The Impact of Shared Assistance between Dermatology and Internal Medicine on Patients with Psoriasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Interdiscinplinary Dermatology and Internal Medicine Clinic for Patients with Psoriasis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Evolution of Metabolic- and Disease-Related Variables throughout the Study Period
3.2.1. Psoriasis Severity and Quality of Life
3.2.2. Body Mass Index
3.2.3. Blood Test Variables
3.2.4. Patient Knowledge of Psoriasis
3.2.5. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raychaudhuri, S.P.; Jiang, W.-Y.; Raychaudhuri, S.K. Revisiting the Koebner phenomenon: Role of NGF and its receptor system in the pathogenesis of psoriasis. Am. J. Pathol. 2008, 172, 961–971. [Google Scholar] [CrossRef]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2016, 38, 11–27. [Google Scholar] [CrossRef]
- Elmets, C.A.; Leonardi, C.L.; Davis, D.M.R.; Gelfand, J.M.; Lichten, J.; Mehta, N.N.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Elewski, B.E.; et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 2019, 80, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Psoriasis Day—Document EB133.R2, Agenda Item 6.2. 30 May 2013. Available online: http://apps.who.int/gb/ebwha/pdf_files/EB133/B133_R2-en.pdf (accessed on 13 March 2015).
- Mease, P.J.; Gladman, D.D.; Papp, K.A.; Khraishi, M.M.; Thaçi, D.; Behrens, F.; Northington, R.; Fuiman, J.; Bananis, E.; Boggs, R.; et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J. Am. Acad. Dermatol. 2013, 69, 729–735. [Google Scholar] [CrossRef]
- López-Ferrer, A.; Láiz-Alonso, A. Psoriasis-Arthritis Units: Three Years on. Actas Dermosifiliogr. 2018, 109, 101–103. [Google Scholar] [CrossRef]
- Luelmo, J.; Gratacós, J.; Moreno Martínez-Losa, M.; Ribera, M.; Romaní, J.; Calvet, J.; Leal, L.; Larrosa, M. Multidisciplinary psoriasis and psoriatic arthritis unit: Report of 4 years’ experience. Actas Dermosifiliogr. 2014, 105, 371–377. [Google Scholar] [CrossRef]
- Ritchlin, C.; Tausk, F. Centers for psoriasis: A comprehensive approach to patient care, education and research. Curr. Opin. Rheumatol. 2008, 20, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Dhana, A.; Yen, H.; Yen, H.; Cho, E. All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Ruiz de Morales, J.G.; Dauden, E.; Andreu, J.L.; Cervera, R.; Adán, A.; Marsal, S.; Escobar, C.; Hinojosa, J.; Palau, J.; et al. Prevalence of ten Immune-mediated inflammatory diseases (IMID) in Spain. Rev. Esp. Salud Publica 2019, 93, e201903013. [Google Scholar]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef]
