Factors Associated with Frailty in Older Adults in Community and Nursing Home Settings: A Systematic Review with a Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistic Analysis
3. Results
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Quality Assessment of the Included Studies
3.4. Publication Bias
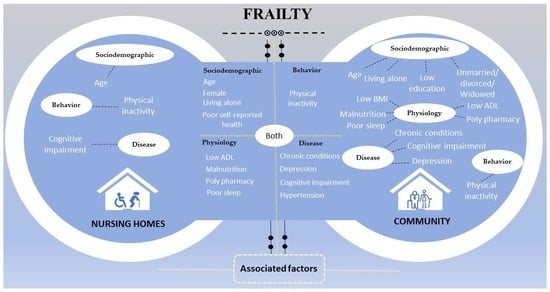
3.5. Associated Factors of Frailty in Older Adults in Nursing Home and Community Settings
4. Discussion
4.1. Socio-Demographic Factors Associated with Frailty in Nursing Homes and Community Settings
4.2. Physiological Factors Associated with Frailty in Nursing Homes and Community Settings
4.3. Behavioral Factors Associated with Frailty in Nursing Homes and Community Settings
4.4. Disease-Related Factors Associated with Frailty in Nursing Homes and Community Settings
4.5. Strengths and Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 26 March 2022).
- Jiao, J.; Wang, Y.; Zhu, C.; Li, F.; Zhu, M.; Wen, X.; Jin, J.; Wang, H.; Lv, D.; Zhao, S.; et al. Prevalence and associated factors for frailty among elder patients in China: A multicentre cross-sectional study. BMC Geriatr. 2020, 20, 100. [Google Scholar] [CrossRef]
- Lee, H.; Lee, E.; Jang, I.Y. Frailty and Comprehensive Geriatric Assessment. J. Korean Med. Sci. 2020, 35, e16. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Maniaci, A.; Ferlito, S.; Lechien, J.R.; Di Luca, M.; Iannella, G.; Cammaroto, G.; Cannavicci, A.; Pollicina, I.; Stilo, G.; Di Mauro, P.; et al. Anxiety, depression and sleepiness in OSA patients treated with barbed reposition pharyngoplasty: A prospective study. Eur. Arch. Otorhinolaryngol. 2022, 8, 4189–4198. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.; Sacha, M.; Soboń, J.; Borysiuk, Z.; Feusette, P. Is It Time to Begin a Public Campaign Concerning Frailty and Pre-frailty? A Review Article. Front. Physiol. 2017, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.; Bergman, H.; Morley, J.E.; Kritchevsky, S.B.; Vellas, B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J. Nutr. Health Aging 2008, 12, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Ovalle, M.; Delgado-Becerra, I.; Molina, X.; Masferrer, D. Instruments to assess functional capacity and the presence of frailty in older people. Rev. Med. Chil. 2022, 150, 930–943. [Google Scholar] [CrossRef]
- Uchmanowicz, I.; Pasieczna, A.H.; Wójta-Kempa, M.; Gobbens, R.J.J.; Młynarska, A.; Faulkner, K.M.; Czapla, M.; Szczepanowski, R. Physical, Psychological and Social Frailty Are Predictive of Heart Failure: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 565. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabbà, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef]
- Gu, D.; Feng, Q. Frailty still matters to health and survival in centenarians: The case of China. BMC Geriatr. 2015, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Etman, A.; Kamphuis, C.B.; van der Cammen, T.J.; Burdorf, A.; van Lenthe, F.J. Do lifestyle, health and social participation mediate educational inequalities in frailty worsening. Eur. J. Public Health 2015, 25, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, M.E.; Zapico, S.C. Frailty, Cognitive Decline, Neurodegenerative Diseases and Nutrition Interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Jayanama, K.; Theou, O.; Godin, J.; Mayo, A.; Cahill, L.; Rockwood, K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022, 20, 404. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.N.; Wilczek, M.P.; Smith, L.H.; Bosse, J.D.; Richard, E.L.; Cavanaugh, R.; Manjourides, J.; Orkaby, A.R.; Olivieri-Mui, B. Frailty Among Sexual and Gender Minority Older Adults: The All of Us Database. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2111–2118. [Google Scholar] [CrossRef]
- Picca, A.; Coelho-Junior, H.J.; Calvani, R.; Marzetti, E.; Vetrano, D.L. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101530. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Joanna Briggs Institute Critical Appraisal Tools. Available online: https://jbi.global/critical-appraisal-tools (accessed on 10 March 2023).
- The Newcastle-Ottawa Scale (NOS). Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 November 2021).
