Maternal–Fetal Compatibility in Recurrent Pregnancy Loss
Abstract
:1. Introduction
2. The Role of Natural Killer Cells in Early Pregnancy
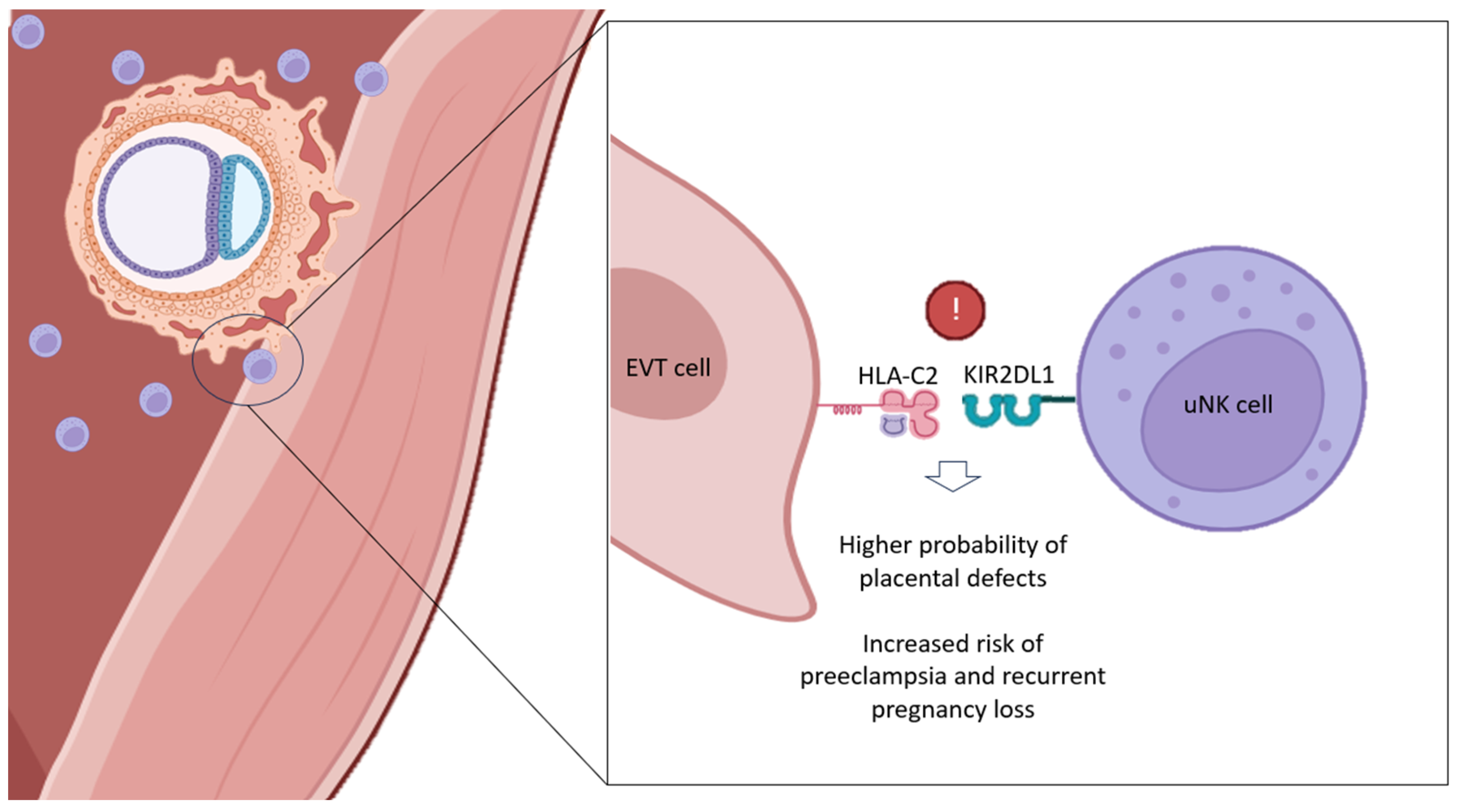
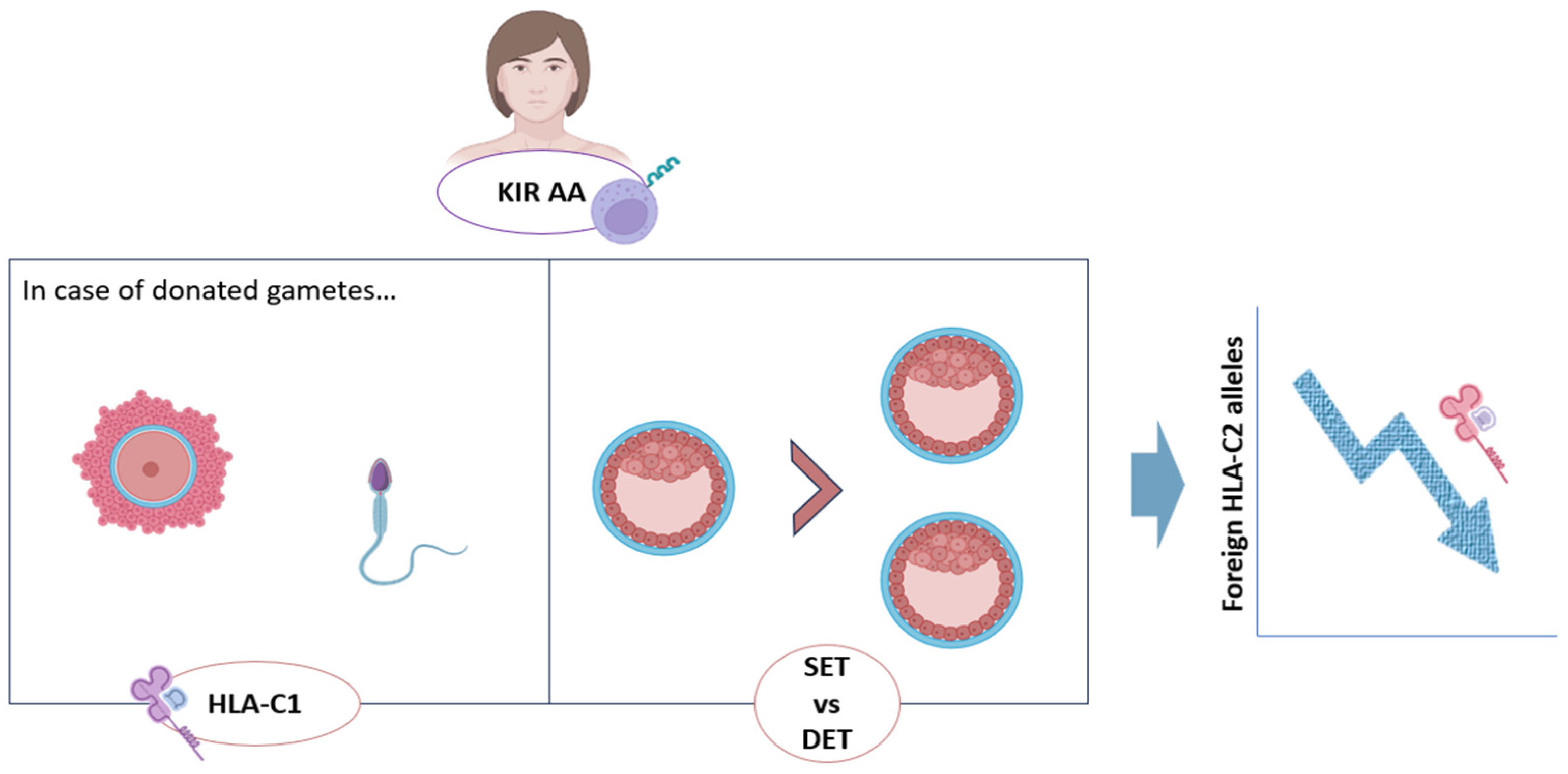
3. KIR–HLA-C Compatibility and RPL
4. Other HLA Molecules at the Maternal–Fetal Interface, and Their Relation to RPL
5. Management of Patients with Immunological Incompatibility
| Immune Therapy | Patients | Results/Conclusions | Publication |
|---|---|---|---|
| Glucocorticoids | Women with history of idiopathic RPL | Prednisolone therapy improves pregnancy outcomes in women with idiopathic RPL | [97] |
| Glucocorticoids | RPL and implantation failure patients | Significant positive effect of prednisolone was found | [98] |
| Glucocorticoids | Women with RPL and RIF | No evidence for a significant beneficial effect for prednisolone therapy on pregnancy outcomes. | [99] |
| Glucocorticoids, combined with aspirin and doxycycline | Women undergoing IVF/ICSI with own oocytes | No benefit of this combined adjuvant strategy in fresh IVF cycles, and possible harm when used in frozen cycles | [100] |
| Glucocorticoids | Subfertile women undergoing IVF or ICSI | There is insufficient evidence that administration of peri-implantation glucocorticoids in IVF/ICSI cycles influenced clinical outcomes | [103] |
| Heparin and aspirin | Women with unexplained RPL | No significant differences in live birth rate were found | [106] |
| Heparin and aspirin | Women with unexplained recurrent miscarriage | Neither aspirin combined with nadroparin nor aspirin alone improved the LBR | [104] |
| Heparin | Patients with history of placenta-mediated pregnancy complications | Low-molecular-weight heparin does not seem to reduce the risk of recurrent placenta-mediated pregnancy complications | [105] |
| Aspirin | Women at risk of preeclampsia | Low-dose aspirin is an efficient method of reducing the incidence of preeclampsia and FGR | [110] |
| IVIG | Women with unexplained RPL | Intravenous immunoglobulin showed no effect on live birth rate compared with placebo | [106] |
| IVIG | Women with history of RPL | No significant difference in the frequency of live birth was found | [111] |
| LIT | Women with history of RPL | Beneficial effect of the use of immunotherapy with lymphocytes in cases of RPL | [112] |
| LIT | Women who had had three or more spontaneous abortions of unknown cause | Immunization with paternal mononuclear cells does not improve pregnancy outcome in women with unexplained recurrent miscarriage | [113] |
| LIT | Women with history of RPL | Lymphocyte immunotherapies do not have the required FDA approval and are considered investigational drugs | [22] |
| TNF-α inhibitor | Women with infertility and T helper 1/T helper 2 cytokine elevation | The use of a TNF-α inhibitor and IVIG significantly improves IVF outcome | [115] |
| TNF-α inhibitor | Patients with rheumatoid arthritis | The rate of spontaneous abortion was highest among patients exposed to anti-TNF at the time of conception | [118] |
| Intralipids | Women with unexplained secondary infertility, RPL, and elevated levels of natural killer cells | Intralipid supplementation did not increase frequency of chemical pregnancy. | [119] |
| Intralipids | Women aged 40–42 years with a previous history of miscarriage | The use of intravenous intralipid to suppress natural killer cell activity does not seem to improve the chance of a live delivery | [120] |
| G-CSF | Women with RPL | Improved LBR and pregnancy rate in the group treated with G-CSF, compared to control group | [121] |