- Strauss, H. Zur Lehre von der neurogenen und der thyreogenen Glykosurie (Schluss aus No. 18.). DMW-Dtsch. Med. Wochenschr. 1897, 23, 309–312. [Google Scholar] [CrossRef]
- Korman, N.J. Management of psoriasis as a systemic disease: What is the evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Karpińska-Mirecka, A.; Bartosińska, J.; Krasowska, D. The Impact of Hypertension, Diabetes, Lipid Disorders, Overweight/Obesity and Nicotine Dependence on Health-Related Quality of Life and Psoriasis Severity in Psoriatic Patients Receiving Systemic Conventional and Biological Treatment. Int. J. Environ. Res. Public. Health 2021, 18, 13167. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Boehncke, S.; Tobin, A.-M.; Kirby, B. The “psoriatic march”: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lwin, S.M.; Snowden, J.A.; Griffiths, C.E.M. The promise and challenges of cell therapy for psoriasis. Br. J. Dermatol. 2021, 185, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Addis, A.; Chimenti, S.; Giannetti, A.; Picardo, M.; Tomino, C.; Maccarone, M.; Chatenoud, L.; Bertuccio, P.; Caggese, E.; et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology 2008, 217, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, R.; Kong, Y.; Zhao, M.; Su, Y. Impact of smoking on psoriasis risk and treatment efficacy: A meta-analysis. J. Int. Med. Res. 2020, 48, 10. [Google Scholar] [CrossRef] [PubMed]
- Conroy, R.M.; Pyörälä, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetière, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; Pintó, X.; Armario, P.; Masana, L.; Ascaso, J.F.; Valdivielso, P. En nombre de la Sociedad Española de Arteriosclerosis; Miembros de la Sociedad Española de Arteriosclerosis Standards for global cardiovascular risk management arteriosclerosis. Clin. Investig. Arterioscler. Publicacion Soc. Espanola Arterioscler. 2019, 31 (Suppl. S1), 1–43. [Google Scholar] [CrossRef]
- Velez, N.F.; Wei-Passanese, E.X.; Husni, M.E.; Mody, E.A.; Qureshi, A.A. Management of psoriasis and psoriatic arthritis in a combined dermatology and rheumatology clinic. Arch. Dermatol. Res. 2012, 304, 7–13. [Google Scholar] [CrossRef]
- Zheng, Y.-X.; Zheng, M. A multidisciplinary team for the diagnosis and management of psoriatic arthritis. Chin. Med. J. 2021, 134, 1387–1389. [Google Scholar] [CrossRef]
- Jadon, D.R.; Helliwell, P.S. The role of the multidisciplinary team in the management of psoriatic arthritis. Musculoskelet. Care 2022, 20 (Suppl. S1), S32–S40. [Google Scholar] [CrossRef]
- Salgado-Boquete, L.; Arias-Santiago, S.; Belinchón-Romero, I.; de la Cuadra-Grande, A.; de la Cueva, P.; Gilaberte, Y.; Notario, J.; Rivera-Díaz, R.; Ruiz-Villaverde, R.; Carrascosa, J.M.; et al. Selection of Quality Indicators for the Certification of Psoriasis Units: The CUDERMA Project Delphi Consensus Study. Actas Dermosifiliogr. 2023, 114, 865–883. [Google Scholar] [CrossRef]
