D.; Mirandola, P.; Neri, L.M. Role of Physical Exercise in the Regulation of Epigenetic Mechanisms in Inflammation, Cancer, Neurodegenerative Diseases, and Aging Process. J. Cell. Physiol. 2019, 234, 14852–14864. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef] [PubMed]
- Raffin, J.; Rolland, Y.; Aggarwal, G.; Nguyen, A.D.; Morley, J.E.; Li, Y.; Bateman, R.J.; Vellas, B.; Barreto, P.D.S. Associations between Physical Activity, Blood-Based Biomarkers of Neurodegeneration, and Cognition in Healthy Older Adults: The MAPT Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Ghezzi, L.; Cross, A.H.; Piccio, L. Effects of Dietary Restriction on Neuroinflammation in Neurodegenerative Diseases. J. Exp. Med. 2021, 218, e20190086. [Google Scholar] [CrossRef] [PubMed]
- Bok, E.; Jo, M.; Lee, S.; Lee, B.R.; Kim, J.; Kim, H.J. Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. Int. J. Mol. Sci. 2019, 20, 464. [Google Scholar] [CrossRef] [PubMed]
- Hadem, I.K.H.; Majaw, T.; Kharbuli, B.; Sharma, R. Beneficial Effects of Dietary Restriction in Aging Brain. J. Chem. Neuroanat. 2019, 95, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.Q.; Yu, L.L.; Chen, W.; Tian, F.W.; Zhai, Q.X. Dietary Patterns Affect Parkinson’s Disease via the Microbiota-Gut-Brain Axis. Trends Food Sci. Technol. 2021, 116, 90–101. [Google Scholar] [CrossRef]
- Arora, S.; Santiago, J.A.; Bernstein, M.; Potashkin, J.A. Diet and Lifestyle Impact the Development and Progression of Alzheimer’s Dementia. Front. Nutr. 2023, 10, 1213223. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Barbagallo, M.; Muñoz-Garcia, M.; Godos, J.; Martinez-Gonzalez, M.A. Dietary Patterns and Cognitive Decline: Key Features for Prevention. Curr. Pharm. Des. 2019, 25, 2428–2442. [Google Scholar] [CrossRef]
- Barbaresko, J.; Lellmann, A.W.; Schmidt, A.; Lehmann, A.; Amini, A.M.; Egert, S.; Schlesinger, S.; Nöthlings, U. Dietary Factors and Neurodegenerative Disorders: An Umbrella Review of Meta-Analyses of Prospective Studies. Adv. Nutr. 2020, 11, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Murnane, K.S.; Edinoff, A.N.; Cornett, E.M.; Kaye, A.D. Updated Perspectives on the Neurobiology of Substance Use Disorders Using Neuroimaging. Subst. Abus. Rehabil. 2023, 14, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, T.; Zhao, B. Statistical Learning Methods for Neuroimaging Data Analysis with Applications. Annu. Rev. Biomed. Data Sci. 2023, 6, 73–104. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Pinto, N.; Serrenho, I.; Pato, M.V.; Baltazar, G. Contribution of Glial Cells to the Neuroprotective Effects Triggered by Repetitive Magnetic Stimulation. Neural Regen. Res. 2024, 19, 116–123. [Google Scholar] [CrossRef]
- Kelly, K.M.; Nadon, N.L.; Morrison, J.H.; Thibault, O.; Barnes, C.A.; Blalock, E.M. The Neurobiology of Aging. Epilepsy Res. 2006, 68, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Al Towairqi, W.; Torres, C.; O’Donnell, H.; Bourque, P.R. MRI Findings in Transient Headache and Neurologic Deficits with Cerebrospinal Lymphocytosis Syndrome. Can. J. Neurol. Sci. 2022, 50, 764–765. [Google Scholar] [CrossRef]
- Veldkamp, R.; Goetschalckx, M.; Hulst, H.E.; Nieuwboer, A.; Grieten, K.; Baert, I.; Leone, C.; Moumdjian, L.; Feys, P. Cognitive-Motor Interference in Individuals with a Neurologic Disorder: A Systematic Review of Neural Correlates. Cogn. Behav. Neurol. 2021, 34, 79–95. [Google Scholar] [CrossRef]
- Feng, M.; Wen, H.; Xin, H.; Zhang, N.; Liang, C.; Guo, L. Altered Spontaneous Brain Activity Related to Neurologic Dysfunction in Patients with Cerebral Small Vessel Disease. Front. Aging Neurosci. 2021, 13, 731585. [Google Scholar] [CrossRef]
- Pancho, A.; Mitsogiannis, M.D.; Aerts, T.; Dalla Vecchia, M.; Ebert, L.K.; Geenen, L.; Noterdaeme, L.; Vanlaer, R.; Stulens, A.; Hulpiau, P.; et al. Modifying PCDH19 Levels Affects Cortical Interneuron Migration. Front. Neurosci. 2022, 16, 887478. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, S.; Kang, W.; Chen, P.; Liu, J. Associations between Polyunsaturated Fatty Acid Concentrations and Parkinson’s Disease: A Two-Sample Mendelian Randomization Study. Front. Aging Neurosci. 2023, 15, 1123239. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Kording, K.P.; Parvizi, J.; Fox, M.D. Causal Mapping of Human Brain Function. Nat. Rev. Neurosci. 2022, 23, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Guo, Z.; Gao, Z.; Cao, Y.; Fu, J. Multiscale Brain Network Models and Their Applications in Neuropsychiatric Diseases. Electronics 2022, 11, 3468. [Google Scholar] [CrossRef]
- Gong, C.; Jing, C.; Chen, X.; Pun, C.M.; Huang, G.; Saha, A.; Nieuwoudt, M.; Li, H.X.; Hu, Y.; Wang, S. Generative AI for Brain Image Computing and Brain Network Computing: A Review. Front. Neurosci. 2023, 17, 1203104. [Google Scholar] [CrossRef] [PubMed]
- Dhifallah, S.; Rekik, I. Estimation of Connectional Brain Templates Using Selective Multi-View Network Normalization. Med. Image Anal. 2020, 59, 101567. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Duan, S.; Wei, P.; Chen, L.; Wang, J.; Zhang, J. Aberrant Brain Spontaneous Activity and Synchronization in Type 2 Diabetes Mellitus Patients: A Resting-State Functional MRI Study. Front. Aging Neurosci. 2020, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sui, C.; Chen, B.; Xin, H.; Che, Y.; Zhang, X.; Wang, N.; Wang, Y.; Liang, C. Voxel-Based Morphometry Reveals the Correlation between Gray Matter Volume and Serum P-Tau-181 in Type 2 Diabetes Mellitus Patients with Different HbA1c Levels. Front. Neurosci. 2023, 17, 1202374. [Google Scholar] [CrossRef] [PubMed]
