Effects of Etching Time and Ethanol Wet Bonding on Bond Strength and Metalloproteinase Activity in Radicular Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- -
- Group 1: 15 s 36% H3PO4 application;
- -
- Group 2: 30 s 36% H3PO4 application;
- -
- Group 3: 15 s 36% H3PO4 application, then post spaces were filled with 100% EtOH for 1 min;
- -
- Group 4: 30 s 36% H3PO4 application, then post spaces were filled with 100% EtOH for 1 min.
2.2. Push-Out Test
2.3. Nanoleakage Evaluation
2.4. In Situ Zymography
2.5. Statistical Analysis
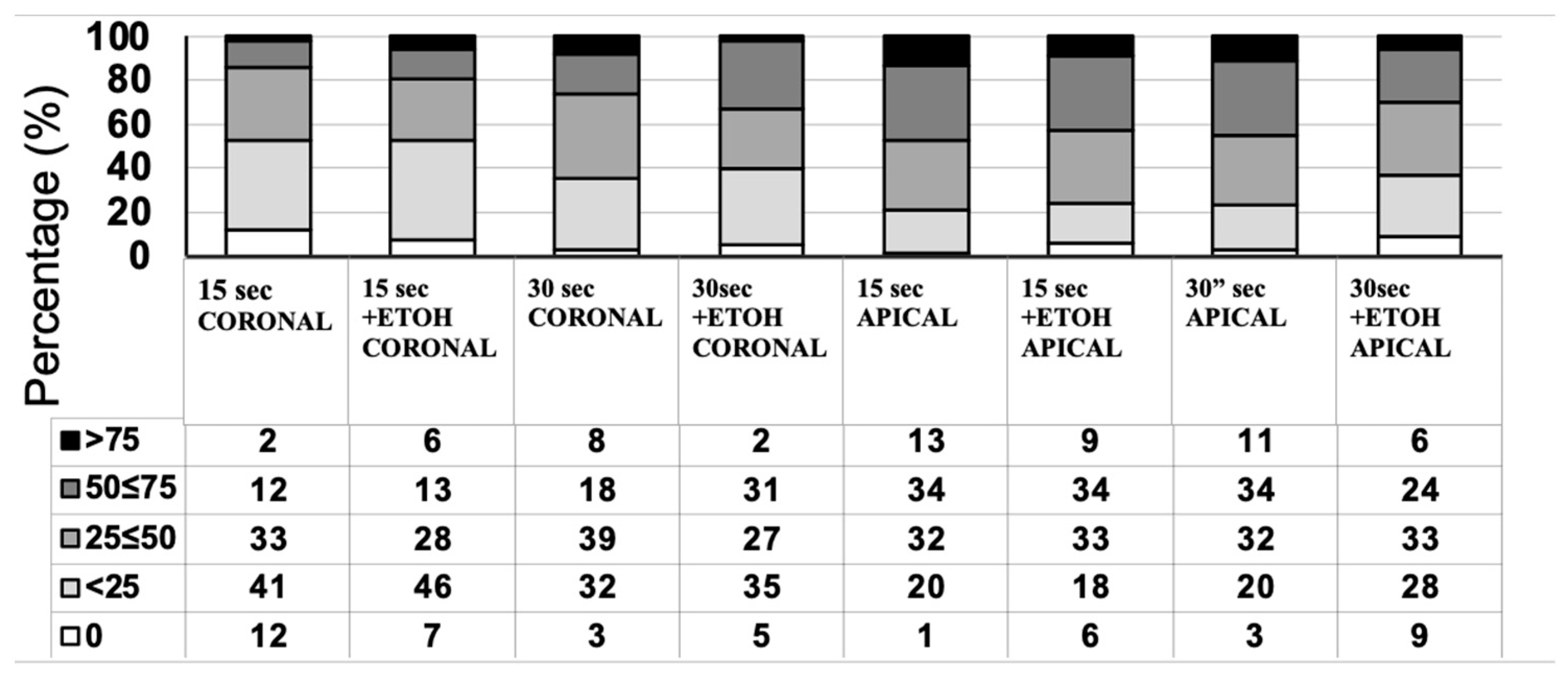
3. Results
3.1. Push-Out Bond Strength Test
3.2. Nanoleakage Analysis
3.3. In Situ Zymography
4. Discussion
5. Conclusions
- -
- The ethanol wet bonding technique did not affect radicular bond strength at baseline for coronal and apical dentin; however, bonding in the apical region was shown to be less effective than in the coronal one;
- -
- Etching time plays an important role in increasing immediate bond strength, irrespective of the use of ethanol. However, extended etching time activates a higher amount of MMPs, possibly leading to faster degradation of the hybrid layer over time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, G.M.; Gomes, O.M.M.; Reis, A.; Gomes, J.C.; Loguercio, A.D.; Calixto, A.L. Regional bond strengths to root canal dentin of fiber posts luted with three cementation systems. Braz. Dent. J. 2011, 22, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S.; Robbins, J.W. Post placement and restoration of endodontically treated teeth: A literature review. J. Endod. 2004, 30, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Bitter, K.; Hambarayan, A.; Neumann, K.; Blunck, U.; Sterzenbach, G. Various irrigation protocols for final rinse to improve bond strengths of fiber posts inside the root canal. Eur. J. Oral Sci. 2013, 121, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Salameh, Z.; Sorrentino, R.; Papacchini, F.; Ounsi, H.F.; Tashkandi, E.; Goracci, C.; Ferrari, M. Fracture resistance and failure patterns of endodontically treated mandibular molars restored using resin composite with or without translucent glass fiber posts. J. Endod. 