- NICE. Psoriasis: Assessment and Management. NICE Clinical Guideline. In Excellence NNIoHaC: Niceorguk/Guidance/cg153, 2012 (Updated 2017). Disponible. Available online: https://www.nice.org.uk/guidance/cg153 (accessed on 1 June 2023).
- Wei, J.; Zhu, J.; Xu, H.; Zhou, D.; Elder, J.T.; Tsoi, L.C.; Patrick, M.T.; Li, Y. Alcohol consumption and smoking in relation to psoriasis: A Mendelian randomization study. Br. J. Dermatol. 2022, 187, 684–691. [Google Scholar] [CrossRef]
- Roszkiewicz, M.; Dopytalska, K.; Szymańska, E.; Jakimiuk, A.; Walecka, I. Environmental risk factors and epigenetic alternations in psoriasis. Ann. Agric. Environ. Med. AAEM 2020, 27, 335–342. [Google Scholar] [CrossRef]
- Musumeci, M.L.; Nasca, M.R.; Boscaglia, S.; Micali, G. The role of lifestyle and nutrition in psoriasis: Current status of knowledge and interventions. Dermatol. Ther. 2022, 35, e15685. [Google Scholar] [CrossRef]
- Huang, S.; Bai, Y. Knowledge Mapping and Research Hotspots of Comorbidities in Psoriasis: A Bibliometric Analysis from 2004 to 2022. Med. Kaunas Lith. 2023, 59, 393. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Kapoor, B.; Gulati, M.; Rani, P.; Gupta, R. Psoriasis: Interplay between dysbiosis and host immune system. Autoimmun. Rev. 2022, 21, 103169. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Papadopoulou, M.-M.; Chaidaki, K.; Georgopoulou, K.-E.; Magaliou, S.; Roussaki Schulze, A.V.; Bogdanos, D.P.; Zafiriou, E. Plaque Psoriasis Exacerbation and COVID-19 Vaccination: Assessing the Characteristics of the Flare and the Exposome Parameters. Vaccines 2024, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Strain, T.; Wijndaele, K.; Sharp, S.J.; Dempsey, P.C.; Wareham, N.; Brage, S. Impact of follow-up time and analytical approaches to account for reverse causality on the association between physical activity and health outcomes in UK Biobank. Int. J. Epidemiol. 2020, 49, 162–172. [Google Scholar] [CrossRef]


| Clinical Variables |
|---|
| Date of birth, level of education, employment status |
| First-degree family history of cardiovascular risk factors |
| Comorbidities: dyslipidemia, hypertension, diabetes mellitus, cardiovascular disease, cancer, hepatopathy, thyroid disease, kidney disease, rheumatologic disease, inflammatory bowel disease, neurological disease, infections, autoimmune disease, psychiatric disease, and other diseases |
| Habits: smoking or drinking alcohol, type of diet (hypocaloric, Mediterranean, etc.), physical exercise |