- Sizonenko, S.V.; Babiloni, C.; Sijben, J.W.; Walhovd, K.B. Brain Imaging and Human Nutrition: Which Measures to Use in Intervention Studies? Adv. Nutr. 2013, 4, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wen, H.; Li, J.; Lv, H.; Cho, J.; Guo, L. Editorial: Neuroimaging of Brain Structure-Function Coupling Mechanism in Neuropsychiatric Disorders. Front. Neurosci. 2023, 17, 1270645. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2003, 58, M176–M180. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Engvig, A.; Fjell, A.M.; Westlye, L.T.; Moberget, T.; Sundseth, O.; Larsen, V.A.; Walhovd, K.B. Effects of Memory Training on Cortical Thickness in the Elderly. Neuroimage 2010, 52, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Boecker, H.; Sprenger, T.; Spilker, M.E.; Henriksen, G.; Koppenhoefer, M.; Wagner, K.J.; Valet, M.; Berthele, A.; Tolle, T.R. The Runner’s High: Opioidergic Mechanisms in the Human Brain. Cereb. Cortex 2008, 18, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Rubio-Arias, J. Dosage and Effectiveness of Aerobic Training on Cardiorespiratory Fitness, Functional Capacity, Balance, and Fatigue in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2021, 102, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Martínez-Rodríguez, A.; Rubio-Arias, J. Effects and Optimal Dosage of Resistance Training on Strength, Functional Capacity, Balance, General Health Perception, and Fatigue in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Disabil. Rehabil. 2022, 45, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhou, W.; Xia, R.; Tao, J.; Chen, L. Aerobic Exercises for Cognition Rehabilitation Following Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2016, 25, 2780–2789. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chen, H.C.; Liou, T.H.; Li, W.; Chen, S.C. Exercise Interventions for Individuals with Neurological Disorders: A Systematic Review of Systematic Reviews. Am. J. Phys. Med. Rehabil. 2019, 98, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, H.; Zhou, N.; Zhang, J.; Wu, Y.; Zhang, Y.; Bai, Y.; Jia, J.; Zhang, Q.; Tian, S.; et al. Early Exercise Improves Cerebral Blood Flow through Increased Angiogenesis in Experimental Stroke Rat Model. J. Neuroeng. Rehabil. 2013, 10, 43. [Google Scholar] [CrossRef]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running Enhances Neurogenesis, Learning, and Long-Term Potentiation in Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Hötting, K.; Röder, B. Beneficial Effects of Physical Exercise on Neuroplasticity and Cognition. Neurosci. Biobehav. Rev. 2013, 37 Pt B, 2243–2257. [Google Scholar] [CrossRef]
- Mackay, C.P.; Kuys, S.S.; Brauer, S.G. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017, 2017, 4716197. [Google Scholar] [CrossRef]
- Gujral, S.; Aizenstein, H.; Reynolds, C.F.; Butters, M.A.; Erickson, K.I. Exercise Effects on Depression: Possible Neural Mechanisms. Gen. Hosp. Psychiatry 2017, 49, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cancela, J.M.; Ayán, C.; Varela, S.; Seijo, M. Effects of a Long-Term Aerobic Exercise Intervention on Institutionalized Patients with Dementia. J. Sci. Med. Sport 2015, 19, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.F.; Yang, T.; Yu, S.X.; Huang, H.D.; Jiang, L.L.; Gu, J.W.; Kuang, Y.Q. Aerobic Exercise for Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2014, 9, e100503. [Google Scholar] [CrossRef]
- Chin, L.M.; Keyser, R.E.; Dsurney, J.; Chan, L. Improved Cognitive Performance Following Aerobic Exercise Training in People with Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2014, 96, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Manonelles, P.; Boas, J.P.V.; Rubio-Arias, J.Á. Fast-Velocity Resistance Training Improves Force Development and Mobility in Multiple Sclerosis. Int. J. Sports Med. 2021, 43, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Manonelles, P.; Abellán-Aynés, O.; Rubio-Arias, J. Effects of Fast-Velocity Concentric Resistance Training in People with Multiple Sclerosis: A Randomized Controlled Trial. Acta Neurol. Scand. 2022, 146, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Signorile, J.F. High-Speed Resistance Training Modifies Load-Velocity and Load-Power Relationships in Parkinson’s Disease. J. Strength. Cond. Res. 2017, 31, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.L.H.; Thilarajah, S.; Tan, D. Effectiveness of Resistance Training on Muscle Strength and Physical Function in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2016, 30, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Veldema, J.; Jansen, P. Resistance Training in Stroke Rehabilitation: Systematic Review and Meta-Analysis. Clin. Rehabil. 2020, 34, 1173–1197. [Google Scholar] [CrossRef]
- Garuffi, M.; Costa, J.L.R.; Hernández, S.S.S.; Vital, T.M.; Stein, A.M.; dos Santos, J.G.; Stella, F. Effects of Resistance Training on the Performance of Activities of Daily Living in Patients with Alzheimer’s Disease. Geriatr. Gerontol. Int. 2013, 13, 322–328. [Google Scholar] [CrossRef]
- Williams, G.; Hassett, L.; Clark, R.; Bryant, A.L.; Morris, M.E.; Olver, J.; Ada, L. Ballistic Resistance Training Has a Similar or Better Effect on Mobility than Non-Ballistic Exercise Rehabilitation in People with a Traumatic Brain Injury: A Randomised Trial. J. Physiother. 2022, 68, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Perrochon, A.; Borel, B.; Istrate, D.; Compagnat, M.; Daviet, J.C. Exercise-Based Games Interventions at Home in Individuals with a Neurological Disease: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil. Med. 2019, 62, 366–378. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef] [PubMed]