| G-CSF | Patients under 40 years old with at least two unexplained pregnancy losses | No significant difference was observed in terms of chemical pregnancy, implantation, clinical pregnancy and abortion | [122] |
| G-CSF | Women with RPL | Improved LBR in the group treated with G-CSF, compared to control group | [123] |
| G-CSF | Women with RPL/RIF | There is evidence that G-CSF increases the pregnancy rate in ART treatment, particularly in patients with RIF, and decreases the abortion rate in patients with RPL | [124] |
| Progesterone | Women with unexplained RPL, aged between 18 and 39 years at randomization, conceiving naturally | There is no evidence that first-trimester progesterone therapy improves outcomes in women with a history of unexplained RPL | [126] |
| Progesterone and progestins | Women with a history of unexplained recurrent miscarriage | Supplementation with progestogens may reduce the incidence of recurrent miscarriages and seem to be safe for the fetuses | [127] |
| Progestogens | Women with a history of one or more previous miscarriages and early pregnancy bleeding | Vaginal micronized progesterone seems to increase the live birth rate | [128] |
6. Discussion
7. Conclusions
8. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers 2020, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- The ESHRE Guideline Group on RPL. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, Y.; Chen, Z.; Li, T. Traditional and molecular chromosomal abnormality analysis of products of conception in spontaneous and recurrent miscarriage. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Collins, G.S.; Salem, S.A.; Liu, X.; Lyle, S.S.; Peck, A.C.; Sills, E.S.; Salem, R.D. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: Results from a randomized pilot study. Mol. Cytogenet. 2012, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gimenez, C.; Alijotas-Reig, J. Recurrent miscarriage: Causes, evaluation and management. Postgrad. Med. J. 2015, 91, 151–162. [Google Scholar] [CrossRef]
- Homer, H.A. Modern management of recurrent miscarriage. Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, M.; Manchorova, D.; Dimova, T. Immunity at maternal–fetal interface: KIR/HLA (Allo)recognition*. Immunol. Rev. 2022, 308, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Barrio, A.; Garrido, N.; Aparicio, P.; Pellicer, A.; Moffett, A.; García -Velasco, J.A. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil. Steril. 2020, 114, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef]
- Semmes, E.C.; Coyne, C.B. Innate immune defenses at the maternal-fetal interface. Curr. Opin. Immunol. 2022, 74, 60–67. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Scott, R.T. Contribution of immunology to implantation failure of euploid embryos. Fertil. Steril. 2017, 107, 1279–1283. [Google Scholar] [CrossRef]
- Diaz-Hernandez, I.; Alecsandru, D.; Garcia-Velasco, J.A.; Dominguez, F. Uterine natural killer cells: From foe to friend in reproduction. Hum. Reprod. Update 2021, 27, 720–746. [Google Scholar] [CrossRef]
- Moffett, A.; Chazara, O.; Colucci, F. Maternal allo-recognition of the fetus. Fertil. Steril. 2017, 107, 1269–1272. [Google Scholar] [CrossRef]
- Parham, P. MHC class I molecules and kirs in human history, health and survival. Nat. Rev. Immunol. 2005, 5, 201–214. [Google Scholar] [CrossRef]
- Hiby, S.E.; Apps, R.; Chazara, O.; Farrell, L.E.; Magnus, P.; Trogstad, L.; Gjessing, H.K.; Carrington, M.; Moffett, A. Maternal KIR in Combination with Paternal HLA-C2 Regulate Human Birth Weight. J. Immunol. 2014, 192, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Apps, R.; Sharkey, A.M.; Farrell, L.E.; Gardner, L.; Mulder, A.; Claas, F.H.; Walker, J.J.; Redman, C.C.; Morgan, L.; et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Investig. 2010, 120, 4102–4110. [Google Scholar] [CrossRef]