| Psoriasis: type of psoriasis, family history, time since onset, previous treatments and current medication, severity (PASI, BSA), quality of life (DLQI, PSO-life) |
| Phototype, weight, height, body mass index, waist circumference, blood pressure |
| Blood test parameters |
| Glucose, HbA1c, cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, estimated glomerular filtration rate, vitamin D3(25-OH), calcium, intact PTH, uric acid, microalbumin/creatinine quotient, creatinine, ALT, AST, GGT, C-peptide, C-reactive protein CRP, ESR, insulin, HOMA index |
| SCORE (Systematic Coronary Risk Evaluation): assesses the 10-year risk of dying from a cardiovascular event, as well as high and low cardiovascular risk charts based on gender, age, total cholesterol, systolic blood pressure, and smoking status. Spain is a country with low cardiovascular risk. Scoring: 1%, low risk; 2–4%, moderate risk; 5–9%, high risk; 9–14%, very high risk; >15%, extremely high risk. |
| Arterial hypertension: blood pressure ≥ 140/90 mmHg or antihypertensive treatment |
| Dyslipidemia: total cholesterol > 200 mg/dL and/or LDL > 130 mg/dL or treatment with a hypolipidemic agent |
| Diabetes mellitus: glycosylated hemoglobin > 6.5% or blood glucose ≥ 126 mg/mL or treatment with oral antidiabetics or insulin |
| Obesity: BMI ≥ 30 and/or waist circumference > 80 cm in women and >94 cm in men. |
| Smoking |
| Cases N (%) | Controls N (%) | p Value | |
|---|---|---|---|
| Sex | 0.630 | ||
| Male | 22 (81.5%) | 18 (72%) | |
| Female | 5 (18.5%) | 7 (28%) | |
| Age (mean ±SD) | 54.19 ± 12.88 | 56.76 ± 14.42 | 0.500 |
| Type of psoriasis | 0.340 | ||
| Plaques | 21 (77.8%) | 15 (60%) | |
| Guttate | 1 (3.7%) | 2 (8%) | |
| Erythrodermic | 0 (0%) | 2 (8%) | |
| Palmoplantar/Nail | 5 (18.5%) | 6 (24%) | |
| Time since onset (years) | 0.971 | ||
| (mean ±SD) | 16.48 ± 10.5 | 16.38 ± 10.31 | |
| Smoker | 0.338 | ||
| Yes | 9 (33.3%) | 10 (40%) | |
| Ex-smoker | 12 (44.4%) | 5 (20%) | |
| No | 6 (22.2%) | 10 (40%) | |
| Alcohol | 0.016 * | ||
| Yes | 13 (48.1%) | 5 (20%) | |
| Ex-drinker | 0 (0%) | 2 (8%) | |
| Social Drinker | 0 (0%) | 4 (16%) | |
| No | 14 (51.9%) | 14 (56%) | |
| Number of comorbidities | 0.184 | ||
| Mean ± SD | 3.41 ± 1.82 | 2.64 ± 2.27 | |
| Median | 3.00 | 3.00 | |
| Dyslipidemia | 0.001 * | ||
| Yes | 24 (88.9%) | 11 (44%) | |
| No | 3 (11.1%) | 14 (56%) | |
| Time since onset (mean ± SD) | 8.44 ± 5.85 | 10.09 ± 4.5 | 0.415 |
| Arterial hypertension | 0.405 | ||
| Yes | 15 (55.6%) | 11 (44%) | |
| No | 12 (44.4%) | 14 (56%) | |
| Time since onset (mean ± SD) | 4.66 ± 6.43 | 11.09 ± 4.48 | 0.005 * |
| Diabetes | 0.612 | ||
| Yes | 7 (25.9%) | 5 (20%) | |
| No | 20 (74.1%) | 20 (80%) | |