- Liu, W.; Puts, M.; Jiang, F.; Zhou, C.; Tang, S.; Chen, S. Physical frailty and its associated factors among elderly nursing home residents in China. BMC Geriatr. 2020, 20, 294. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, J.; Li, M.; Wang, K. Relationship Between Loneliness and Frailty Among Older Adults in Nursing Homes: The Mediating Role of Activity Engagement. J. Am. Med. Dir. Assoc. 2019, 20, 759–764. [Google Scholar] [CrossRef]
- Murukesu, R.R.; Singh, D.; Subramaniam, P.; Tan, X.V.; Mohamd Izhar, I.A.; Ponvel, P.; Mohd Rasdi, H.F. Prevalence of Frailty and its Association with Cognitive Status and Functional Fitness among Ambulating Older Adults Residing in Institutions within West Coast of Peninsular Malaysia. Int. J. Environ. Res. Public Health 2019, 16, 4716. [Google Scholar] [CrossRef]
- Medeiros, M.M.D.; Figueredo, O.M.C.; Pinheiro, M.A.; Oliveira, L.F.S.; Wanderley, R.L.; Cavalcanti, Y.W.; Rodrigues Garcia, R.C.M. Factors associated with the overlap of frailty and nutrition in institutionalized older adults: A multicenter study. Arch. Gerontol. Geriatr. 2020, 90, 104150. [Google Scholar] [CrossRef]
- Marisa de la Rica-Escuín, J.G.; Rosana Varela-Pérez, M.D.A.; Marta Silva-Iglesias, J.L.O.; Abizanda, P. Frailty and mortality or incident disability in institutionalized older adults: The FINAL Study. Maturitas 2014, 78, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Paes de Andrade, F.L.J.; Jerez-Roig, J.; Belém, L.N.M.; de Lima, K.C. Frailty among institutionalized older people: A cross-sectional study in Natal (Brazil). J. Frailty Sarcopenia Falls 2019, 4, 51–56. [Google Scholar] [CrossRef]
- Okamura, T.; Sugiyama, M.; Inagaki, H.; Miyamae, F.; Ura, C.; Sakuma, N.; Edahiro, A.; Taga, T.; Tsuda, S.; Awata, S. Depressed mood and frailty among older people in Tokyo during the COVID-19 pandemic. Psychogeriatrics 2021, 21, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Rizka, A.; Indrarespati, A.; Dwimartutie, N.; Muhadi, M. Frailty among Older Adults Living in Nursing Homes in Indonesia: Prevalence and Associated Factors. Ann. Geriatr. Med. Res. 2021, 25, 93–97. [Google Scholar] [CrossRef]
- Alqahtani, B.A.; Alenazi, A.M.; Alshehri, M.M.; Osailan, A.M.; Alsubaie, S.F.; Alqahtani, M.A. Prevalence of frailty and associated factors among Saudi community-dwelling older adults: A cross-sectional study. BMC Geriatr. 2021, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shi, Z.; Wang, D.; Niu, Y.; Xu, C.; Ma, Y.; Liu, H.; Guo, H.; Li, M.; Zhang, Y. Prevalence and associated factors of frailty among community dwelling older adults in Northwest China: A cross-sectional study. BMJ Open 2022, 12, e060089. [Google Scholar] [CrossRef]
- Jang, A.R.; Won, C.W.; Sagong, H.; Bae, E.; Park, H.; Yoon, J.Y. Social factors predicting improvement of frailty in community-dwelling older adults: Korean Frailty and Aging Cohort Study. Geriatr. Gerontol. Int. 2021, 21, 465–471. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Hairi, N.N.; Said, M.A.; Kamaruzzaman, S.B.; Choo, W.Y.; Hairi, F.; Othman, S.; Ismail, N.; Peramalah, D.; Kandiben, S.; et al. Prevalence, transitions and factors predicting transition between frailty states among rural community-dwelling older adults in Malaysia. PLoS ONE 2018, 13, e0206445. [Google Scholar] [CrossRef]
- Batko-Szwaczka, A.; Dudzińska-Griszek, J.; Hornik, B.; Janusz-Jenczeń, M.; Włodarczyk, I.; Wnuk, B.; Szołtysek, J.; Durmała, J.; Wilczyński, K.; Cogiel, A.; et al. Frailty Phenotype: Evidence of Both Physical and Mental Health Components in Community-Dwelling Early-Old Adults. Clin. Interv. Aging 2020, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Makizako, H.; Tsutsumimoto, K.; Nakakubo, S.; Kim, M.J.; Kurita, S.; Hotta, R.; Shimada, H. Transitional status and modifiable risk of frailty in Japanese older adults: A prospective cohort study. Geriatr. Gerontol. Int. 2018, 18, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Cacciatore, F.; Komici, K.; Rengo, G.; Vitale, D.F.; Furgi, G.; Pagano, G.; Bencivenga, L.; Davinelli, S.; Ferrara, N. Inter-relationships between Gender, Frailty and 10-Year Survival in Older Italian Adults: An observational longitudinal study. Sci. Rep. 2019, 9, 18416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X.; Gao, X.; Wei, Y.; Ye, L.; Liu, S.; Ye, J.; Qiu, Y.; Zheng, X.; Chen, C.; et al. Leisure Activities, Genetic Risk, and Frailty: Evidence from the Chinese Adults Aged 80 Years or Older. Gerontology 2023, 69, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Sharifi, F.; Fadayevatan, R.; Kamrani, A.; Moodi, M.; Khorashadizadeh, M.; Kazemi, T.; Khodabakhshi, H.; Fakhrzadeh, H.; Arzaghi, M.; et al. Low physical activity is the strongest factor associated with frailty phenotype and frailty index: Data from baseline phase of Birjand Longitudinal Aging Study (BLAS). BMC Geriatr. 2022, 22, 498. [Google Scholar] [CrossRef]
- Ge, L.; Yap, C.W.; Heng, B.H. Associations of social isolation, social participation, and loneliness with frailty in older adults in Singapore: A panel data analysis. BMC Geriatr. 2022, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fan, W.; Zhu, B.; Ma, C.; Tan, X.; Gu, Y. Frailty Risk Prediction Model among Older Adults: A Chinese Nation-Wide Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 8410. [Google Scholar] [CrossRef]
- Usher, T.; Buta, B.; Thorpe, R.J.; Huang, J.; Samuel, L.J.; Kasper, J.D.; Bandeen-Roche, K. Dissecting the Racial/Ethnic Disparity in Frailty in a Nationally Representative Cohort Study with Respect to Health, Income, and Measurement. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 69–76. [Google Scholar] [CrossRef]
- Alves, M.; Oliveira, N.; Pegorari, M.S.; Tavares, D.; Rodrigues, M.; Bolina, A.F. Evidence of association between the use of drugs and community-dwelling older people frailty: A cross-sectional study. Sao Paulo Med. J. 2020, 138, 465–474. [Google Scholar] [CrossRef]
- Peters, L.L.; Boter, H.; Burgerhof, J.G.; Slaets, J.P.; Buskens, E. Construct validity of the Groningen Frailty Indicator established in a large sample of home-dwelling elderly persons: Evidence of stability across age and gender. Exp. Gerontol. 2015, 69, 129–141. [Google Scholar] [CrossRef]
- Tang, J.Y.; Luo, H.; Tse, M.; Lum, T.Y.; Wong, G.H.; Li, S.X. The relationship between insomnia symptoms and frailty in community-dwelling older persons: A path analysis. Sleep. Med. 2021, 84, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Poli, S.; Cella, A.; Puntoni, M.; Musacchio, C.; Pomata, M.; Torriglia, D.; Vello, N.; Molinari, B.; Pandolfini, V.; Torrigiani, C.; et al. Frailty is associated with socioeconomic and lifestyle factors in community-dwelling older subjects. Aging Clin. Exp. Res. 2016, 29, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H.; Mera, R.M.; Peinado, C.D.; Zambrano, M.; Sedler, M.J. Frailty and Risk of Falls in Community-Dwelling Older Adults Living in a Rural Setting. The Atahualpa Project. J. Frailty Aging 2020, 9, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, Y.; Wang, Q.; Zhao, H.; Kong, L.; Li, J. Association of insomnia and multidimensional frailty in community-dwelling older adults: A cross-sectional survey. J. Clin. Nurs. 2021, 31, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Henchoz, Y.; Büla, C.; Guessous, I.; Santos-Eggimann, B. Association between Physical Frailty and Quality of Life in a Representative Sample of Community-Dwelling Swiss Older People. J. Nutr. Health Aging 2016, 21, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsuboya, T.; Aida, J.; Matsuyama, Y.; Koyama, S.; Sugiyama, K.; Kondo, K.; Osaka, K. Income and education are associated with transitions in health status among community-dwelling older people in Japan: The JAGES cohort study. Fam. Pract. 2019, 36, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Kim, J.H. Factors Associated with Frailty According to Gender of Older Adults Living Alone. Healthcare 2021, 9, 475. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.; Lee, Y.; Won, C.W. Prevalence of Physical Frailty and Its Multidimensional Risk Factors in Korean Community-Dwelling Older Adults: Findings from Korean Frailty and Aging Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 7883. [Google Scholar] [CrossRef]