- Loong, S.; Barnes, S.; Gatto, N.M.; Chowdhury, S.; Lee, G.J. Omega-3 Fatty Acids, Cognition, and Brain Volume in Older Adults. Brain Sci. 2023, 13, 1278. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Marino, A.; Cuzzocrea, S. N-3 Fatty Acids: Role in Neurogenesis and Neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary Omega-3 Fatty Acid Supplementation Increases the Rate of Muscle Protein Synthesis in Older Adults: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty Acid Analysis of Blood Plasma of Patients with Alzheimer’s Disease, Other Types of Dementia, and Cognitive Impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef]
- Scrimgeour, A.G.; Condlin, M.L.; Loban, A.; DeMar, J.C. Omega-3 Fatty Acids and Vitamin D Decrease Plasma T-Tau, GFAP, and UCH-L1 in Experimental Traumatic Brain Injury. Front. Nutr. 2021, 8, 685220. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Logsdon, A.F.; Nguyen, L.; Eltanahay, A.; Turner, R.C.; Bonasso, P.; Knotts, C.; Moeck, A.; Maroon, J.C.; Bailes, J.E.; et al. Supplements, Nutrition, and Alternative Therapies for the Treatment of Traumatic Brain Injury. Nutr. Neurosci. 2018, 21, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Jaca, A.; Durão, S.; Harbron, J. Omega-3 Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. S. Afr. Med. J. 2005, 64, 2040–2045. [Google Scholar] [CrossRef]
- De Lau, L.M.L.; Bornebroek, M.; Witteman, J.C.M.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Dietary Fatty Acids and the Risk of Parkinson Disease: The Rotterdam Study. Neurology 2005, 64, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, M.; Loria, G.; Salvati, S.; Di Biase, A.; Conte, G.; Villella, C.; Righino, E.; Ciciarelli, C.; Bria, P.; La Torre, G.; et al. DHA Effects in Parkinson Disease Depression. Basal Ganglia 2014, 4, 61–66. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Clinical and Metabolic Status in Patients with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.; Al Nimer, F.; Frostell, A.; Zetterberg, H.; Blennow, K.; Nyström, H.; Svensson, M.; Bellander, B.M.; Piehl, F.; Nelson, D.W. A Serum Protein Biomarker Panel Improves Outcome Prediction in Human Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2850–2862. [Google Scholar] [CrossRef]
- Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Aging. Curr. Neuropharmacol. 2017, 15, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological Benefits of Omega-3 Fatty Acids. NeuroMolecular Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of Polyunsaturated Fatty Acids in Human Brain Structure and Function across the Lifespan: An Update on Neuroimaging Findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Frautschy, S.A. DHA May Prevent Age-Related Dementia. J. Nutr. 2010, 140, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E. Ascorbate Regulation and Its Neuroprotective Role in the Brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kangisser, L.; Tan, E.; Bellomo, R.; Deane, A.M.; Plummer, M.P. Neuroprotective Properties of Vitamin C: A Scoping Review of Pre-Clinical and Clinical Studies. J. Neurotrauma 2021, 38, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Joksovic, P.M.; Su, P.; Kang, H.W.; Van Deusen, A.; Baumgart, J.P.; David, L.S.; Snutch, T.P.; Barrett, P.Q.; Lee, J.H.; et al. Molecular Mechanisms of Subtype-Specific Inhibition of Neuronal T-Type Calcium Channels by Ascorbate. J. Neurosci. 2007, 27, 12577–12583. [Google Scholar] [CrossRef] [PubMed]
- Sutor, B.; ten Bruggencate, G. Ascorbic Acid: A Useful Reductant to Avoid Oxidation of Catecholamines in Electrophysiological Experiments in Vitro? Neurosci. Lett. 1990, 116, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Calero, C.I.; Vickers, E.; Cid, G.M.; Aguayo, L.G.; von Gersdorff, H.; Calvo, D.J. Allosteric Modulation of Retinal GABA Receptors by Ascorbic Acid. J. Neurosci. 2011, 31, 9672–9682. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A.; London, E.D. Regulation of the NMDA Receptor by Redox Phenomena: Inhibitory Role of Ascorbate. Brain Res. 1990, 537, 328–332. [Google Scholar] [CrossRef]
- Moretti, M.; Budni, J.; Ribeiro, C.M.; Rieger, D.K.; Leal, R.B.; Rodrigues, A.L.S. Subchronic Administration of Ascorbic Acid Elicits Antidepressant-like Effect and Modulates Cell Survival Signaling Pathways in Mice. J. Nutr. Biochem. 2016, 38, 50–56. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Johansen, L.K.; Raida, Z.; Villumsen, C.K.; Larsen, J.O.; Lykkesfeldt, J. Vitamin C Deficiency in Early Postnatal Life Impairs Spatial Memory and Reduces the Number of Hippocampal Neurons in Guinea Pigs. Am. J. Clin. Nutr. 2009, 90, 540–546. [Google Scholar] [CrossRef]
- Salazar, K.; Martínez, M.; Ulloa, V.; Bertinat, R.; Martínez, F.; Jara, N.; Espinoza, F.; Bongarzone, E.R.; Nualart, F. SVCT2 Overexpression in Neuroblastoma Cells Induces Cellular Branching That Is Associated with ERK Signaling. Mol. Neurobiol. 2015, 53, 6668–6679. [Google Scholar] [CrossRef]
- Murakami, K.; Murata, N.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Vitamin C Restores Behavioral Deficits and Amyloid-β Oligomerization without Affecting Plaque Formation in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Seo, M.; Seo, J.S.; Rhim, H.; Nahm, S.S.; Cho, I.H.; Chang, B.J.; Kim, H.J.; Choi, S.H.; Nah, S.Y. Ascorbic Acid Mitigates D-Galactose-Induced Brain Aging by Increasing Hippocampal Neurogenesis and Improving Memory Function. Nutrients 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Delrobaei, F.; Fatemi, I.; Shamsizadeh, A.; Allahtavakoli, M. Ascorbic Acid Attenuates Cognitive Impairment and Brain Oxidative Stress in Ovariectomized Mice. Pharmacol. Rep. 2019, 71, 133–138. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Lau, F.C.; Carey, A.N.; Galli, R.L.; Spangler, E.L.; Ingram, D.K.; Joseph, J.A. Blueberry Polyphenols Attenuate Kainic Acid-Induced Decrements in Cognition and Alter Inflammatory Gene Expression in Rat Hippocampus. Nutr. Neurosci. 2008, 11, 172–182. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.G.; Smith, M.A.; Joseph, J.A. Modulation of Hippocampal Plasticity and Cognitive Behavior by Short-Term Blueberry Supplementation in Aged Rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef]