| Time since onset (mean ± SD) | 3 ± 6.97 | 12 ± 2.96 | 0.005 * |
| Metabolic syndrome | |||
| Yes | 17 (62.9%) | ||
| No | 10 (37.03%) | ||
| Cardiovascular disease | 0.262 | ||
| Yes (ischemic heart disease, heart failure) | 1 (3.7%) | 3 (12%) | |
| No | 26 (96.3%) | 22 (88%) | |
| Hepatopathy | 0.138 | ||
| Hepatic steatosis | 3 (11.1%) | 0 (0%) | |
| Alcoholic liver cirrhosis | 3 (11.1%) | 2 (8%) | |
| HBV | 2 (7.4%) | 0 (0%) | |
| No | 19 (70.4%) | 23 (92%) | |
| Rheumatologic disease | 0.749 | ||
| Psoriatic arthritis | 9 (33.3%) | 8 (32%) | |
| Osteoarthritis | 1 (3.7%) | 0 (0%) | |
| Spondyloarthropathy | 2 (7.4%) | 3 (12%) | |
| No | 15 (55.6%) | 14 (56%) |
| (a) | |||||||
| N | Mean | Standard Deviation | p Value | ||||
| PASI_t1 | Cases | 27 | 4.43 | 8.74 | 0.826 | ||
| Controls | 24 | 5.21 | 6.09 | ||||
| PASI_t2 | Cases | 24 | 3.52 | 7.21 | 0.264 | ||
| Controls | 25 | 2.22 | 4.02 | ||||
| PASI_t3 | Cases | 23 | 2.20 | 3.38 | 0.256 | ||
| Controls | 25 | 1.14 | 2.56 | ||||
| BSA_t1 | Cases | 27 | 3.99 | 6.17 | 0.333 | ||
| Controls | 24 | 6.12 | 7.76 | ||||
| BSA_t2 | Cases | 24 | 3.19 | 6.91 | 0.483 | ||
| Controls | 25 | 2.82 | 5.55 | ||||
| BSA_t3 | Cases | 23 | 2.29 | 3.52 | 0.345 | ||
| Controls | 25 | 1.28 | 3.26 | ||||
| Controls | 22 | 26.9 | 3.78 | ||||
| (b) | |||||||
| N | Mean | Standard Deviation | Median | IQR | p Value | ||
| DLQI_t1 | 27 | 4.04 | 5.85 | 2.00 | [1.00; 4.50] | 0.936 | |
| DLQI_t2 | 24 | 2.67 | 4.38 | 1.00 | [0.00; 3.00] | ||
| DLQI_t3 | 24 | 2.83 | 3.38 | 1.50 | [1.00; 3.00] | ||
| PSOLIFE_t1 | 27 | 69.7 | 26.5 | 73.0 | [58.5; 91.0] | 0.070 | |
| PSOLIFE_t2 | 24 | 83.0 | 12.2 | 84.5 | [76.5; 93.2] | ||
| PSOLIFE_t3 | 24 | 84.6 | 11.8 | 84.0 | [78.8; 94.0] | ||
| N | Mean | Standard Deviation | IQR | p Value | ||
|---|---|---|---|---|---|---|
| BMI_t1 (kg/m2) | Cases | 27 | 33.3 | 5.9 | [28.8; 36.3] | - |
| Controls | - | - | - | |||
| BMI_t2 (kg/m2) | Cases | 24 | 36.1 | 15.5 | [30.3; 35.9] | 0.003 * |
| Controls | 23 | 27.6 | 4.23 | [25.0; 29.4] | ||
| BMI_t3 (kg/m2) | Cases | 23 | 33.9 | 6.11 | [30.0; 36.8] | 0.001 * |
| Controls | 22 | 26.9 | 3.78 | 26.7 |
| (a) | ||||||||
| N | Mean | Standard Deviation | IQR | p Value | ||||
| Glucose_t1 (mg/dL) | Cases | 27 | 119 | 29.8 | [98.5; 136] | 0.051 | ||
| Controls | 24 | 110 | 32.6 | [93.8; 109] | ||||
| Glucose_t2 (mg/dL) | Cases | 24 | 118 | 33.8 | [97.2; 124] | 0.267 | ||
| Controls | 25 | 111 | 29.5 | [92.0; 125] | ||||
| Glucose_t3 (mg/dL) | Cases | 23 | 129 | 36.1 | [109; 138] | 0.011 * | ||
| Controls | 24 | 111 | 35.2 | [90.0; 110] | ||||
| Hb1Ac_t1 (%) | Cases | 22 | 6.25 | 1.06 | [5.60; 6.55] | 0.235 | ||
| Controls | 6 | 6.88 | 1.39 | [5.80; 7.42] | ||||