2006, 32, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Have dentin adhesives become too hydrophilic? J. Can. Dent. Assoc. 2003, 69, 726–731. [Google Scholar] [PubMed]
- Gupta, A.; Tavane, P.; Gupta, P.K.; Tejolatha, B.; Lakhani, A.A.; Tiwari, R.; Kashyap, S.; Garg, G. Evaluation of Microleakage with Total Etch, Self Etch and Universal Adhesive Systems in Class V Restorations: An In vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC53–ZC56. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H.C. Immediate bonding properties of universal adhesives to dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; De Stefano Dorigo, E.; et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef]
- Mazzoni, A.; Pashley, D.H.; Nishitani, Y.; Breschi, L.; Mannello, F.; Tjäderhane, L.; Toledano, M.; Pashley, E.L.; Tay, F.R. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 2006, 27, 4470–4476. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Zhang, L.; Jiao, K.; Li, F.; Ding, Y.X.; Wang, D.Y.; Wang, M.Q.; Tay, F.R.; Chen, J.C. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J. Dent. 2011, 39, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Mutluay, M.M.; Agee, K.A.; Seseogullari-Dirihan, R.; Hoshika, T.; Cadenaro, M.; Breschi, L.; Vallittu, P.; Tay, F.R.; Pashley, D.H. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. J. Dent. Res. 2012, 91, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Self-Etching Adhesives Increase Collagenolytic Activity in Radicular Dentin—PubMed [Internet]. [Citato]. Available online: https://pubmed-ncbi-nlm-nih-gov.bibliopass.unito.it/16934629/ (accessed on 30 October 2023).