- Kume, Y.; Takahashi, T.; Itakura, Y.; Lee, S.; Makizako, H.; Ono, T.; Shimada, H.; Ota, H. Polypharmacy and Lack of Joy Are Related to Physical Frailty among Northern Japanese Community-Dwellers from the ORANGE Cohort Study. Gerontology 2021, 67, 184–193. [Google Scholar] [CrossRef]
- Liu, M.; Hou, T.; Nkimbeng, M.; Li, Y.; Taylor, J.L.; Sun, X.; Tang, S.; Szanton, S.L. Associations between symptoms of pain, insomnia and depression, and frailty in older adults: A cross-sectional analysis of a cohort study. Int. J. Nurs. Stud. 2021, 117, 103873. [Google Scholar] [CrossRef]
- Oyon, J.; Serra-Prat, M.; Ferrer, M.; Llinares, A.; Pastor, N.; Limón, E.; Rejón, T.; Ramírez, S.; Salietti, A. Psychosocial factors associated with frailty in the community-dwelling aged population with depression. A cross-sectional study. Aten. Primaria 2021, 53, 102048. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, G.; Ates Bulut, E.; Gurpinar, B.; Ilcin, N.; Isik, A.T. Determination of an Optimal Frailty Cutoff Score of Tilburg Frailty Indicator and Frailty Associated Factors in Community-Dwelling Turkish Older Adults. Ann. Geriatr. Med. Res. 2021, 25, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Setiati, S.; Soejono, C.H.; Harimurti, K.; Dwimartutie, N.; Aryana, I.; Sunarti, S.; Budiningsih, F.; Mulyana, R.; Dwipa, L.; Sudarso, A.; et al. Frailty and Its Associated Risk Factors: First Phase Analysis of Multicentre Indonesia Longitudinal Aging Study. Front. Med. 2021, 8, 658580. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhang, M.; Sun, X. Sex-Specific Association Between Socioeconomic Status, Lifestyle, and the Risk of Frailty Among the Elderly in China. Front. Med. 2021, 8, 775518. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, Y.; Xu, S.; Bao, L.; Parker, D.; Xu, X.; Li, J. Evaluation of frailty status among older people living in urban communities by Edmonton Frail Scale in Wuhu, China: A cross-sectional study. Contemp. Nurse 2018, 54, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Tamayo, K.; Manrique-Espinoza, B.; Rosas-Carrasco, O.; Pérez-Moreno, A.; Salinas-Rodríguez, A. Sleep complaints are associated with frailty in Mexican older adults in a rural setting. Geriatr. Gerontol. Int. 2017, 17, 2573–2578. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Kojima, G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 940–945. [Google Scholar] [CrossRef]
- Liau, S.J.; Lalic, S.; Visvanathan, R.; Dowd, L.A.; Bell, J.S. The FRAIL-NH Scale: Systematic Review of the Use, Validity and Adaptations for Frailty Screening in Nursing Homes. J. Nutr. Health Aging 2021, 25, 1205–1216. [Google Scholar] [CrossRef]
- Chong, E.; Huang, Y.; Chan, M.; Tan, H.N.; Lim, W.S. Concurrent and Predictive Validity of FRAIL-NH in Hospitalized Older Persons: An Exploratory Study. J. Am. Med. Dir. Assoc. 2021, 22, 1664–1669.e4. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.K.; Lee, W.J.; Chen, L.Y.; Hwang, A.C.; Lin, M.H.; Peng, L.N.; Chen, L.K. Association between Frailty, Osteoporosis, Falls and Hip Fractures among Community-Dwelling People Aged 50 Years and Older in Taiwan: Results from I-Lan Longitudinal Aging Study. PLoS ONE 2015, 10, e0136968. [Google Scholar] [CrossRef]
- Jung, H.W.; Kim, S.W.; Ahn, S.; Lim, J.Y.; Han, J.W.; Kim, T.H.; Kim, K.W.; Kim, K.I.; Kim, C.H. Prevalence and outcomes of frailty in Korean elderly population: Comparisons of a multidimensional frailty index with two phenotype models. PLoS ONE 2014, 9, e87958. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.H.; Hubbard, R.E. Frailty: Understanding the difference between age and ageing. Age Ageing 2022, 51, afac185. [Google Scholar] [CrossRef]