| Hb1Ac_t2 (%) | Cases | 24 | 6.06 | 0.85 | [5.60; 6.25] | 0.762 | ||
| Controls | 25 | 6.7 | 1.55 | [6.15; 7.25] | ||||
| Hb1Ac_t3 (%) | Cases | 23 | 6.17 | 0.98 | [5.60; 6.40] | 0.959 | ||
| Controls | 24 | 6.51 | 1.42 | [5.45; 7.50] | ||||
| (b) | ||||||||
| N | Mean | Standard Deviation | Median | IQR | p Value | |||
| Insulin_t1 (µU/mL) | 21 | 21.87 | 15.85 | 19.4 | [15.2; 21.7] | 0.042 * | ||
| Insulin_t2 (µU/mL) | 22 | 17.76 | 7.98 | 14.8 | [12.7; 20.6] | |||
| Insulin_t3 (µU/mL) | 19 | 14.24 | 5.14 | 15.3 | [10.2; 17.1] | |||
| C-Peptide_t1 (ng/mL) | 21 | 2.76 | 1.31 | 2.74 | [1.90; 3.18] | 0.011 * | ||
| C-Peptide _t2 (ng/mL) | 21 | 2.95 | 1.35 | 3.10 | [1.89; 3.49] | |||
| C-Peptide _t2 (ng/mL) | 19 | 3.85 | 1.53 | 3.59 | [3.08; 4.55] | |||
| HOMA-IR_t1 | 20 | 6.14 | 3.17 | 5.27 | [3.99; 7.78] | 0.138 | ||
| HOMA-IR_t2 | 20 | 5.30 | 3.26 | 3.81 | [3.17; 5.97] | |||
| HOMA-IR_t3 | 18 | 4.93 | 2.70 | 4.27 | [3.34; 6.29] | |||
| N | Mean | Standard Deviation | IQR | p Value | ||
|---|---|---|---|---|---|---|
| Cholesterol total_t1 (mg/dL) | Cases | 27 | 201 | 43.1 | [170;234] | 0.599 |
| Controls | 24 | 197 | 36.2 | [182; 221] | ||
| Cholesterol total_t2 (mg/dL) | Cases | 24 | 176 | 35.6 | [148; 202] | 0.047 * |
| Controls | 25 | 200 | 39.6 | [180; 223] | ||
| Cholesterol total_t3 (mg/dL) | Cases | 23 | 182 | 37.3 | [157; 206] | 0.437 |
| Controls | 23 | 191 | 41.7 | [168; 218] | ||
| HDL_t1 (mg/dL) | Cases | 27 | 48.7 | 13.9 | [39.0; 58.5] | 0.037 * |
| Controls | 21 | 53.7 | 12.6 | [47.0; 56.0] | ||
| HDL_t2 (mg/dL) | Cases | 24 | 46.8 | 12.7 | [38.8; 48.2] | 0.062 |
| Controls | 23 | 54.3 | 9.94 | [48.0; 58.5] | ||
| HDL_t3 (mg/dL) | Cases | 19 | 46.5 | 11.7 | [38.5; 51.0] | 0.030 * |
| Controls | 23 | 54.3 | 11.4 | [47.0; 61.0] | ||
| LDL_t1 (mg/dL) | Cases | 27 | 117 | 36.6 | [90.2; 145] | 0.971 |
| Controls | 21 | 115 | 43.1 | [85.6; 148] | ||
| LDL_t2 (mg/dL) | Cases | 24 | 103 | 38.9 | [70.7; 124] | 0.015 * |
| Controls | 23 | 124 | 42.2 | [92.4; 155] | ||
| LDL_t3 (mg/dL) | Cases | 24 | 98.5 | 27 | [75.0; 112] | 0.028 * |
| Controls | 19 | 122 | 30.6 | [106; 139] | ||
| TRG_t1 (mg/dL) | Cases | 27 | 185 | 126 | [106; 196] | 0.004 * |
| Controls | 21 | 96 | 47.9 | [72.0; 113] | ||
| TRG_t2 (mg/dL) | Cases | 24 | 176 | 90.5 | [90.0; 218] | 0.050 |
| Controls | 23 | 115 | 55.6 | [77.5; 133] | ||
| TRG_t3 (mg/dL) | Cases | 19 | 147 | 54.5 | [103; 162] | <0.001 * |
| Controls | 23 | 103 | 39.8 | [77.5; 122] | ||
|
GGT_t1
(UI/L) | Cases | 27 | 80.4 | 110 | [25.0; 59.5] | 0.288 |
| Controls | 22 | 59.9 | 114 | [17.0; 34.5] | ||
|
GGT_t2
(UI/L) | Cases | 24 | 72.3 | 77.0 | [28.5; 72.2] | 0.153 |
| Controls | 24 | 56.3 | 96.8 | [16.8; 39.5] | ||
|
GGT_t3