- Zheng, Q.; Kebede, M.T.; Kemeh, M.M.; Islam, S.; Lee, B.; Bleck, S.D.; Wurfl, L.A.; Lazo, N.D. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules 2019, 24, 2316. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Schneider, J.A.; Li, H.; Tangney, C.C.; Nag, S.; Bennett, D.A.; Honer, W.G.; Barnes, L.L. Brain Tocopherols Related to Alzheimer’s Disease Neuropathology in Humans. Alzheimer’s Dement. 2014, 11, 32–39. [Google Scholar] [CrossRef]
- Wengreen, H.J.; Munger, R.G.; Corcoran, C.D.; Zandi, P.; Hayden, K.M.; Fotuhi, M.; Skoog, I.; Norton, M.C.; Tschanz, J.; Breitner, J.C.S.; et al. Antioxidant Intake and Cognitive Function of Elderly Men and Women: The Cache County Study. J. Nutr. Health Aging 2007, 11, 230–237. [Google Scholar] [PubMed]
- Miller, D.J.; Sargent, C.; Roach, G.D. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors 2022, 22, 6317. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J. Multidisciplinary Intervention in the Treatment of Mixed Anxiety and Depression Disorder. Physiol. Behav. 2020, 219, 112858. [Google Scholar] [CrossRef]
- Myers, K.A.; Sivathamboo, S.; Perucca, P. Heart Rate Variability Measurement in Epilepsy: How Can We Move from Research to Clinical Practice? Epilepsia 2018, 59, 2169–2178. [Google Scholar] [CrossRef]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-Specific Seizure Prediction Based on Heart Rate Variability and Recurrence Quantification Analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef]
- Leal, A.; Pinto, M.F.; Lopes, F.; Bianchi, A.M.; Henriques, J.; Ruano, M.G.; de Carvalho, P.; Dourado, A.; Teixeira, C.A. Heart Rate Variability Analysis for the Identification of the Preictal Interval in Patients with Drug-Resistant Epilepsy. Sci. Rep. 2021, 11, 5987. [Google Scholar] [CrossRef]
- Kim, M.S.; Yoon, J.H.; Hong, J.M. Early Differentiation of Dementia with Lewy Bodies and Alzheimer’s Disease: Heart Rate Variability at Mild Cognitive Impairment Stage. Clin. Neurophysiol. 2018, 129, 1570–1578. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Hillman, C. The Influence of Exercise on Cognitive Abilities. Compr. Physiol. 2013, 3, 403–428. [Google Scholar] [CrossRef]
- Erickson, K.I.; Raji, C.A.; Lopez, O.L.; Becker, J.T.; Rosano, C.; Newman, A.B.; Gach, H.M.; Thompson, P.M.; Ho, A.J.; Kuller, L.H. Physical Activity Predicts Gray Matter Volume in Late Adulthood: The Cardiovascular Health Study. Neurology 2010, 75, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic Fitness Is Associated with Hippocampal Volume in Elderly Humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J. An Introduction to the Event-Related Potential Technique (Cognitive Neuroscience); MIT Press: Cambridge, MA, USA, 2005; Volume 89. [Google Scholar]
- Themanson, J.R.; Hillman, C.H.; Curtin, J.J. Age and Physical Activity Influences on Action Monitoring during Task Switching. Neurobiol. Aging 2006, 27, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Matsumoto, S.; Kawasaki, N.; Masaki, M.; Fukui, I.; Kaiya, H. The Anxious-Depressive Attack Severity Scale: Development and Initial Validation and Reliability. Biopsychosoc. Med. 2021, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Turgut, B.A.; Naz, İ. Physical Activity in Neurological Disorders: A Narrative Review. Eur. J. Ther. 2023, 29, 97–102. [Google Scholar] [CrossRef]
- Farrell, J.W.; Merkas, J.; Pilutti, L.A. The Effect of Exercise Training on Gait, Balance, and Physical Fitness Asymmetries in Persons with Chronic Neurological Conditions: A Systematic Review of Randomized Controlled Trials. Front. Physiol. 2020, 11, 585765. [Google Scholar] [CrossRef] [PubMed]
- Adamson, B.C.; Ensari, I.; Motl, R.W. Effect of Exercise on Depressive Symptoms in Adults with Neurologic Disorders: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Tsutsumimoto, K.; Doi, T.; Hotta, R.; Nakakubo, S.; Liu-Ambrose, T.; Shimada, H. Effects of Exercise and Horticultural Intervention on the Brain and Mental Health in Older Adults with Depressive Symptoms and Memory Problems: Study Protocol for a Randomized Controlled Trial [UMIN000018547]. Trials 2015, 16, 499. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S.; Cuesta, M.; Bieler-Aeschlimann, M.; Giacomino, K.; Widmer, A.; Mittaz Hager, A.G.; Perez-Marcos, D.; Cardin, S.; Boller, B.; Bier, N.; et al. Rationale and Protocol of the StayFitLonger Study: A Multicentre Trial to Measure Efficacy and Adherence of a Home-Based Computerised Multidomain Intervention in Healthy Older Adults. BMC Geriatr. 2020, 20, 315. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, H.; Wang, X.; Huang, K.; Zuo, Y.; Wu, X.; Abdullah, A.S.; Yang, L. The Efficacy of Cognitive Training for Elderly Chinese Individuals with Mild Cognitive Impairment. Biomed. Res. Int. 2019, 2019, 4347281. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients with Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Emig, M.; George, T.; Zhang, J.K.; Soudagar-Turkey, M. The Role of Exercise in Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2021, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.Y.; Chen, S.C.; Peng, C.W.; Lin, Y.N.; Chang, Y.T.; Lai, C.H. Effects of Interactive Video-Game-Based Exercise on Balance in Older Adults with Mild-to-Moderate Parkinson’s Disease. J. Neuroeng. Rehabil. 