Comparison between Imaging and Physiology in Guiding Coronary Revascularization: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria, Databases, and Search Strategy
2.2. Assessment of Risk of Bias
2.3. Statistical Analysis
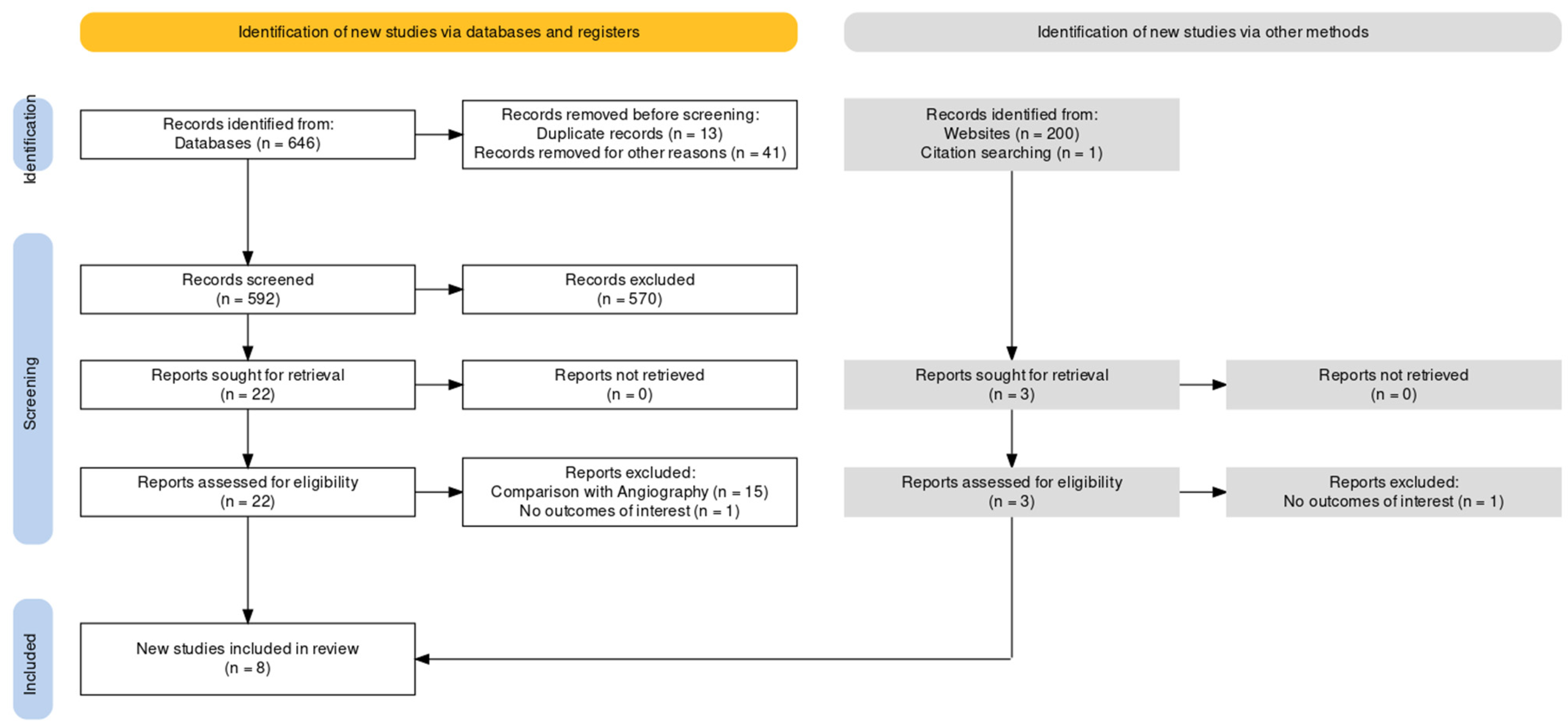
3. Results