- Shi, S.M.; Olivieri-Mui, B.; McCarthy, E.P.; Kim, D.H. Changes in a Frailty Index and Association with Mortality. J. Am. Geriatr. Soc. 2021, 69, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Bellelli, F.; Consorti, E.; Hettiarachchige, T.; Rossi, P.; Lucchi, T.; Froldi, M.; Cesari, M. Relationship among Age, Education and Frailty in Older Persons. J. Frailty Aging 2023, 12, 326–328. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Liang, M. Analysis of frailty status and influencing factors among 4378 elderly people. Chin. Elder. Care Med. 2021, 19, 4. [Google Scholar]
- Herr, M.; Robine, J.M.; Pinot, J.; Arvieu, J.J.; Ankri, J. Polypharmacy and frailty: Prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol. Drug Saf. 2015, 24, 637–646. [Google Scholar] [CrossRef]
- Ye, L.; Elstgeest, L.; Zhang, X.; Alhambra-Borrás, T.; Tan, S.S.; Raat, H. Factors associated with physical, psychological and social frailty among community-dwelling older persons in Europe: A cross-sectional study of Urban Health Centres Europe (UHCE). BMC Geriatr. 2021, 21, 422. [Google Scholar] [CrossRef]
- Sánchez-García, S.; Sánchez-Arenas, R.; García-Peña, C.; Rosas-Carrasco, O.; Avila-Funes, J.A.; Ruiz-Arregui, L.; Juárez-Cedillo, T. Frailty among community-dwelling elderly Mexican people: Prevalence and association with sociodemographic characteristics, health state and the use of health services. Geriatr. Gerontol. Int. 2014, 14, 395–402. [Google Scholar] [CrossRef]
- Pegorari, M.S.; Tavares, D.M. Factors associated with the frailty syndrome in elderly individuals living in the urban area. Rev. Lat. Am. Enferm. 2014, 22, 874–882. [Google Scholar] [CrossRef]
- Arakawa Martins, B.; Visvanathan, R.; Barrie, H.; Huang, C.H.; Matsushita, E.; Okada, K.; Satake, S.; Uno, C.; Kuzuya, M. Frailty prevalence using Frailty Index, associated factors and level of agreement among frailty tools in a cohort of Japanese older adults. Arch. Gerontol. Geriatr. 2019, 84, 103908. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Coppinger, T.; McCarthy, A.L. The role of nutrition and physical activity in frailty: A review. Clin. Nutr. ESPEN 2020, 35, 1–11. [Google Scholar] [CrossRef]
- Gordon, E.H.; Hubbard, R.E. Differences in frailty in older men and women. Med. J. Aust. 2020, 212, 183–188. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.C.; Máximo, R.O.; Ramírez, P.C.; de Souza, A.F.; Luiz, M.M.; Delinocente, M.; Steptoe, A.; de Oliveira, C.; Alexandre, T. Does the incidence of frailty differ between men and women over time. Arch. Gerontol. Geriatr. 2023, 106, 104880. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Fried, L.P. Frailty and the older man. Med. Clin. N. Am. 1999, 83, 1173. [Google Scholar] [CrossRef] [PubMed]
- Carcaillon, L.; Blanco, C.; Alonso-Bouzón, C.; Alfaro-Acha, A.; Garcia-García, F.J.; Rodriguez-Mañas, L. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: The Toledo Study for Healthy Aging. PLoS ONE 2012, 7, e32401. [Google Scholar] [CrossRef] [PubMed]
- García-Peña, C.; Ávila-Funes, J.A.; Dent, E.; Gutiérrez-Robledo, L.; Pérez-Zepeda, M. Frailty prevalence and associated factors in the Mexican health and aging study: A comparison of the frailty index and the phenotype. Exp. Gerontol. 2016, 79, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Campitelli, M.A.; Bronskill, S.E.; Hogan, D.B.; Diong, C.; Amuah, J.E.; Gill, S.; Seitz, D.; Thavorn, K.; Wodchis, W.P.; Maxwell, C.J. The prevalence and health consequences of frailty in a population-based older home care cohort: A comparison of different measures. BMC Geriatr. 2016, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Dorosty, A.; Arero, G.; Chamar, M.; Tavakoli, S. Prevalence of Sarcopenia and Its Association with Socioeconomic Status among the Elderly in Tehran. Ethiop. J. Health Sci. 2016, 26, 389–396. [Google Scholar] [CrossRef] [PubMed]