2020, 17, 91. [Google Scholar] [CrossRef]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of Home-Based and Remotely Supervised Aerobic Exercise in Parkinson’s Disease: A Double-Blind, Randomised Controlled Trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, A.; Pickering, R.; McIntosh, E.; Hulbert, S.; Rochester, L.; Roberts, H.C.; Nieuwboer, A.; Kunkel, D.; Goodwin, V.A.; Lamb, S.E.; et al. Exercise-and Strategy-Based Physiotherapy-Delivered Intervention for Preventing Repeat Falls in People with Parkinson’s: The PDSAFE RCT. Health Technol. Assess. 2019, 23, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Brandín-De la Cruz, N.; Secorro, N.; Calvo, S.; Benyoucef, Y.; Herrero, P.; Bellosta-López, P. Immersive Virtual Reality and Antigravity Treadmill Training for Gait Rehabilitation in Parkinson’s Disease: A Pilot and Feasibility Study. Rev. Neurol. 2020, 71, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple Sclerosis: Clinical Aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Molhemi, F.; Monjezi, S.; Mehravar, M.; Shaterzadeh-Yazdi, M.J.; Salehi, R.; Hesam, S.; Mohammadianinejad, E. Effects of Virtual Reality vs Conventional Balance Training on Balance and Falls in People with Multiple Sclerosis: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 102, 290–299. [Google Scholar] [CrossRef]
- Grazioli, E.; Tranchita, E.; Borriello, G.; Cerulli, C.; Minganti, C.; Parisi, A. The Effects of Concurrent Resistance and Aerobic Exercise Training on Functional Status in Patients with Multiple Sclerosis. Curr. Sports Med. Rep. 2019, 18, 452–457. [Google Scholar] [CrossRef]
- Amedoro, A.; Berardi, A.; Conte, A.; Pelosin, E.; Valente, D.; Maggi, G.; Tofani, M.; Galeoto, G. The Effect of Aquatic Physical Therapy on Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2020, 41, 102022. [Google Scholar] [CrossRef] [PubMed]
- Beata, B.K.; Wojciech, J.; Johannes, K.; Piotr, L.; Barbara, M. Alzheimer’s Disease—Biochemical and Psychological Background for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1059. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Umegaki, H.; Ando, M.; Cheng, X.W.; Ishida, K.; Akima, H.; Oshida, Y.; Yoshida, Y.; Uemura, K.; Shimada, H.; et al. Effects of Aerobic, Resistance, or Combined Exercise Training among Older Adults with Subjective Memory Complaints: A Randomized Controlled Trial. J. Alzheimer’s Dis. 2021, 82, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Inskip, M.J.; Mavros, Y.; Sachdev, P.S.; Hausdorff, J.M.; Hillel, I.; Singh, M.A.F. Promoting Independence in Lewy Body Dementia through Exercise: The PRIDE Study. BMC Geriatr. 2022, 22, 650. [Google Scholar] [CrossRef] [PubMed]
- Bouça-Machado, R.; Rosário, A.; Caldeira, D.; Castro Caldas, A.; Guerreiro, D.; Venturelli, M.; Tinazzi, M.; Schena, F.; Ferreira, J.J. Physical Activity, Exercise, and Physiotherapy in Parkinson’s Disease: Defining the Concepts. Mov. Disord. Clin. Pract. 2019, 7, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, G.N.; Sanlier, N. The Relationship between Nutrition and Depression in the Life Process: A Mini-Review. Exp. Gerontol. 2023, 172, 112072. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients 2023, 15, 3106. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain Foods: The Effects of Nutrients on Brain Function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Beyer, J.L.; Payne, M.E. Nutrition and Bipolar Depression. Psychiatr. Clin. N. Am. 2016, 39, 75–86. [Google Scholar] [CrossRef]
- Lee, H. The Importance of Nutrition in Neurological Disorders and Nutrition Assessment Methods. Brain Neurorehabilit. 2022, 15, e1. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hou, Y.; Sudersan, P.; Lam, C.W.E.; Poulikakos, D.; Butt, H.J.; Yeung, K.L. Inhibition of Condensation-Induced Droplet Wetting by Nano-Hierarchical Surfaces. Chem. Eng. J. 2023, 460, 141761. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kawashima, R. Nutrients and Dementia: Prospective Study. Nutrients 2023, 15, 842. [Google Scholar] [CrossRef] [PubMed]
- Van Der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A Ketogenic Diet Reduces Amyloid Beta 40 and 42 in a Mouse Model of Alzheimer’s Disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A Ketone Ester Diet Exhibits Anxiolytic and Cognition-Sparing Properties, and Lessens Amyloid and Tau Pathologies in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson Disease with Diet-Induced Hyperketonemia: A Feasibility Study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Shahid, S.M.; Bishop, K.S. Comprehensive Approaches to Improving Nutrition: Future Prospects. Nutrients 2019, 11, 1760. [Google Scholar] [CrossRef]
- Kola, A.; Nencioni, F.; Valensin, D. Bioinorganic Chemistry of Micronutrients Related to Alzheimer’s and Parkinson’s Diseases. Molecules 2023, 28, 5467. [Google Scholar] [CrossRef]
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jésus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN Guideline Clinical Nutrition in Neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Nazarewicz, R.; Dikalov, S. Ascorbic Acid Efficiently Enhances Neuronal Synthesis of Norepinephrine from Dopamine. Brain Res. Bull. 2012, 90, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Micinski, D. Vitamin D Upregulates Glutamate Cysteine Ligase and Glutathione Reductase, and GSH Formation, and Decreases ROS and MCP-1 and IL-8 Secretion in High-Glucose Exposed U937 Monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef]
- Masjedi, F.; Keshtgar, S.; Zal, F.; Talaei-Khozani, T.; Sameti, S.; Fallahi, S.; Kazeroni, M. Effects of Vitamin D on Steroidogenesis, Reactive Oxygen Species Production, and Enzymatic Antioxidant Defense in Human Granulosa Cells of Normal and Polycystic Ovaries. J. Steroid Biochem. Mol. Biol. 2020, 197, 105521. [Google Scholar] [CrossRef]