- Hiby, S.E.; Regan, L.; Lo, W.; Farrell, L.; Carrington, M.; Moffett, A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 2008, 23, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Hiby, S.E.; Walker, J.J.; O’Shaughnessy, K.M.; Redman, C.W.; Carrington, M.; Trowsdale, J.; Moffett, A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 2004, 200, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, A.; Tzonis, P.; Perros, G.; Graphou, O.; Keramitsoglou, T.; Koussoulakos, S.; Margaritis, L.; Varla-Leftherioti, M. Comparative analysis of peripheral natural killer cells in the two phases of the ovarian cycle. Am. J. Reprod. Immunol. 2010, 63, 46–53. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Moffett, A.; Shreeve, N. First do no harm: Uterine natural killer (NK) cells in assisted reproduction. Hum. Reprod. 2015, 30, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.F.; Porter, T.F.; Scott, J.R. Immunotherapy for recurrent miscarriage. Cochrane Database Syst. Rev. 2014, 2014, Cd000112. [Google Scholar] [CrossRef] [PubMed]
- Trundley, A.; Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Garcia-Velasco, J.A. Why natural killer cells are not enough: A further understanding of killer immunoglobulin-like receptor and human leukocyte antigen. Fertil. Steril. 2017, 107, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, J.; Male, V.; Ghazal, P.; Forster, T.; Gibson, D.A.; Williams, A.R.; Brito-Mutunayagam, S.L.; Craigon, M.; Lourenco, P.; Cameron, I.T.; et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. J. Immunol. 2013, 191, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Robson, S.C.; Bulmer, J.N. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010, 31, S87–S92. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-W.; Zhang, Y.-C.; Wu, F.; Tian, F.-J.; Lin, Y. The role of extravillous trophoblasts and uterine NK cells in vascular remodeling during pregnancy. Front. Immunol. 2022, 13, 951482. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sharkey, A.; Sheridan, M.; Magistrati, E.; Arutyunyan, A.; Huhn, O.; Sancho-Serra, C.; Anderson, H.; McGovern, N.; Esposito, L.; et al. Human uterine natural killer cells regulate differentiation of extravillous trophoblast early in pregnancy. Cell Stem Cell 2024, 31, 181–195.e9. [Google Scholar] [CrossRef]
- Lash, G.E.; Schiessl, B.; Kirkley, M.; Innes, B.A.; Cooper, A.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J. Leukoc. Biol. 2006, 80, 572–580. [Google Scholar] [CrossRef]
- Su, M.T.; Lin, S.H.; Chen, Y.C.; Kuo, P.L. Genetic association studies of ACE and PAI-1 genes in women with recurrent pregnancy loss: A systematic review and meta-analysis. Thromb. Haemost. 2013, 109, 8–15. [Google Scholar] [CrossRef]
- Xie, M.; Li, Y.; Meng, Y.-Z.; Xu, P.; Yang, Y.-G.; Dong, S.; He, J.; Hu, Z. Uterine Natural Killer Cells: A Rising Star in Human Pregnancy Regulation. Front. Immunol. 2022, 13, 918550. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, C.; Carbone, F.; Longobardi, S.; Matarese, G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 2014, 162, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Farshchi, M.; Abdollahi, E.; Saghafi, N.; Hosseini, A.; Fallahi, S.; Rostami, S.; Rostami, P.; Rafatpanah, H.; Habibagahi, M. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. J. Clin. Transl. Res. 2022, 8, 256–265. [Google Scholar] [PubMed]