- González-Pichardo, A.M.; Navarrete-Reyes, A.P.; Adame-Encarnación, H.; Aguilar-Navarro, S.; García-Lara, J.M.; Amieva, H.; Avila-Funes, J.A. Association between Self-Reported Health Status and Frailty in Community-Dwelling Elderly. J. Frailty Aging 2014, 3, 104–108. [Google Scholar] [CrossRef]
- Beasley, J.M.; Shikany, J.M.; Thomson, C.A. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr. Clin. Pract. 2013, 28, 684–690. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Chen, C.; Winterstein, A.G.; Fillingim, R.B.; Wei, Y.J. Body weight, frailty, and chronic pain in older adults: A cross-sectional study. BMC Geriatr. 2019, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Yoshida, T.; Watanabe, Y.; Yamada, Y.; Kimura, M.; Group, K.S. A U-Shaped Relationship Between the Prevalence of Frailty and Body Mass Index in Community-Dwelling Japanese Older Adults: The Kyoto-Kameoka Study. J. Clin. Med. 2020, 9, 1367. [Google Scholar] [CrossRef]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, T.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic obesity: Epidemiology, pathophysiology, cardiovascular disease, mortality, and management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Richter, D.; Guasti, L.; Walker, D.; Lambrinou, E.; Lionis, C.; Abreu, A.; Savelieva, I.; Fumagalli, S.; Bo, M.; Rocca, B.; et al. Frailty in cardiology: Definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Association for Acute Cardio Vascular Care (ACVC), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio-Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e-Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur. J. Prev. Cardiol. 2022, 29, 216–227. [Google Scholar]
- Uchmanowicz, I. Oxidative Stress, Frailty and Cardiovascular Diseases: Current Evidence. Adv. Exp. Med. Biol. 2020, 1216, 65–77. [Google Scholar] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Valencia, M.; Izquierdo, M.; Cesari, M.; Casas-Herrero, Á.; Inzitari, M.; Martínez-Velilla, N. The relationship between frailty and polypharmacy in older people: A systematic review. Br. J. Clin. Pharmacol. 2018, 84, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Veronese, N.; Maggi, S.; Baggio, G.; Toffanello, E.D.; Zambon, S.; Sartori, L.; Musacchio, E.; Perissinotto, E.; Crepaldi, G.; et al. Factors Influencing Transitions Between Frailty States in Elderly Adults: The Progetto Veneto Anziani Longitudinal Study. J. Am. Geriatr. Soc. 2017, 65, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Cumming, R.G.; Handelsman, D.J.; McLachlan, A.J.; Abernethy, D.R.; Banks, E.; Le Couteur, D.G. High-risk prescribing and incidence of frailty among older community-dwelling men. Clin. Pharmacol. Ther. 2012, 91, 521–528. [Google Scholar] [CrossRef]
- Moulis, F.; Moulis, G.; Balardy, L.; Gérard, S.; Sourdet, S.; Rougé-Bugat, M.E.; Lapeyre-Mestre, M.; Montastruc, J.L.; Rolland, Y.; Vellas, B. Searching for a polypharmacy threshold associated with frailty. J. Am. Med. Dir. Assoc. 2015, 16, 259–261. [Google Scholar] [CrossRef]
- Derhem, B.; Özsari, S. Frailty and Polypharmacy in Primary Care. Biol. Res. Nurs. 2023, 25, 658–663. [Google Scholar] [CrossRef]
- Shih, A.C.; Chen, L.H.; Tsai, C.C.; Chen, J.Y. Correlation between Sleep Quality and Frailty Status among Middle-Aged and Older Taiwanese People: A Community-Based, Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 9457. [Google Scholar] [CrossRef]
- Fu, P.; Zhou, C.; Meng, Q. Associations of Sleep Quality and Frailty among the Older Adults with Chronic Disease in China: The Mediation Effect of Psychological Distress. Int. J. Environ. Res. Public Health 2020, 17, 5240. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Rawtaer, I.; Fam, J.; Jiang, M.J.; Feng, L.; Kua, E.H.; Mahendran, R. Sleep correlates of depression and anxiety in an elderly Asian population. Psychogeriatrics 2016, 16, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Yuhang, L. Frailty index and its influencing factors among the elderly in China. Sci. Res. Aging 2022, 10, 42–55. [Google Scholar]