- Holton, K.F. Micronutrients May Be a Unique Weapon Against the Neurotoxic Triad of Excitotoxicity, Oxidative Stress and Neuroinflammation: A Perspective. Front. Neurosci. 2021, 15, 726457. [Google Scholar] [CrossRef]
- Rahimmi, A.; Tozandehjani, S.; Daraei, M.; Khademerfan, M. The Neuroprotective Roles of Dietary Micronutrients on Parkinson’s Disease: A Review. Mol. Biol. Rep. 2022, 49, 8051–8060. [Google Scholar] [CrossRef]
- Pacifici, F.; Salimei, C.; Pastore, D.; Malatesta, G.; Ricordi, C.; Donadel, G.; Bellia, A.; Rovella, V.; Tafani, M.; Garaci, E.; et al. The Protective Effect of a Unique Mix of Polyphenols and Micronutrients against Neurodegeneration Induced by an In Vitro Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 3110. [Google Scholar] [CrossRef]
- Schirinzi, T.; Martella, G.; Imbriani, P.; Di Lazzaro, G.; Franco, D.; Colona, V.L.; Alwardat, M.; Salimei, P.S.; Mercuri, N.B.; Pierantozzi, M.; et al. Dietary Vitamin E as a Protective Factor for Parkinson’s Disease: Clinical and Experimental Evidence. Front. Neurol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Kheirouri, S.; Keramati, M. What Dietary Vitamins and Minerals Might Be Protective against Parkinson’s Disease? Brain Sci. 2023, 13, 1119. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, J.; Shim, E.; Chung, E.J.; Jang, S.H.; Koh, S.B. Association of Serum Carotenoid, Retinol, and Tocopherol Concentrations with the Progression of Parkinson’s Disease. Nutr. Res. Pract. 2017, 11, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Jamali, B.; Entezari, M.; Babaei, N.; Hashemi, M.; Heidari, M. β-Carotene Has the Neuroprotective Effects in Parkinson’s Disease by Regulating Mitochondrial Apoptotic Pathway Genes. J. Hum. Genet. Genom. 2022, 4, 1. [Google Scholar] [CrossRef]
- Wu, L.Y.; Chen, J.X.; Chen, G.S.; Gao, H.; Huo, J.H.; Pang, Y.F.; Gao, Q.H. Dietary β-Carotene and Vitamin A and Risk of Parkinson Disease: A Protocol for Systematic Review and Meta-Analysis. Medicine 2022, 101, e31002. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Rimm, E.B.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of Antioxidant Vitamins and Risk of Parkinson’s Disease. Mov. Disord. 2016, 31, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.G.; Salas, A.A.; Ballestín, S.S. Vitamin Supplementation and Dementia: A Systematic Review. Nutrients 2022, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.C.; Williams, B.J.; Gaussoin, S.A.; Baker, L.D.; Manson, J.A.E.; Espeland, M.A.; Sesso, H.D.; Shumaker, S.A.; Rapp, S.R. Impact of Multivitamin-Mineral and Cocoa Extract on Incidence of Mild Cognitive Impairment and Dementia: Results from the COcoa Supplement and Multivitamin Outcomes Study for the Mind (COSMOS-Mind). Alzheimer’s Dement. 2023, 19, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 Fatty Acid, Carotenoid and Vitamin E Supplementation Improves Working Memory in Older Adults: A Randomised Clinical Trial. Clin. Nutr. 2021, 41, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan Oligosaccharides Alleviate Cognitive Deficits in an Amyloid-Β1-42-Induced Rat Model of Alzheimer’s Disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef]
- Maugard, M.; Vigneron, P.A.; Bolaños, J.P.; Bonvento, G. L-Serine Links Metabolism with Neurotransmission. Prog. Neurobiol. 2020, 197, 101896. [Google Scholar] [CrossRef]
- Porter, R.J.; Lunn, B.S.; Walker, L.L.M.; Gray, J.M.; Ballard, C.G.; O’Brien, J.T. Cognitive Deficit Induced by Acute Tryptophan Depletion in Patients with Alzheimer’s Disease. Am. J. Psychiatry 2000, 157, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated Long-Chain Fatty Acids Activate Inflammatory Signaling in Astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.J.; Rucklidge, J.J.; Kennedy, M.A. Epigenetics, Nutrition and Mental Health. Is There a Relationship? Nutr. Neurosci. 2018, 21, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; El Sharkawy, M. Epigenetics, Nutrition, and Growth. In World Review of Nutrition and Dietetics; Karger: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Naureen, Z.; Dhuli, K.; Medori, M.C.; Caruso, P.; Manganotti, P.; Chiurazzi, P.; Bertelli, M. Dietary Supplements in Neurological Diseases and Brain Aging. J. Prev. Med. Hyg. 2022, 63, E174–E188. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Canning, C.G.; Almeida, L.R.S.; Bloem, B.R.; Keus, S.H.J.; Löfgren, N.; Nieuwboer, A.; Verheyden, G.S.A.F.; Yamato, T.P.; Sherrington, C. Interventions for Preventing Falls in Parkinson’s Disease. Cochrane Database Syst. Rev. 2022, 2022, CD011574. [Google Scholar] [CrossRef]
- Coulter, E.H.; Bond, S.; Dalgas, U.; Paul, L. The Effectiveness of Interventions Targeting Physical Activity and/or Sedentary Behaviour in People with Multiple Sclerosis: A Systematic Review. Disabil. Rehabil. 2018, 42, 594–612. [Google Scholar] [CrossRef]
- Bhalsing, K.S.; Abbas, M.M.; Tan, L.C.S. Role of Physical Activity in Parkinson’s Disease. Ann. Indian Acad. Neurol. 2018, 21, 242–249. [Google Scholar] [CrossRef]
- Sparrow, D.; De Angelis, T.R.; Hendron, K.; Thomas, C.A.; Saint-Hilaire, M.; Ellis, T. Highly Challenging Balance Program Reduces Fall Rate in Parkinson Disease. J. Neurol. Phys. Ther. 2016, 40, 24–30. [Google Scholar] [CrossRef]
- Afzal, R.; Dowling, J.K.; McCoy, C.E. Impact of Exercise on Immunometabolism in Multiple Sclerosis. J. Clin. Med. 2020, 9, 3038. [Google Scholar] [CrossRef]