- Zidan, H.E.; Abdul-Maksoud, R.S.; Mowafy, H.E.; Elsayed, W.S.H. The association of IL-33 and Foxp3 gene polymorphisms with recurrent pregnancy loss in Egyptian women. Cytokine 2018, 108, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Crespo, Â.C.; Mulik, S.; Dotiwala, F.; Ansara, J.A.; Sen Santara, S.; Ingersoll, K.; Ovies, C.; Junqueira, C.; Tilburgs, T.; Strominger, J.L.; et al. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts. Cell 2020, 182, 1125–1139.e18. [Google Scholar] [CrossRef] [PubMed]
- Sen Santara, S.; Crespo, Â.C.; Mulik, S.; Ovies, C.; Boulenouar, S.; Strominger, J.L.; Lieberman, J. Decidual NK cells kill Zika virus–infected trophoblasts. Proc. Natl. Acad. Sci. USA 2021, 118, e2115410118. [Google Scholar] [CrossRef] [PubMed]
- Jonjic, S.; Siewiera, J.; El Costa, H.; Tabiasco, J.; Berrebi, A.; Cartron, G.; Bouteiller, P.; Jabrane-Ferrat, N. Human Cytomegalovirus Infection Elicits New Decidual Natural Killer Cell Effector Functions. PLoS Pathog. 2013, 9, e1003257. [Google Scholar] [CrossRef]
- Alecsandru, D.; Garcia-Velasco, J.A. Immunology and human reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 231–234. [Google Scholar] [CrossRef]
- Colucci, F. The role of KIR and HLA interactions in pregnancy complications. Immunogenetics 2017, 69, 557–565. [Google Scholar] [CrossRef]
- Adams, E.J.; Parham, P. Species-specific evolution ofMHCclass I genes in the higher primates. Immunol. Rev. 2001, 183, 41–64. [Google Scholar] [CrossRef]
- Colucci, F.; Moffett, A.; Trowsdale, J. Medawar and the immunological paradox of pregnancy: 60 years on. Eur. J. Immunol. 2014, 44, 1883–1885. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.L.; Hviid, T.V.F. HLA Class Ib-receptor interactions during embryo implantation and early pregnancy. Hum. Reprod. Update 2022, 28, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, A.; Geraghty, D.E. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. USA 1992, 89, 3947–3951. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Burrows, T.D.; Hiby, S.E.; Bowen, J.M.; Joseph, S.; Verma, S.; Lim, P.B.; Gardner, L.; Le Bouteiller, P.; Ziegler, A.; et al. Surface Expression of HLA-C Antigen by Human Extravillous Trophoblast. Placenta 2000, 21, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Parham, P. NK cells and trophoblasts: Partners in pregnancy. J. Exp. Med. 2004, 200, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Faridi, R.M.; Agrawal, S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum. Reprod. 2011, 26, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Samaridis, J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science 1995, 268, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Vilches, C.; Parham, P. KIR: Diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 2002, 20, 217–251. [Google Scholar] [CrossRef]
- Anthony Nolan Research Institute. IPD-KIR Database. Available online: https://www.ebi.ac.uk/ipd/kir/ (accessed on 22 March 2024).
- Biassoni, R.; Cantoni, C.; Falco, M.; Verdiani, S.; Bottino, C.; Vitale, M.; Conte, R.; Poggi, A.; Moretta, A.; Moretta, L. The human leukocyte antigen (HLA)-C-specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J. Exp. Med. 1996, 183, 645–650. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, X.; Lu, P.; Song, Y.; Lin, Q. Killer immunoglobulin-like receptor repertoire on uterine natural killer cell subsets in women with recurrent spontaneous abortions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Chazara, O.; Colucci, F.; Johnson, M.H. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: Too early for clinical intervention. Reprod. Biomed. Online 2016, 33, 763–769. [Google Scholar] [CrossRef]
- Anthony Nolan Research Institute. HLA Nomenclature. Available online: http://hla.alleles.org/nomenclature/stats.html (accessed on 22 March 2024).