- Mazzoni, A.; Pashley, D.H.; Tay, F.R.; Gobbi, P.; Orsini, G.; Ruggeri, A.; Carrilho, M.; Tjäderhane, L.; Di Lenarda, R.; Breschi, L. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin: Correlative FEI-SEM/TEM analysis. J. Biomed. Mater. Res. A 2009, 88, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Tersariol, I.L.; Geraldeli, S.; Minciotti, C.L.; Nascimento, F.D.; Pääkkönen, V.; Martins, M.T.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Salo, T.; et al. Cysteine cathepsins in human dentin-pulp complex. J. Endod. 2010, 36, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Carrilho, M.; Tervahartiala, T.; Sorsa, T.; Breschi, L.; Mazzoni, A.; Pashley, D.; Tay, F.; Ferraz, C.; Tjäderhane, L. Determination of matrix metalloproteinases in human radicular dentin. J. Endod. 2009, 35, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Papa, V.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Ruggeri, A.; Gobbi, P.; Mazzotti, G.; Tay, F.R.; Pashley, D.H.; et al. Immunohistochemical and biochemical assay of MMP-3 in human dentine. J. Dent. 2011, 39, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Basting, R.T.; Bridi, E.C.; França, F.M.G.; do Amaral, F.L.B.; Basting, R.T. Wet-bonding technique with ethanol may reduce protease activity in dentin-resin interface following application of universal adhesive system. J. Clin. Exp. Dent. 2023, 15, e403–e410. [Google Scholar]
- Wang, Y.; Spencer, P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J. Dent. Res. 2003, 82, 141–145. [Google Scholar] [CrossRef]
- Kanca, J.; Sandrik, J. Bonding to dentin. Clues to the mechanism of adhesion. Am. J. Dent. 1998, 11, 154–159. [Google Scholar]
- Jacobsen, T.; Söderholm, K.J. Some effects of water on dentin bonding. Dent. Mater. 1995, 11, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef]
- Mazzoni, A.; Nascimento, F.D.; Carrilho, M.; Tersariol, I.; Papa, V.; Tjäderhane, L.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 2012, 91, 467–472. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Apolonio, F.M.; Saboia, V.P.A.; Santi, S.; Angeloni, V.; Checchi, V.; Curci, R.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; et al. Carbodiimide inactivation of MMPs and effect on dentin bonding. J. Dent. Res. 2014, 93, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kern, C.M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int. J. Oral Sci. 2009, 1, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.R.; Keller, J.C.; Boyer, D.B. Mode of failure in the dentin-adhesive resin-resin composite bonded joint as determined by strength-based (muTBS) and fracture-based (CNSB) mechanical testing. Dent. Mater. 2001, 17, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Czonstkowsky, M.; Wilson, E.G.; Holstein, F.A. The smear layer in endodontics. Dent. Clin. N. Am. 1990, 34, 13–25. [Google Scholar] [CrossRef]
- Serafino, C.; Gallina, G.; Cumbo, E.; Ferrari, M. Surface debris of canal walls after post space preparation in endodontically treated teeth: A scanning electron microscopic study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 381–387. [Google Scholar] [CrossRef]
- Brännström, M.; Nordenvall, K.J. The effect of acid etching on enamel, dentin, and the inner surface of the resin restoration: A scanning electron microscopic investigation. J. Dent. Res. 1977, 56, 917–923. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Volpato, C.A.M. Current perspectives on dental adhesion: (3) Adhesion to intraradicular dentin: Concepts and applications. Jpn. Dent. Sci. Rev. 2020, 56, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mannocci, F.; Vichi, A.; Cagidiaco, M.C.; Mjör, I.A. Bonding to root canal: Structural characteristics of the substrate. Am. J. Dent. 2000, 13, 255–260. [Google Scholar]