- Acosta-Gallego, A.; Hernández-Beltrán, V.; Gámez-Calvo, L.; Muñoz-Jiménez, J.; Gamonals, J.M. Analysis of Aquatic Exercise Programmes for People with Fibromyalgia. Retos 2023, 48, 988–999. [Google Scholar] [CrossRef]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise Therapy for Fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Zarapuz, A.; Apolo-Arenas, M.D.; Clemente-Suárez, V.J.; Costa, A.R.; Pardo-Caballero, D.; Parraca, J.A. Acute Effects of a Session with The EXOPULSE Mollii Suit in a Fibromyalgia Patient: A Case Report. Int. J. Environ. Res. Public Health 2023, 20, 2209. [Google Scholar] [CrossRef] [PubMed]
- Sadaqa, M.; Németh, Z.; Makai, A.; Prémusz, V.; Hock, M. Effectiveness of Exercise Interventions on Fall Prevention in Ambulatory Community-Dwelling Older Adults: A Systematic Review with Narrative Synthesis. Front. Public Health 2023, 11, 1209319. [Google Scholar] [CrossRef] [PubMed]
- Mittaz Hager, A.G.; Mathieu, N.; Lenoble-Hoskovec, C.; Swanenburg, J.; De Bie, R.; Hilfiker, R. Effects of Three Home-Based Exercise Programmes Regarding Falls, Quality of Life and Exercise-Adherence in Older Adults at Risk of Falling: Protocol for a Randomized Controlled Trial. BMC Geriatr. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.W.; Foley, N.C.; Bhogal, S.K.; Speechley, M.R. An Evidence-Based Review of Stroke Rehabilitation. Top. Stroke Rehabil. 2003, 10, 29–58. [Google Scholar] [CrossRef]
- Hogg, J. Understanding Psychological Preparation for Sport: Theory and Practice of Elite Performers. Sport Psychol. 1997, 11, 355–356. [Google Scholar] [CrossRef]
- Dawes, H. Psychological Approaches to Sports Injury Rehabilitation. Physiotherapy 1998, 84, 99–100. [Google Scholar] [CrossRef]
- English, M.; Wallace, L.; Evans, J.; Diamond, S.; Caperchione, C.M. The Impact of Sport and Physical Activity Programs on the Mental Health and Social and Emotional Wellbeing of Young Aboriginal and Torres Strait Islander Australians: A Systematic Review. Prev. Med. Rep. 2021, 25, 101676. [Google Scholar] [CrossRef]
- Moran, A.P. Sport and Exercise Psychology: A Critical Introduction (2nd Edition). Sport Exerc. Psychol. Rev. 2012, 8, 92–93. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.; Polonio-López, B.; Corregidor-Sánchez, A.I.; Martín-Conty, J.L.; Mohedano-Moriano, A.; Criado-Álvarez, J.J. Effects of Specific Virtual Reality-Based Therapy for the Rehabilitation of the Upper Limb Motor Function Post-Ictus: Randomized Controlled Trial. Brain Sci. 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Schaffert, N.; Janzen, T.B.; Mattes, K.; Thaut, M.H. A Review on the Relationship between Sound and Movement in Sports and Rehabilitation. Front. Psychol. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Chaput, M.; Palimenio, M.; Farmer, B.; Katsavelis, D.; Bagwell, J.J.; Turman, K.A.; Wichman, C.; Grindstaff, T.L. Quadriceps Strength Influences Patient Function More than Single Leg Forward Hop during Late-Stage Acl Rehabilitation. Int. J. Sports Phys. Ther. 2021, 16, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Cederström, N.; Granér, S.; Ageberg, E. Addressing Psychological Factors in Sports Injury Rehabilitation—What Is a Physical Therapist to Do? Int. J. Sports Phys. Ther. 2022, 17, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Rollo, I.; Carter, J.M.; Close, G.L.; Leyes, J.Y.; Gomez, A.; Leal, D.M.; Duda, J.L.; Holohan, D.; Erith, S.J.; Podlog, L. Role of Sports Psychology and Sports Nutrition in Return to Play from Musculoskeletal Injuries in Professional Soccer: An Interdisciplinary Approach. Eur. J. Sport Sci. 2020, 21, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Moumdjian, L.; Van Halewyck, F.; Wens, I.; Eijnde, B.O.; Van Wijmeersch, B.; Popescu, V.; Van Asch, P. Effects of an Individual 12-Week Community-Located “Start-to-Run” Program on Physical Capacity, Walking, Fatigue, Cognitive Function, Brain Volumes, and Structures in Persons with Multiple Sclerosis. Mult. Scler. J. 2017, 25, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, F.; Geurts, E.; Abasıyanık, Z.; Coninx, K.; Feys, P. Feasibility Study of a 10-Week Community-Based Program Using the WalkWithMe Application on Physical Activity, Walking, Fatigue and Cognition in Persons with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2020, 42, 102067. [Google Scholar] [CrossRef] [PubMed]
- Ferrusola-Pastrana, A.; Davison, G.; Meadows, S.N. The Therapeutic Effects of Multimodal Exercise for People with Parkinson’s: A Longitudinal Community-Based Study. Park. Relat. Disord. 2023, 110, 105366. [Google Scholar] [CrossRef] [PubMed]
- Marsden, D.L.; Dunn, A.; Callister, R.; McElduff, P.; Levi, C.R.; Spratt, N.J. A Home- and Community-Based Physical Activity Program Can Improve the Cardiorespiratory Fitness and Walking Capacity of Stroke Survivors. J. Stroke Cerebrovasc. Dis. 2016, 25, 2386–2398. [Google Scholar] [CrossRef]
- Devine, J.M.; Wong, B.; Gervino, E.; Pascual-Leone, A.; Alexander, M.P. Independent, Community-Based Aerobic Exercise Training for People with Moderate-to-Severe Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2016, 97, 1392–1397. [Google Scholar] [CrossRef]
- Arumugam, A.; Thiyagarajan, D. Role of Nutrition in Pathogenesis of Neurological Disorders; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Tidman, M. Effects of a Ketogenic Diet on Symptoms, Biomarkers, Depression, and Anxiety in Parkinson’s Disease: A Case Study. Cureus 2022, 14, e23684. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.; K Kale, D.; Naik, V.; Thakker, F.; Bailur, S. Dietary Therapy in Secondary Progressive Multiple Sclerosis: A Case Report. Cureus 2019, 11, e5341. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Lee, V.; Bundy, R.; Gillings, R.; Jennings, A.; Stephan, B.; Hornberger, M.; Balanos, G.; Paddick, S.M.; Hanson, S.; et al. Feasibility and acceptability of a multi-domain intervention to increase Mediterranean diet adherence and physical activity in older UK adults at risk of dementia: Protocol for the MedEx-UK randomised controlled trial. BMJ Open 2021, 11, e042823. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. Physical Activity and Lifestyle Modifications in the Treatment of Neurodegenerative Diseases. Front. Aging Neurosci. 2023, 15, 1185671. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Su, S.-C.; Chen, C.-W.; Kang, Y.-W.; Hu, M.-H.; Hsu, L.-L.; Wu, S.-Y.; Chen, L.; Chang, H.-Y.; Chuang, S.-Y.; et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: A randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 119. [Google Scholar] [CrossRef]