- Mandelboim, O.; Reyburn, H.T.; Vales-Gomez, M.; Pazmany, L.; Colonna, M.; Borsellino, G.; Strominger, J.L. Protection from lysis by natural killer cells of group 1 and 2 specificity is mediated by residue 80 in human histocompatibility leukocyte antigen C alleles and also occurs with empty major histocompatibility complex molecules. J. Exp. Med. 1996, 184, 913–922. [Google Scholar] [CrossRef]
- Winter, C.C.; Gumperz, J.E.; Parham, P.; Long, E.O.; Wagtmann, N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J. Immunol. 1998, 161, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Alecsandru, D.; Garrido, N.; Vicario, J.L.; Barrio, A.; Aparicio, P.; Requena, A.; Garcia-Velasco, J.A. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum. Reprod. 2014, 29, 2637–2643. [Google Scholar] [CrossRef]
- Xiong, S.; Sharkey, A.M.; Kennedy, P.R.; Gardner, L.; Farrell, L.E.; Chazara, O.; Bauer, J.; Hiby, S.E.; Colucci, F.; Moffett, A. Maternal uterine NK cell–activating receptor KIR2DS1 enhances placentation. J. Clin. Investig. 2013, 123, 4264–4272. [Google Scholar] [CrossRef]
- Wallace, A.E.; Whitley, G.S.; Thilaganathan, B.; Cartwright, J.E. Decidual natural killer cell receptor expression is altered in pregnancies with impaired vascular remodeling and a higher risk of pre-eclampsia. J. Leukoc. Biol. 2015, 97, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Pires, C.R.; Moron, A.F.; Araujo, E.; Traina, E.; Mattar, R. Doppler assessment of uterine blood flow in recurrent pregnancy loss. Int. J. Gynecol. Obstet. 2007, 98, 115–119. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the Maternal-Fetal Interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]
- Meuleman, T.; Drabbels, J.; van Lith, J.M.M.; Dekkers, O.M.; Rozemuller, E.; Cretu-Stancu, M.; Claas, F.H.J.; Bloemenkamp, K.W.M.; Eikmans, M. Lower frequency of the HLA-G UTR-4 haplotype in women with unexplained recurrent miscarriage. J. Reprod. Immunol. 2018, 126, 46–52. [Google Scholar] [CrossRef]
- Ober, C.; Aldrich, C.L.; Chervoneva, I.; Billstrand, C.; Rahimov, F.; Gray, H.L.; Hyslop, T. Variation in the HLA-G Promoter Region Influences Miscarriage Rates. Am. J. Hum. Genet. 2003, 72, 1425–1435. [Google Scholar] [CrossRef]
- Thomsen, C.K.; Steffensen, R.; Nielsen, H.S.; Kolte, A.M.; Krog, M.C.; Egerup, P.; Larsen, E.C.; Hviid, T.V.; Christiansen, O.B. HLA-DRB1 polymorphism in recurrent pregnancy loss: New evidence for an association to HLA-DRB1*07. J. Reprod. Immunol. 2021, 145, 103308. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Wei, H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front. Immunol. 2020, 11, 592010. [Google Scholar] [CrossRef]
- Rouas-Freiss, N.; Moreau, P.; LeMaoult, J.; Papp, B.; Tronik-Le Roux, D.; Carosella, E.D. Role of the HLA-G immune checkpoint molecule in pregnancy. Hum. Immunol. 2021, 82, 353–361. [Google Scholar] [CrossRef]
- Zhuang, B.; Shang, J.; Yao, Y. HLA-G: An Important Mediator of Maternal-Fetal Immune-Tolerance. Front. Immunol. 2021, 12, 744324. [Google Scholar] [CrossRef]
- Alegre, E.; Díaz-Lagares, A.; LeMaoult, J.; López-Moratalla, N.; Carosella, E.D.; González, A. Maternal antigen presenting cells are a source of plasmatic HLA-G during pregnancy: Longitudinal study during pregnancy. Hum. Immunol. 2007, 68, 661–667. [Google Scholar] [CrossRef]
- Zidi, I.; Rizzo, R.; Bouaziz, A.; Laaribi, A.B.; Zidi, N.; Di Luca, D.; Tlili, H.; Bortolotti, D. sHLA-G1 and HLA-G5 levels are decreased in Tunisian women with multiple abortion. Hum. Immunol. 2016, 77, 342–345. [Google Scholar] [CrossRef]
- Madduru, D.; Dirsipam, K.; Goli, M.; Ramana Devi, V.; Jahan, P. Association of reduced maternal sHLA-G5 isoform levels and elevated TNF-α/IL-4 cytokine ratio with Recurrent Pregnancy Loss: A study on South Indian women. Scand. J. Immunol. 2021, 94, e13095. [Google Scholar] [CrossRef]
- Barbaro, G.; Inversetti, A.; Cristodoro, M.; Ticconi, C.; Scambia, G.; Di Simone, N. HLA-G and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2023, 24, 2557. [Google Scholar] [CrossRef]
- Bae, S.-C.; Lee, Y.H. Association of HLA-G polymorphisms with systemic lupus erythematosus and correlation between soluble HLA-G levels and the disease: A meta-analysis. Z. Für Rheumatol. 2020, 80, 96–102. [Google Scholar] [CrossRef]
- de Carvalho, J.F.; de Oliveira, R.M.; Rodrigues, C.E.; Glezer, A.; Bonfa, E.; Pereira, R.M. Heparin increases HLA-G levels in primary antiphospholipid syndrome. Clin. Dev. Immunol. 2012, 2012, 232390. [Google Scholar] [CrossRef]
- Hylenius, S. Association between HLA-G genotype and risk of pre-eclampsia: A case-control study using family triads. Mol. Hum. Reprod. 2004, 10, 237–246. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, W.; Zhang, D. Association of 14-bp insertion/deletion polymorphism of HLA-G gene with unexplained recurrent spontaneous abortion: A meta-analysis. Tissue Antigens 2013, 81, 108–115. [Google Scholar] [CrossRef]