(UI/L) | Cases | 19 | 62.5 | 68.5 | [25.5; 63.5] | 0.649 |
| Controls | 23 | 52.9 | 87.2 | [16.0; 42.5] | ||
|
Vitamin D_t1 (ng/mL) | Cases | 22 | 22.7 | 8.96 | [17.9; 27.5] | 0.008 * |
| Controls | 7 | 33.5 | 29.8 | [16.5; 37.5] | ||
|
Vitamin D_t2 (ng/mL) | Cases | 24 | 21.2 | 8.36 | [16.0; 25.6] | 0.649 |
| Controls | 8 | 24.8 | 16.4 | [14.0; 28.9] | ||
|
Vitamin D_t3 (ng/mL) | Cases | 19 | 26.0 | 11.2 | [18.9; 28.6] | 0.215 |
| Controls | 9 | 20.9 | 9.54 | [13.1; 24.5] |
| N | Mean | Standard Deviation | Median | IQR | p Value | ||
|---|---|---|---|---|---|---|---|
| SCORE_t1 | Cases | 26 | 2.62 | 1.96 | 2 | [1.00; 3.00] | - |
| Controls | - | - | - | - | - | ||
| SCORE_t2 | Cases | 24 | 2.33 | 1.49 | 2 | [1.00; 3.25] | 0.594 |
| Controls | 24 | 2.88 | 3.30 | 2 | [0.75; 3.25] | ||
| SCORE_t3 | Cases | 19 | 1.89 | 1.05 | 2 | [1.00; 2.00] | 0.205 |
| Controls | 24 | 2.83 | 3.34 | 2 | [0.75; 4.00] |
| (a) | |||
| Cases N (%) | Controls N (%) | p Value | |
| Do you think psoriasis has a genetic predisposition? | 0.359 | ||
| - Yes | 9 (33.3%) | 13 (52%) | |
| - No | 9 (33.3%) | 7 (28%) | |
| - Do not know | 9 (33.3%) | 5 (20%) | |
| Do you think psoriasis only affects the skin? | 0.731 | ||
| - Yes | 4 (14.8%) | 5 (20%) | |
| - No | 14 (56%) | 14 (56%) | |
| - Do not know | 5 (18.5%) | 6 (24%) | |
| Can psoriasis be affected by cholesterol, blood pressure, sugar, or weight? | 0.559 | ||
| - Yes | 9 (33.3%) | 9 (36%) | |
| - No | 2 (4.4%) | 4 (16%) | |
| - Do not know | 16 (59.3%9 | 12 (48%) | |
| Can cholesterol, blood pressure, sugar, or weight be affected by psoriasis? | 0.875 | ||
| - Yes | 8 (29.6%) | 9 (36%) | |
| - No | 4 (14.8%) | 3 (12%) | |
| - Do not know | 15 (55.6%) | 13 (52%) | |
| Can all patients with psoriasis have psoriatic arthritis? | 0.297 | ||
| - Yes | 4 (14.8%) | 8 (32%) | |
| - No | 10 (37%) | 6 (24%) | |
| - Do not know | 13 (48.1%) | 11 (44%) | |
| Do you think that the follow-up in this consultation of Dermatology and Internal Medicine will help you control your psoriasis? | |||
| - Yes | 20 (74.1%) | ||
| - No | 0 (0%) | ||
| - Do not know | 7 (25.9%) | ||
| Do you think that the follow-up in this joint consultation of Dermatology and Internal Medicine will help you control your cholesterol, tension …? | |||
| - Yes | 20 (74.1%) | ||
| - No | 0 (0%) | ||
| - Do not know | 7 (25.9%) | ||
| (b) | |||
| Before N (%) | After N (%) | p | |
| Do you think psoriasis has a genetic predisposition? | 0.201 | ||
| - Yes | 9 (33.3%) | 14 (58.3%) | |
| - No | 9 (33.3%) | 5 (20.8%) | |
| - Do not know | 9 (33.3%) | 5 (20.8%) | |
| Do you think psoriasis only affects the skin? | 0.028 * | ||
| - Yes | 4 (14.8%) | 0 (0%) | |