| Authors | Study Desing | Study Participants | Disorder | Type of Exercise | Results |
|---|---|---|---|---|---|
| Yuan et al. [165] | Clinic trial | N = 24. Aged more than 65 years | Parkinson Disease | Interactive video-game-based (IVGB) exercise program for 30 min three times per week the first 6 weeks | Improved the balance, postural stability, and confidence |
| Kolk et al. [166] | Clinic trial | N = 65 Aged from 30 to 65 years | Parkinson Disease | 30–45 min training three times per week for 6 months | Aerobic exercise can be done at home by patients with Parkinson’s disease with mild disease severity and it attenuates off-state motor signs |
| Brandín de la Cruz et al. [168] | Clinical Trial | N = 12 Aged more than 65 years | Parkinson Disease | 12 sessions of 30 min, evenly distributed over a period of four consecutive weeks. | The viability of integrating an antigravity treadmill with an immersive virtual reality system for the rehabilitation of individuals with PD. |
| Molhemi et al. [170] | Clinic trial | N = 35 Aged from 18 to 64 years | Multiple Sclerosis | 35 min training three times per week during 6 consecutive weeks | Both the Virtual Reality-based and conventional balance exercises improved balance and mobility in people with MS |
| Grazioli et al. [171] | Clinic trial | N = 20 Aged from 25 to 55 years | Multiple Sclerosis | 12 weeks combined training intervention (resistance and aerobic exercise) | Improvement in walking and balance ability as well as reduced depression and fatigue |
| Shimada et al. [162] | Clinic trial | N = 945 Aged more than 65 years | Mild Cognitive Impairment | 40 weeks program of combined cognitive and physical activity with those of a health education program | Combined physical and cognitive activity improves or maintains cognitive and physical performance in older adults with mild cognitive impairment |
| Makino et al. [174] | Clinic trial | N = 415 Aged more than 70 years | Alzheimer Disease | 26 weeks of aerobic exercise training, resistance exercise training and combined exercise training | Improve delayed memory in community-dwelling older adults |
| Inskip et al. [175] | Clinic trial | N = 9 Aged from 66 to 84 years | Lewy Body Dementia | 8 weeks three times per week static balance, dynamic balance, functional practice, and progressive resistive exercise. | Improved clinically meaningful amounts in functional independence, cognition, physical function, and strength |
| Authors | Study Desing | Disorder | Supplement | Results |
|---|---|---|---|---|
| Pacifici et al.’s [199] | In vitro Clinical-Trial | Parkinson’s Disease | A5+, a combination of polyphenols and micronutrients | A novel mixture of polyphenol and micronutrients known as A5+, by acting in a synergic manner, can counteract the noxious processes |
| Alizadeh et al. [201] | Case-Control | Parkinson’s Disease | β-carotene, vitamin C, riboflavin, vitamin B6, and biotin | An adequate dietary intake of vitamins and minerals may have a preventive effect on developing PD |
| Jamali et al. [203] | Clinical-Trial | Parkinson’s Disease | β-carotene | There is a positive effect of β-carotene administration in PD rats |
| Sachs et al. [207] | Randomized-controlled clinical trial | Mild Cognitive Impairment | Multivitamin-mineral treatment and cocoa supplementation | Over 3 years, 110 incident MCI and 14 incident dementia cases were adjudicated. Incidence rates did not vary by assignment to multivitamin-mineral or cocoa extract |
| Jia et al. [210] | Clinical-Trial | Alzheimer Disease | Prebiotic chitosan oligosaccharide (COS) | Orally administered COS at 200, 400, or 800 mg/kg doses were effective at reducing the learning and memory deficits in Aβ1-42-induced rats |
| Gupta et al. [213] | In vitro Clinical-Trial | Alzheimer Disease | ω-3 fatty acid docosahexaenoic acid | Essential ω-3 fatty acid docosahexaenoic acid acts in a dose-dependent manner to prevent the actions of palmitic acid on inflammatory signaling in astrocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Belinchón-deMiguel, P.; Ramos-Campo, D.J.; Curiel-Regueros, A.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. The Interplay of Sports and Nutrition in Neurological Health and Recovery. J. Clin. Med. 2024, 13, 2065. https://doi.org/10.3390/jcm13072065
Clemente-Suárez VJ, Redondo-Flórez L, Beltrán-Velasco AI, Belinchón-deMiguel P, Ramos-Campo DJ, Curiel-Regueros A, Martín-Rodríguez A, Tornero-Aguilera JF. The Interplay of Sports and Nutrition in Neurological Health and Recovery. Journal of Clinical Medicine. 2024; 13(7):2065. https://doi.org/10.3390/jcm13072065
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Laura Redondo-Flórez, Ana Isabel Beltrán-Velasco, Pedro Belinchón-deMiguel, Domingo Jesús Ramos-Campo, Agustín Curiel-Regueros, Alexandra Martín-Rodríguez, and José Francisco Tornero-Aguilera. 2024. "The Interplay of Sports and Nutrition in Neurological Health and Recovery" Journal of Clinical Medicine 13, no. 7: 2065. https://doi.org/10.3390/jcm13072065