- Nilsson, L.L.; Djurisic, S.; Andersen, A.M.N.; Melbye, M.; Bjerre, D.; Ferrero-Miliani, L.; Hackmon, R.; Geraghty, D.E.; Hviid, T.V.F. Distribution of HLA-G extended haplotypes and one HLA-E polymorphism in a large-scale study of mother–child dyads with and without severe preeclampsia and eclampsia. Hla 2016, 88, 172–186. [Google Scholar] [CrossRef]
- Fan, W.; Li, S.; Huang, Z.; Chen, Q. Relationship between HLA-G polymorphism and susceptibility to recurrent miscarriage: A meta-analysis of non-family-based studies. J. Assist. Reprod. Genet. 2013, 31, 173–184. [Google Scholar] [CrossRef]
- Monti, M.; Lupoli, R.; Sosa Fernandez, L.M.; Cirillo, F.; Di Minno, M.N.D. Association of human leukocyte antigen-G 14 bp polymorphism with recurrent pregnancy loss in European countries: A meta-analysis of literature studies. Fertil. Steril. 2019, 112, 577–585.e3. [Google Scholar] [CrossRef]
- Kalotra, V.; Lall, M.; Verma, I.C.; Kaur, A.; Kaur, A. The HLA-G 14 bp insertion/deletion polymorphism and its association with soluble HLA-G levels in women with recurrent miscarriages. Hla 2018, 91, 167–174. [Google Scholar] [CrossRef]
- Agrawal, D.; Prakash, S.; Misra, M.K.; Phadke, S.R.; Agrawal, S. Implication of HLA-G 5′ upstream regulatory region polymorphisms in idiopathic recurrent spontaneous abortions. Reprod. BioMed. Online 2015, 30, 82–91. [Google Scholar] [CrossRef]
- Hviid, T.; Rizzo, R.; Christiansen, O.; Melchiorri, L.; Lindhard, A.; Baricordi, O. HLA-G and IL-10 in serum in relation to HLA-G genotype and polymorphisms. Immunogenetics 2004, 56, 135–141. [Google Scholar] [CrossRef]
- Dias, F.C.; Bertol, B.C.; Poras, I.; Souto, B.M.; Mendes-Junior, C.T.; Castelli, E.C.; Gineau, L.; Sabbagh, A.; Rouas-Freiss, N.; Carosella, E.D.; et al. The genetic diversity within the 1.4 kb HLA-G 5′ upstream regulatory region moderately impacts on cellular microenvironment responses. Sci. Rep. 2018, 8, 5652. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Gasbarrini, A.; Castellani, R.; Rocchetti, S.; Sisti, L.G.; Scambia, G.; Di Simone, N. Human leukocyte antigen (HLA) DQ2/DQ8 prevalence in recurrent pregnancy loss women. Autoimmun. Rev. 2016, 15, 638–643. [Google Scholar] [CrossRef]
- Liu, E.; Lee, H.-S.; Aronsson, C.A.; Hagopian, W.A.; Koletzko, S.; Rewers, M.J.; Eisenbarth, G.S.; Bingley, P.J.; Bonifacio, E.; Simell, V.; et al. Risk of Pediatric Celiac Disease According to HLA Haplotype and Country. N. Engl. J. Med. 2014, 371, 42–49. [Google Scholar] [CrossRef]
- Romanos, J.; van Diemen, C.C.; Nolte, I.M.; Trynka, G.; Zhernakova, A.; Fu, J.; Bardella, M.T.; Barisani, D.; McManus, R.; van Heel, D.A.; et al. Analysis of HLA and Non-HLA Alleles Can Identify Individuals at High Risk for Celiac Disease. Gastroenterology 2009, 137, 834–840.e3. [Google Scholar] [CrossRef]
- Królik, M.; Wrześniak, M.; Jezela-Stanek, A. Possible effect of the HLA-DQ2/DQ8 polymorphism on autoimmune parameters and lymphocyte subpopulation in recurrent pregnancy losses. J. Reprod. Immunol. 2022, 149, 103467. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Ticconi, C.; Tersigni, C.; Garofalo, S.; Martino, C.; Lanzone, A.; Scambia, G.; Di Simone, N. The pathogenic role of autoantibodies in recurrent pregnancy loss. Am. J. Reprod. Immunol. 2019, 83, e13200. [Google Scholar] [CrossRef]
- Twig, G.; Shina, A.; Amital, H.; Shoenfeld, Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J. Autoimmun. 2012, 38, J275–J281. [Google Scholar] [CrossRef]
- Andreoli, L.; Fredi, M.; Nalli, C.; Reggia, R.; Lojacono, A.; Motta, M.; Tincani, A. Pregnancy implications for systemic lupus erythematosus and the antiphospholipid syndrome. J. Autoimmun. 2012, 38, J197–J208. [Google Scholar] [CrossRef]
- Dong, A.C.; Morgan, J.; Kane, M.; Stagnaro-Green, A.; Stephenson, M.D. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: A systematic review and meta-analysis. Fertil. Steril. 2020, 113, 587–600.e1. [Google Scholar] [CrossRef]
- Gleicher, N. Maternal autoimmunity and adverse pregnancy outcomes. J. Autoimmun. 2014, 50, 83–86. [Google Scholar] [CrossRef]
- Hsiao, T.-W.; Chung, M.-T.; Wen, J.-Y.; Lin, Y.-L.; Lin, L.-Y.; Tsai, Y.-C. HLA sharing and maternal HLA expression in couples with recurrent pregnancy loss in Taiwan. Taiwan. J. Obstet. Gynecol. 2022, 61, 854–857. [Google Scholar] [CrossRef]
- Persson, G.; Melsted, W.N.; Nilsson, L.L.; Hviid, T.V.F. HLA class Ib in pregnancy and pregnancy-related disorders. Immunogenetics 2017, 69, 581–595. [Google Scholar] [CrossRef]
- Vassiliadou, N.; Bulmer, J.N. Immunohistochemical evidence for increased numbers of c‘lassic’ CD57+ natural killer cells in the endometrium of women suffering spontaneous early pregnancy loss. Hum. Reprod. 1996, 11, 1569–1574. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, Y.; Kase, N.; Mori, H. Decidual natural killer cells in recurrent spontaneous abortion with normal chromosomal content. Am. J. Reprod. Immunol. 1999, 41, 337–342. [Google Scholar] [CrossRef]