- Wu Nuoyi, W.Y.; He Haiyan, L.S.; Cai Mingyu, Z.J.; Wang, L. Analysis of influencing factors and construction of risk prediction model for frailty in elderly patients with chronic diseases. Chongqing Med. 2023, 52, 3730–3736. [Google Scholar]
- Dent, E.; Daly, R.M.; Hoogendijk, E.O.; Scott, D. Exercise to Prevent and Manage Frailty and Fragility Fractures. Curr. Osteoporos. Rep. 2023, 21, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Álvarez-Bustos, A.; Rodríguez-Mañas, L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020, 35, 101513. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019, 132, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sadjapong, U.; Yodkeeree, S.; Sungkarat, S.; Siviroj, P. Multicomponent Exercise Program Reduces Frailty and Inflammatory Biomarkers and Improves Physical Performance in Community-Dwelling Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3760. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Vahedi, A.; Eriksdotter, M.; Ihle-Hansen, H.; Wyller, T.B.; Øksengård, A.R.; Fure, B. Cognitive impairment in people with physical frailty using the phenotype model: A systematic review and meta analysis. Int. J. Geriatr. Psychiatry 2022, 37. [Google Scholar] [CrossRef]
- Brigola, A.G.; Rossetti, E.S.; Dos Santos, B.R.; Neri, A.L.; Zazzetta, M.S.; Inouye, K.; Pavarini, S. Relationship between cognition and frailty in elderly: A systematic review. Dement. Neuropsychol. 2015, 9, 110–119. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Blanco-Fandiño, J.; Cibeira, N.; Buján, A.; López-López, R.; Maseda, A.; Millán-Calenti, J.C. Clinical and Neuropsychological Correlates of Prefrailty Syndrome. Front. Med. 2020, 7, 609359. [Google Scholar] [CrossRef]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Pei Li, T.X.; Haoying, D. Research progress on frailty and chronic diseases in the elderly. Chin. J. Gerontol. 2020, 40, 4471–4473. [Google Scholar]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.L.; Walston, J.D.; Kasper, J.D. Frailty in Older Adults: A Nationally Representative Profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef]
- Cho, I.Y.; Kang, J.; Ko, H.; Sung, E.; Chung, P.W.; Kim, C. Association Between Frailty-Related Factors and Depression among Older Adults. Clin. Gerontol. 2022, 45, 366–375. [Google Scholar] [CrossRef]
- Deng, M.G.; Liu, F.; Liang, Y.; Wang, K.; Nie, J.Q.; Liu, J. Association between frailty and depression: A bidirectional Mendelian randomization study. Sci. Adv. 2023, 9, eadi3902. [Google Scholar] [CrossRef]
- Maștaleru, A.; Abdulan, I.M.; Ștefăniu, R.; Lefter, N.; Sandu, I.A.; Pîslaru, A.I.; Leon-Constantin, M.M.; Alexa, I.D.; Ilie, A.C. Relationship between Frailty and Depression in a Population from North-Eastern Romania. Int. J. Environ. Res. Public Health 2022, 19, 5731. [Google Scholar] [CrossRef]
- Nerobkova, N.; Park, Y.S.; Park, E.C.; Shin, J. Frailty transition and depression among community-dwelling older adults: The Korean Longitudinal Study of Aging (2006–2020). BMC Geriatr. 2023, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Na, W.R.; Sohn, C.M. The effects of socioeconomic, psychological, and health behavior factors of the elderly on Frailty: Mediating subjective health status and multimorbidity. Korean J. Hum. Ecol. 2021, 30, 429–440. [Google Scholar] [CrossRef]
- Jang, K.O. Effects of the elderly frailty prevention program on the subjective health status, depression, physical fitness, and quality of life of the elderly per center. Korean Soc. Ind. Technol. 2017, 18, 47–58. [Google Scholar]
- Ribeiro, O.; Duarte, N.; Teixeira, L.; Paul, C. Frailty and depression in centenarians. Int. Psychogeriatr. 2018, 30, 115–124. [Google Scholar] [CrossRef]