| - No | 18(66.7%) | 23 (95.8%) | |
| - Do not know | 5 (18.5%) | 1 (4.2%) | |
| Can psoriasis be affected by cholesterol, blood pressure, sugar, or weight? | 0.001 * | ||
| - Yes | 9 (33.3%) | 20 (83.3) | |
| - No | 2 (7.4%) | 0 (0%) | |
| - Do not know | 16 (59.3%) | 4 (16.7%) | |
| Can cholesterol, blood pressure, sugar, or weight be affected by psoriasis? | <0.001 * | ||
| - Yes | 8 (29.6%) | 20 (83.3%) | |
| - No | 4 (14.8%) | 0 (0%) | |
| - Do not know | 15 (55.6%) | 4 (16.7%) | |
| Can all patients with psoriasis have psoriatic arthritis? | <0.001 * | ||
| - Yes | 4 (14.8%) | 21 (87.5%) | |
| - No | 10 (37%) | 0 (0%) | |
| - Do not know | 13 (48.1%) | 3 (12.5) | |
| Do you think that the follow-up in this consultation of Dermatology and Internal Medicine will help you control your psoriasis? | 0.007 * | ||
| - Yes | 20 (74.1%) | 24 (100%) | |
| - No | 0 (0%) | 0 (0%) | |
| - Do not know | 7 (25.9%) | 0 (0%) | |
| Do you think that the follow-up in this joint consultation of Dermatology and Internal Medicine will help you control your cholesterol, tension …? | 0.007 * | ||
| - Yes | 20 (74.1%) | 24 (100%) | |
| - No | 0 (0%) | 0 (0%) | |
| - Do not know | 7 (25.9%) | 0 (0%) | |
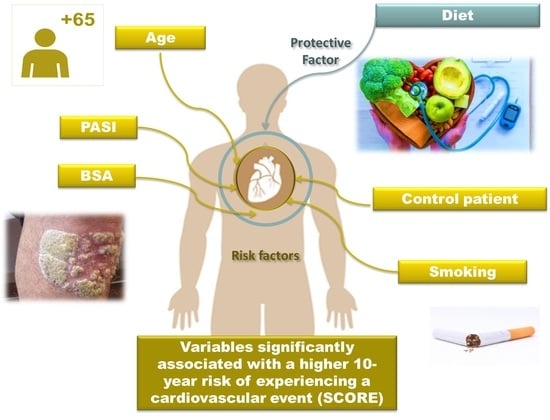
| Coefficients | Estimation | Standard Error | p | Odds Ratio (CI95%) |
|---|---|---|---|---|
| β1 Age | 0.290 | 0.052 | <0.001 | 1.33 (1.21–1.50) |
| ||||
| −0.606 | 0.879 | 0.451 | 0.54 (0.14–4.05) |
| 1.621 | 0.815 | 0.047 | 5.05 (1.07–27.37) |
| β3 PASI | 2.077 | 0.696 | 0.003 | 7.98 (2.32–35.86) |
| β4 BSA | 0.197 | 0.097 | 0.044 | 1.22 (1.01–1.49) |
| β5 Controls–Cases | 1.183 | 0.7015 | 0.029 | 3.26 (0.84–13.56) |
| β6 Diet | −2.669 | 1.335 | 0.046 | 0.06 (0.03–0.83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Malinis, A.J.; Pérez-Gilaberte, J.B.; Gracia-Cazaña, T.; González García, M.P.; Planas Linares, D.; Gilaberte, Y. The Impact of Shared Assistance between Dermatology and Internal Medicine on Patients with Psoriasis. J. Clin. Med. 2024, 13, 2441. https://doi.org/10.3390/jcm13082441
García-Malinis AJ, Pérez-Gilaberte JB, Gracia-Cazaña T, González García MP, Planas Linares D, Gilaberte Y. The Impact of Shared Assistance between Dermatology and Internal Medicine on Patients with Psoriasis. Journal of Clinical Medicine. 2024; 13(8):2441. https://doi.org/10.3390/jcm13082441
Chicago/Turabian StyleGarcía-Malinis, Ana Julia, Juan Blas Pérez-Gilaberte, Tamara Gracia-Cazaña, Maria Pilar González García, Dolores Planas Linares, and Yolanda Gilaberte. 2024. "The Impact of Shared Assistance between Dermatology and Internal Medicine on Patients with Psoriasis" Journal of Clinical Medicine 13, no. 8: 2441. https://doi.org/10.3390/jcm13082441