- Woon, E.V.; Day, A.; Bracewell-Milnes, T.; Male, V.; Johnson, M. Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: A systematic review and meta-analysis. J. Reprod. Immunol. 2020, 142, 103189. [Google Scholar] [CrossRef]
- Dan, S.; Wei, W.; Yichao, S.; Hongbo, C.; Shenmin, Y.; Jiaxiong, W.; Hong, L. Effect of Prednisolone Administration on Patients with Unexplained Recurrent Miscarriage and in Routine Intracytoplasmic Sperm Injection: A Meta-Analysis. Am. J. Reprod. Immunol. 2015, 74, 89–97. [Google Scholar] [CrossRef]
- Mekinian, A.; Cohen, J.; Alijotas-Reig, J.; Carbillon, L.; Nicaise-Roland, P.; Kayem, G.; Daraï, E.; Fain, O.; Bornes, M. Unexplained Recurrent Miscarriage and Recurrent Implantation Failure: Is There a Place for Immunomodulation? Am. J. Reprod. Immunol. 2016, 76, 8–28. [Google Scholar] [CrossRef]
- Cooper, S.; Laird, S.M.; Mariee, N.; Li, T.C.; Metwally, M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J. Reprod. Immunol. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Motteram, C.; Vollenhoven, B.; Hope, N.; Osianlis, T.; Rombauts, L.J. Live birth rates after combined adjuvant therapy in IVF–ICSI cycles: A matched case-control study. Reprod. BioMed. Online 2015, 30, 340–348. [Google Scholar] [CrossRef]
- Toth, B.; Würfel, W.; Bohlmann, M.; Zschocke, J.; Rudnik-Schöneborn, S.; Nawroth, F.; Schleußner, E.; Rogenhofer, N.; Wischmann, T.; von Wolff, M.; et al. Recurrent Miscarriage: Diagnostic and Therapeutic Procedures. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry Number 015/050). Geburtshilfe Frauenheilkd. 2018, 78, 364–381. [Google Scholar] [CrossRef]
- Robertson, S.A.; Jin, M.; Yu, D.; Moldenhauer, L.M.; Davies, M.J.; Hull, M.L.; Norman, R.J. Corticosteroid therapy in assisted reproduction—Immune suppression is a faulty premise. Hum. Reprod. 2016, 31, 2164–2173. [Google Scholar] [CrossRef]
- Boomsma, C.M.; Kamath, M.S.; Keay, S.D.; Macklon, N.S. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst. Rev. 2022, 2022, CD005996. [Google Scholar] [CrossRef]
- Kaandorp, S.P.; Goddijn, M.; van der Post, J.A.M.; Hutten, B.A.; Verhoeve, H.R.; Hamulyák, K.; Mol, B.W.; Folkeringa, N.; Nahuis, M.; Papatsonis, D.N.M.; et al. Aspirin plus Heparin or Aspirin Alone in Women with Recurrent Miscarriage. N. Engl. J. Med. 2010, 362, 1586–1596. [Google Scholar] [CrossRef]
- Rodger, M.A.; Gris, J.-C.; de Vries, J.I.P.; Martinelli, I.; Rey, É.; Schleussner, E.; Middeldorp, S.; Kaaja, R.; Langlois, N.J.; Ramsay, T.; et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet 2016, 388, 2629–2641. [Google Scholar] [CrossRef]
- Rasmark Roepke, E.; Hellgren, M.; Hjertberg, R.; Blomqvist, L.; Matthiesen, L.; Henic, E.; Lalitkumar, S.; Strandell, A. Treatment efficacy for idiopathic recurrent pregnancy loss—A systematic review and meta-analyses. Acta Obstet. Et Gynecol. Scand. 2018, 97, 921–941. [Google Scholar] [CrossRef]
- Quenby, S.; Booth, K.; Hiller, L.; Coomarasamy, A.; de Jong, P.G.; Hamulyák, E.N.; Scheres, L.J.; van Haaps, T.F.; Ewington, L.; Tewary, S.; et al. Heparin for women with recurrent miscarriage and inherited thrombophilia (ALIFE2): An international open-label, randomised controlled trial. Lancet 2023, 402, 54–61. [Google Scholar] [CrossRef]
- Alecsandru, D.; Klimczak, A.M.; Garcia Velasco, J.A.; Pirtea, P.; Franasiak, J.M. Immunologic causes and thrombophilia in recurrent pregnancy loss. Fertil. Steril. 2021, 115, 561–566. [Google Scholar] [CrossRef]
- Petri, M. Antiphospholipid syndrome. Transl. Res. 2020, 225, 70–81. [Google Scholar] [CrossRef]
- Bujold, E.; Roberge, S.; Lacasse, Y.; Bureau, M.; Audibert, F.; Marcoux, S.; Forest, J.-C.; Giguère, Y. Prevention of Preeclampsia and Intrauterine Growth Restriction With Aspirin Started in Early Pregnancy. Obstet. Gynecol. 2010, 116, 402–414. [Google Scholar] [CrossRef]
- Egerup, P.; Lindschou, J.; Gluud, C.; Christiansen, O.B. The Effects of Intravenous Immunoglobulins in Women with Recurrent Miscarriages: A Systematic Review of Randomised Trials with Meta-Analyses and Trial Sequential Analyses Including Individual Patient Data. PLoS ONE 2015, 10, e0141588. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Sarno, M.; Araujo Júnior, E.; Da Silva Costa, F.; Barini, R. Lymphocyte immunotherapy in the treatment of recurrent miscarriage: Systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 295, 511–518. [Google Scholar] [CrossRef]
- Ober, C.; Karrison, T.; Odem, R.R.; Barnes, R.B.; Branch, D.W.; Stephenson, M.D.; Baron, B.; Walker, M.A.; Scott, J.R.; Schreiber, J.R. Mononuclear-cell immunisation in prevention of recurrent miscarriages: A randomised trial. Lancet 1999, 354, 365–369. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; El-Toukhy, T.; Ahuja, S.; Taranissi, M. Degree of TNF-alpha/IL-10 cytokine elevation correlates with IVF success rates in women undergoing treatment with Adalimumab (Humira) and IVIG. Am. J. Reprod. Immunol. 2011, 65, 610–618. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L.; Ashoush, S.; Ahuja, S.; El-Toukhy, T.; Taranissi, M. Treatment with adalimumab (Humira) and intravenous immunoglobulin improves pregnancy rates in women undergoing IVF. Am. J. Reprod. Immunol. 2009, 61, 113–120. [Google Scholar] [CrossRef]