- Maniaci, A.; Riela, P.M.; Iannella, G.; Lechien, J.R.; La Mantia, I.; De Vincentiis, M.; Cammaroto, G.; Calvo-Henriquez, C.; Di Luca, M.; Chiesa Estomba, C.; et al. Machine Learning Identification of Obstructive Sleep Apnea Severity through the Patient Clinical Features: A Retrospective Study. Life 2023, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Pansini, A.; Calabrò, F.; De Gennaro, S.; Esposito, M.; Rinaldi, P.; Colin, A.; Minicucci, F.; Coppola, A.; Frullone, S.; et al. Global cognitive function correlates with P-wave dispersion in frail hypertensive older adults. J. Clin. Hypertens. 2022, 24, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 8, 1487–1492. [Google Scholar] [CrossRef] [PubMed]

| Associated Factors | Setting | Number of Studies | Heterogeneity | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| I2 | p | |||||
| Sociodemographic factors | Age | NH | 6 | 97 | 0.002 | 1.83 (1.26–2.66) |
| Behavior factors | Physical inactivity | NH | 4 | 6 | <0.001 | 2.27 (1.85–2.80) |
| Disease factors | Cognitive impairment | NH | 3 | 87 | 0.26 | 1.11 (0.92–1.34) |
| Sociodemographic factors | Age | Community | 14 | 97 | 0.17 | 1.09 (0.97–1.22) |
| Living alone | Community | 5 | 75 | 0.01 | 1.68 (1.13–2.51) | |
| Low education | Community | 9 | 83 | 0.23 | 0.89 (0.74–1.08) | |
| Unmarried/divorced/ Widowed | Community | 4 | 47 | <0.001 | 2.26 (1.65–3.09) | |
| Physiological factors | Low BMI | Community | 3 | 95 | 0.14 | 2.14 (0.77–5.93) |
| Polypharmacy | Community | 9 | 77 | <0.001 | 1.48 (1.27–1.72) | |
| Malnutrition | Community | 4 | 99 | 0.87 | 1.16 (0.20–6.65) | |
| Low ADL | Community | 8 | 96 | <0.001 | 1.71 (1.25–2.34) | |
| Poor sleep | Community | 6 | 88 | <0.001 | 2.40 (1.51–3.83) | |
| Behavioral factors | Physical inactivity | Community | 10 | 95 | 0.01 | 1.58 (1.11–2.25) |
| Disease factors | Chronic conditions | Community | 9 | 93 | <0.001 | 1.94 (1.40–2.70) |
| Cognitive impairment | Community | 4 | 98 | 0.11 | 1.81 (0.87–3.75) | |
| Depression | Community | 9 | 94 | <0.001 | 2.02 (1.45–2.82) | |
| Sociodemographic factors | Age | NH and Community | 20 | 100 | <0.001 | 1.43 (1.16–1.75) |
| Female | NH and Community | 4 | 91 | 0.98 | 0.98 (0.22–4.40) | |
| Living alone | NH and Community | 6 | 84 | 0.009 | 2.11 (1.21–3.70) | |
| Poor self-reported health | NH and Community | 3 | 50 | <0.001 | 2.04 (1.50–2.76) | |
| Physiological factors | Low ADL | NH and Community | 10 | 95 | 0.003 | 1.65 (1.19–2.30) |
| Malnutrition | NH and Community | 5 | 99 | 0.66 | 1.40 (0.31–6.37) | |
| Polypharmacy | NH and Community | 10 | 98 | <0.001 | 1.63 (1.25–2.13) | |
| Poor sleep | NH and Community | 7 | 85 | <0.001 | 2.37 (1.58–3.56) | |
| Behavioral factors | Physical inactivity | NH and Community | 14 | 94 | 0.003 | 1.57 (1.17–2.10) |
| Disease factors | Chronic conditions | NH and Community | 10 | 93 | <0.001 | 2.14 (1.54–2.99) |
| Cognitive impairment | NH and Community | 7 | 96 | 0.003 | 1.33 (1.10–1.61) | |
| Depression | NH and Community | 11 | 92 | <0.001 | 1.99 (1.48–2.67) | |
| Hypertension | NH and Community | 3 | 94 | 0.67 | 0.65 (0.09–4.51) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhu, Y.; Tan, J.K.; Ismail, A.H.; Ibrahim, R.; Hassan, N.H. Factors Associated with Frailty in Older Adults in Community and Nursing Home Settings: A Systematic Review with a Meta-Analysis. J. Clin. Med. 2024, 13, 2382. https://doi.org/10.3390/jcm13082382
Liu J, Zhu Y, Tan JK, Ismail AH, Ibrahim R, Hassan NH. Factors Associated with Frailty in Older Adults in Community and Nursing Home Settings: A Systematic Review with a Meta-Analysis. Journal of Clinical Medicine. 2024; 13(8):2382. https://doi.org/10.3390/jcm13082382
Chicago/Turabian StyleLiu, Jia, Yuezhi Zhu, Jen Kit Tan, Azera Hasra Ismail, Roszita Ibrahim, and Nor Haty Hassan. 2024. "Factors Associated with Frailty in Older Adults in Community and Nursing Home Settings: A Systematic Review with a Meta-Analysis" Journal of Clinical Medicine 13, no. 8: 2382. https://doi.org/10.3390/jcm13082382