3.1. Study Selection and Characteristics
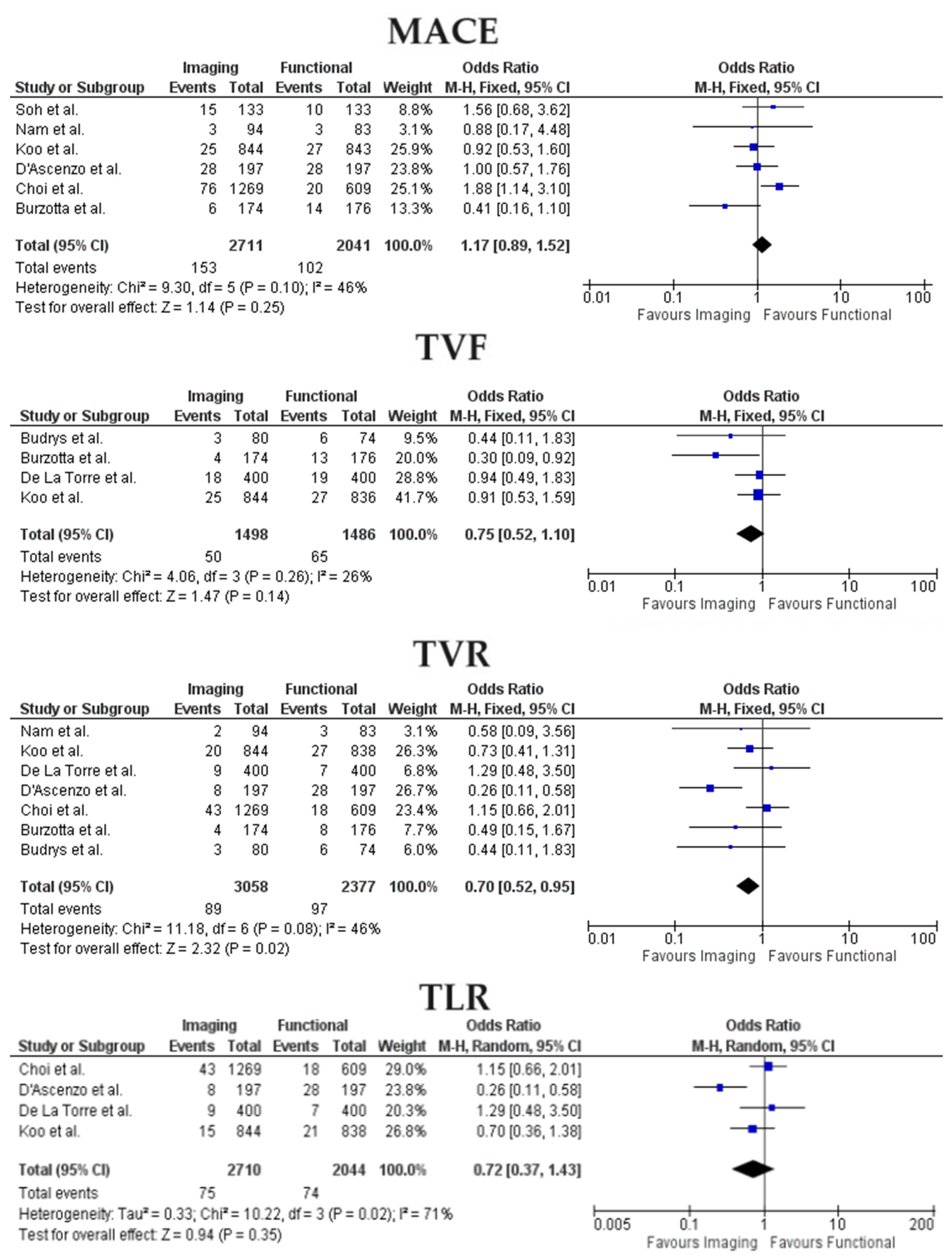
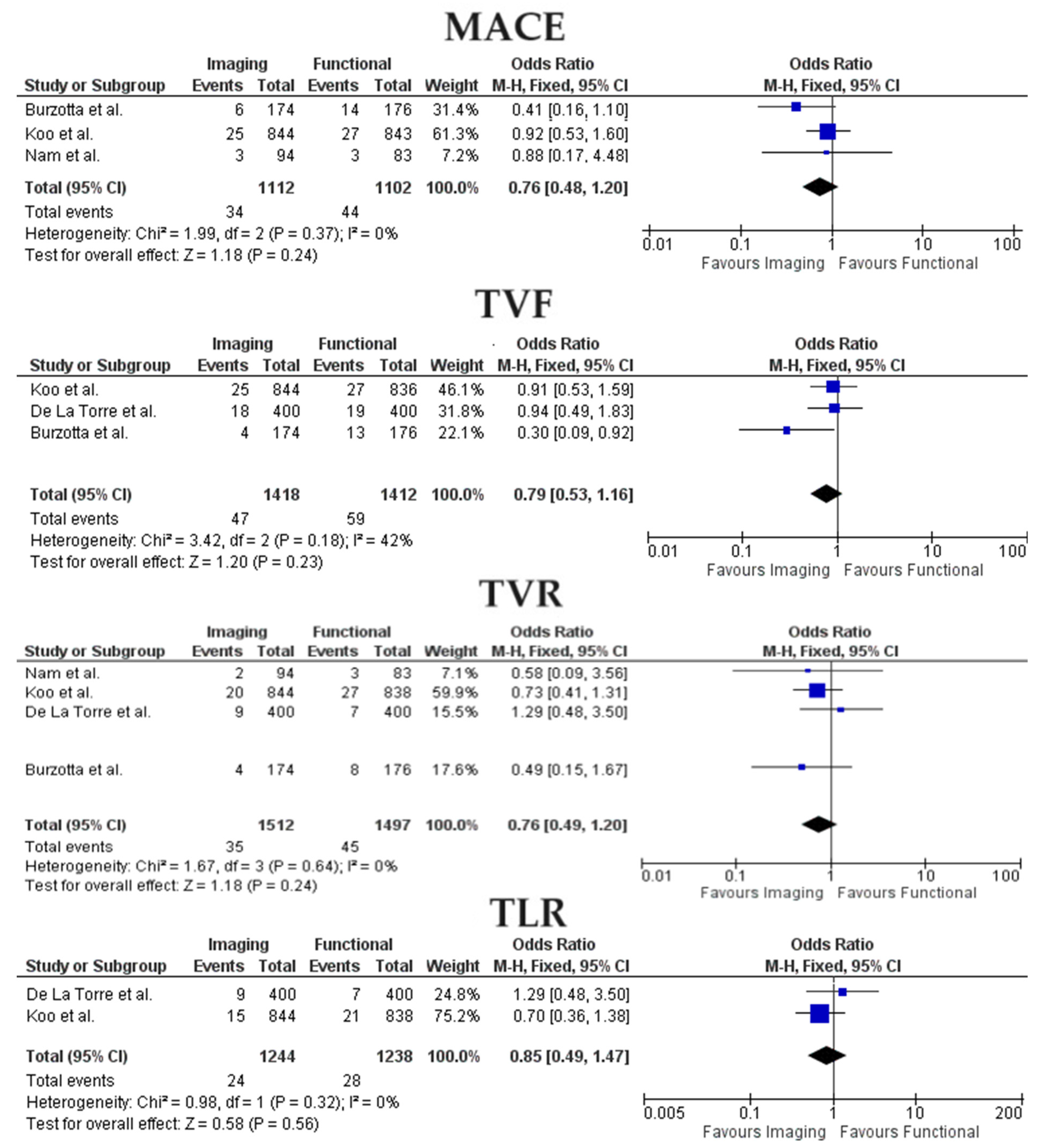
3.2. Clinical Outcomes
4. Discussion
- Between functional and imaging-guided PCI, there were no differences in rates of MACE, TVF, and TLR;
- The incidence of TVR was lower in the imaging-PCI group;
- When considering only studies focused on ICLs management, no significant differences were found between the two analyzed populations concerning MACE, TVR, and TVF;
- Presentation with acute coronary syndrome was not a significant moderator for MACE and TVR across the two groups.