- Hviid, M.M.; Macklon, N. Immune modulation treatments-where is the evidence? Fertil. Steril. 2017, 107, 1284–1293. [Google Scholar] [CrossRef]
- Dai, F.-F.; Hu, M.; Zhang, Y.-W.; Zhu, R.-H.; Chen, L.-P.; Li, Z.-D.; Huang, Y.-J.; Hu, W.; Cheng, Y.-X. TNF-α/anti-TNF-αdrugs and its effect on pregnancy outcomes. Expert Rev. Mol. Med. 2022, 24, e26. [Google Scholar] [CrossRef]
- Verstappen, S.M.M.; King, Y.; Watson, K.D.; Symmons, D.P.M.; Hyrich, K.L. Anti-TNF therapies and pregnancy: Outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 2011, 70, 823–826. [Google Scholar] [CrossRef]
- Dakhly, D.M.; Bayoumi, Y.A.; Sharkawy, M.; Gad Allah, S.H.; Hassan, M.A.; Gouda, H.M.; Hashem, A.T.; Hatem, D.L.; Ahmed, M.F.; El-Khayat, W. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells. Int. J. Gynaecol. Obs. 2016, 135, 324–327. [Google Scholar] [CrossRef]
- Check, J.H.; Check, D.L. Intravenous intralipid therapy is not beneficial in having a live delivery in women aged 40–42 years with a previous history of miscarriage or failure to conceive despite embryo transfer undergoing in vitro fertilization-embryo transfer. Clin Exp Obs. Gynecol 2016, 43, 14–15. [Google Scholar] [CrossRef]
- Scarpellini, F.; Sbracia, M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: A randomised controlled trial. Hum. Reprod. 2009, 24, 2703–2708. [Google Scholar] [CrossRef]
- Zafardoust, S.; Akhondi, M.M.; Sadeghi, M.R.; Mohammadzadeh, A.; Karimi, A.; Jouhari, S.; Ansaripour, S. Efficacy of Intrauterine Injection of Granulocyte Colony Stimulating Factor (G-CSF) on Treatment of Unexplained Recurrent Miscarriage: A Pilot RCT Study. J. Reprod. Infertil. 2017, 18, 379–385. [Google Scholar]
- Santjohanser, C.; Knieper, C.; Franz, C.; Hirv, K.; Meri, O.; Schleyer, M.; Würfel, W.; Toth, B. Granulocyte-Colony Stimulating Factor as Treatment Option in Patients with Recurrent Miscarriage. Arch. Immunol. Ther. Exp. 2013, 61, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Würfel, W. Treatment with granulocyte colony-stimulating factor in patients with repetitive implantation failures and/or recurrent spontaneous abortions. J. Reprod. Immunol. 2015, 108, 123–135. [Google Scholar] [CrossRef]
- Cruz, M.; Alecsandru, D.; García-Velasco, J.A.; Requena, A. Use of granulocyte colony-stimulating factor in ART treatment does not increase the risk of adverse perinatal outcomes. Reprod. BioMed. Online 2019, 39, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Coomarasamy, A.; Williams, H.; Truchanowicz, E.; Seed, P.T.; Small, R.; Quenby, S.; Gupta, P.; Dawood, F.; Koot, Y.E.M.; Bender Atik, R.; et al. A Randomized Trial of Progesterone in Women with Recurrent Miscarriages. N. Engl. J. Med. 2015, 373, 2141–2148. [Google Scholar] [CrossRef]
- Saccone, G.; Schoen, C.; Franasiak, J.M.; Scott, R.T.; Berghella, V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: A systematic review and meta-analysis of randomized, controlled trials. Fertil. Steril. 2017, 107, 430–438.e3. [Google Scholar] [CrossRef]
- Devall, A.J.; Papadopoulou, A.; Podesek, M.; Haas, D.M.; Price, M.J.; Coomarasamy, A.; Gallos, I.D. Progestogens for preventing miscarriage: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 2021, CD013792. [Google Scholar] [CrossRef]
- Cuadrado-Torroglosa, I.; Pacheco, A.; Barrio, A.; Garrido, N.; Aparicio, P.; Pellicer, N.; García-Velasco, J.A.; Alecsandru, D. Increased cytotoxic natural killer cells in the endometrium alone cannot be considered the immunological cause of recurrent miscarriage. Fertil. Steril. 2023, 120, 101–110. [Google Scholar] [CrossRef]
- Lachapelle, M.H.; Miron, P.; Hemmings, R.; Roy, D.C. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J. Immunol. 1996, 156, 4027–4034. [Google Scholar] [CrossRef]
- Von Woon, E.; Greer, O.; Shah, N.; Nikolaou, D.; Johnson, M.; Male, V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 548–582. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadrado-Torroglosa, I.; García-Velasco, J.A.; Alecsandru, D. Maternal–Fetal Compatibility in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 2379. https://doi.org/10.3390/jcm13082379
Cuadrado-Torroglosa I, García-Velasco JA, Alecsandru D. Maternal–Fetal Compatibility in Recurrent Pregnancy Loss. Journal of Clinical Medicine. 2024; 13(8):2379. https://doi.org/10.3390/jcm13082379
Chicago/Turabian StyleCuadrado-Torroglosa, Isabel, Juan A. García-Velasco, and Diana Alecsandru. 2024. "Maternal–Fetal Compatibility in Recurrent Pregnancy Loss" Journal of Clinical Medicine 13, no. 8: 2379. https://doi.org/10.3390/jcm13082379