- Mjör, I.A.; Smith, M.R.; Ferrari, M.; Mannocci, F. The structure of dentine in the apical region of human teeth. Int. Endod. J. 2001, 34, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Vichi, A.; Grandini, S. Efficacy of different adhesive techniques on bonding to root canal walls: An SEM investigation. Dent. Mater. 2001, 17, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, A.J. Quantitative contribution of resin infiltration/hybridization to dentin bonding. Am. J. Dent. 1993, 6, 7–9. [Google Scholar] [PubMed]
- Kurtz, J.S.; Perdigão, J.; Geraldeli, S.; Hodges, J.S.; Bowles, W.R. Bond strengths of tooth-colored posts, effect of sealer, dentin adhesive, and root region. Am. J. Dent. 2003, 16, 31A–36A. [Google Scholar] [PubMed]
- Pashley, D.H.; Tay, F.R.; Carvalho, R.M.; Rueggeberg, F.A.; Agee, K.A.; Carrilho, M.; Donnelly, A.; García-Godoy, F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am. J. Dent. 2007, 20, 7–20. [Google Scholar] [PubMed]
- Nishitani, Y.; Yoshiyama, M.; Donnelly, A.M.; Agee, K.A.; Sword, J.; Tay, F.R.; Pashley, D.H. Effects of resin hydrophilicity on dentin bond strength. J. Dent. Res. 2006, 85, 1016–1021. [Google Scholar] [CrossRef]
- Sadek, F.T.; Pashley, D.H.; Ferrari, M.; Tay, F.R. Tubular occlusion optimizes bonding of hydrophobic resins to dentin. J. Dent. Res. 2007, 86, 524–528. [Google Scholar] [CrossRef]
- Bitter, K.; Aschendorff, L.; Neumann, K.; Blunck, U.; Sterzenbach, G. Do chlorhexidine and ethanol improve bond strength and durability of adhesion of fiber posts inside the root canal? Clin. Oral Investig. 2014, 18, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef]
- Sauro, S.; Watson, T.F.; Mannocci, F.; Miyake, K.; Huffman, B.P.; Tay, F.R.; Pashley, D.H. Two-photon laser confocal microscopy of micropermeability of resin-dentin bonds made with water or ethanol wet bonding. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Perdigão, J.; Gobbi, P.; Mazzotti, G.; Falconi, M.; Lopes, M. Immunocytochemical identification of type I collagen in acid-etched dentin. J. Biomed. Mater. Res. A 2003, 66, 764–769. [Google Scholar] [CrossRef] [PubMed]
- George, S.J.; Johnson, J.L. In situ zymography. Methods Mol. Biol. 2010, 622, 271–277. [Google Scholar] [PubMed]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Uchiyama, T.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Thompson, J.M.; McCracken, C.E.; Looney, S.W.; et al. Inhibition of MMPs by alcohols. Dent. Mater. 2011, 27, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Loushine, R.J.; Weller, R.N.; Monticelli, F.; Osorio, R. Self-etching adhesives increase collagenolytic activity in radicular dentin. J. Endod. 2006, 32, 862–868. [Google Scholar] [CrossRef]
- Brichko, J.; Burrow, M.F.; Parashos, P. Design Variability of the Push-out Bond Test in Endodontic Research: A Systematic Review. J. Endod. 2018, 44, 1237–1245. [Google Scholar] [CrossRef]



| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| coronal | 10.35 a ± 3.64 | 13.86 b ± 3.60 | 18.91 b ± 4.34 | 21.47 b ± 6.94 |
| apical | 6.92 a ± 1.99 | 14.68 b ± 2.83 | 11.39 b ± 3.49 | 12.24 b ± 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comba, A.; Baldi, A.; Pucci, R.; Rolando, C.; Alovisi, M.; Pasqualini, D.; Scotti, N. Effects of Etching Time and Ethanol Wet Bonding on Bond Strength and Metalloproteinase Activity in Radicular Dentin. J. Clin. Med. 2024, 13, 2474. https://doi.org/10.3390/jcm13092474
Comba A, Baldi A, Pucci R, Rolando C, Alovisi M, Pasqualini D, Scotti N. Effects of Etching Time and Ethanol Wet Bonding on Bond Strength and Metalloproteinase Activity in Radicular Dentin. Journal of Clinical Medicine. 2024; 13(9):2474. https://doi.org/10.3390/jcm13092474
Chicago/Turabian StyleComba, Allegra, Andrea Baldi, Riccardo Pucci, Chiara Rolando, Mario Alovisi, Damiano Pasqualini, and Nicola Scotti. 2024. "Effects of Etching Time and Ethanol Wet Bonding on Bond Strength and Metalloproteinase Activity in Radicular Dentin" Journal of Clinical Medicine 13, no. 9: 2474. https://doi.org/10.3390/jcm13092474