5. Limitations
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escaned, J.; Berry, C.; De Bruyne, B.; Shabbir, A.; Collet, C.; Lee, J.M.; Appelman, Y.; Barbato, E.; Biscaglia, S.; Buszman, P.P.; et al. Applied coronary physiology for planning and guidance of percutaneous coronary interventions. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology. EuroIntervention 2023, 19, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. Prognostic Implications of Fractional Flow Reserve after Coronary Stenting: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2232842. [Google Scholar] [CrossRef] [PubMed]
- Piroth, Z.; Otsuki, H.; Zimmermann, F.M.; Ferenci, T.; Keulards, D.C.; Yeung, A.C.; Pijls, N.H.; De Bruyne, B.; Fearon, W.F. Prognostic Value of Measuring Fractional Flow Reserve after Percutaneous Coronary Intervention in Patients with Complex Coronary Artery Disease: Insights from the FAME 3 Trial. Circ. Cardiovasc. Interv. 2022, 15, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; Ijsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: The COMBINE OCT-FFR trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Koo, B.-K.; Hwang, D.; Zhang, J.; Hoshino, M.; Lee, J.M.; Murai, T.; Park, J.; Shin, E.-S.; Doh, J.-H.; et al. High-Risk Morphological and Physiological Coronary Disease Attributes as Outcome Markers after Medical Treatment and Revascularization. JACC Cardiovasc. Imaging 2021, 14, 1977–1989. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Leone, A.M.; Aurigemma, C.; Zambrano, A.; Zimbardo, G.; Arioti, M.; Vergallo, R.; De Maria, G.L.; Cerracchio, E.; Romagnoli, E.; et al. Fractional Flow Reserve or Optical Coherence Tomography to Guide Management of Angiographically Intermediate Coronary Stenosis: A Single-Center Trial. JACC Cardiovasc. Interv. 2020, 13, 49–58. [Google Scholar] [CrossRef] [PubMed]
- de la Torre Hernandez, J.M.; Lopez-Palop, R.; Camarero, T.G.; Saez, P.C.; Gorria, G.M.; Garcia, A.F.; Corada, B.A.; Fort, A.C.; Delgado, J.M.G.; Quilez, P.A.; et al. Clinical outcomes after intravascular ultrasound and fractional flow reserve assessment of intermediate coronary lesions. Propensity score matching of large cohorts from two institutions with a differential approach. EuroIntervention 2013, 9, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.H.R.; Teo, Y.H.; Djohan, A.H.; Ho, S.Y.J.; Sukmawati, I.; A Chan, K.; Sim, H.W.; Yeo, T.C.; Tan, H.C.; Chan, Y.Y.M.; et al. Real-world comparison of intracoronary imaging and fractional flow reserve measurements on outcomes of semi-urgent and elective percutaneous coronary intervention in a multi-ethnic asian population. Eur. Heart J. 2023, 44 (Suppl. S1), ehac779.054. [Google Scholar] [CrossRef]
- Koo, B.-K.; Hu, X.; Kang, J.; Zhang, J.; Jiang, J.; Hahn, J.-Y.; Nam, C.-W.; Doh, J.-H.; Lee, B.-K.; Kim, W.; et al. Fractional Flow Reserve or Intravascular Ultrasonography to Guide PCI. N. Engl. J. Med. 2022, 387, 779–789. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Iannaccone, M.; De Filippo, O.; Leone, A.M.; Niccoli, G.; Zilio, F.; Ugo, F.; Cerrato, E.; Fineschi, M.; Mancone, M.; et al. Optical coherence tomography compared with fractional flow reserve guided approach in acute coronary syndromes: A propensity matched analysis. Int. J. Cardiol. 2017, 244, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Budrys, P.; Peace, A.; Baranauskas, A.; Davidavicius, G. Intravascular Ultrasound vs. Fractional Flow Reserve for Percutaneous Coronary Intervention Optimization in Long Coronary Artery Lesions. Diagnostics 2023, 13, 2921. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.-W.; Yoon, H.-J.; Cho, Y.-K.; Park, H.-S.; Kim, H.; Hur, S.-H.; Kim, Y.-N.; Chung, I.-S.; Koo, B.-K.; Tahk, S.-J.; et al. Outcomes of percutaneous coronary intervention in intermediate coronary artery disease: Fractional flow reserve-guided versus intravascular ultrasound-guided. JACC Cardiovasc. Interv. 2010, 3, 812–817. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.; Lee, J.; Kang, D.; Ahn, J.; Park, D.; Park, S. Routine functional testing in high-risk patients after imaging- or physiology-guided percutaneous coronary intervention. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655.1245. [Google Scholar] [CrossRef]
- Mrevlje, B.; McFadden, E.; de la Torre Hernández, J.M.; Testa, L.; De Maria, G.L.; Banning, A.P.; Spitzer, E. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in unprotected left main coronary artery disease: A systematic review. Cardiovasc. Revasc. Med. 2023, 59, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jain, A.; Goel, S.; Garg, A.; Chaudhary, R.; Tantry, U.S.; Gurbel, P.A. Role of Intravascular Imaging in Complex Percutaneous Coronary Intervention: A Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiol. 2023, 208, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Jawed, K.; Moeed, A.; Ali, S.H. Efficacy of Intravascular Imaging-Guided Drug-Eluting Stent Implantation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Curr. Probl. Cardiol. 2023, 49, 102002. [Google Scholar] [CrossRef]
- Stone, G.W.; Christiansen, E.H.; Ali, Z.A.; Andreasen, L.N.; Maehara, A.; Ahmad, Y.; Landmesser, U.; Holm, N.R. Intravascular imaging-guided coronary drug-eluting stent implantation: An updated network meta-analysis. Lancet 2024, 403, 824–837. [Google Scholar] [CrossRef] [PubMed]
- van Zandvoort, L.J.; Ali, Z.; Kern, M.; van Mieghem, N.M.; Mintz, G.S.; Daemen, J. Improving PCI Outcomes Using Postprocedural Physiology and Intravascular Imaging. JACC Cardiovasc. Interv. 2021, 14, 2415–2430. [Google Scholar] [CrossRef]
- Liu, J.; Maehara, A.; Mintz, G.S.; Weissman, N.J.; Yu, A.; Wang, H.; Mandinov, L.; Popma, J.J.; Ellis, S.G.; Grube, E.; et al. An integrated TAXUS IV, V, and VI intravascular ultrasound analysis of the predictors of edge restenosis after bare metal or paclitaxel-eluting stents. Am. J. Cardiol. 2009, 103, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Romagnoli, E.; La Manna, A.; Burzotta, F.; Gatto, L.; Marco, V.; Fineschi, M.; Fabbiocchi, F.; Versaci, F.; Trani, C.; et al. Long-term consequences of optical coherence tomography findings during percutaneous coronary intervention: The Centro Per La Lotta Contro L’infarto-Optimization Of Percutaneous Coronary Intervention (CLI-OPCI) LATE study. EuroIntervention 2018, 14, e443–e451. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Abdirashid, M.; Annone, U.; Saint-Hilary, G.; Meier, P.; Chieffo, A.; Chen, S.; di Mario, C.; Conrotto, F.; Omedè, P.; et al. Comparison between functional and intravascular imaging approaches guiding percutaneous coronary intervention: A network meta-analysis of randomized and propensity matching studies. Catheter. Cardiovasc. Interv. 2020, 95, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Kiyohara, Y.; Maehara, A.; Ueyama, H.A.; Kampaktsis, P.N.; Takagi, H.; Mehran, R.; Stone, G.W.; Bhatt, D.L.; Mintz, G.S.; et al. Comparison of Intravascular Imaging, Functional, or Angiographically Guided Coronary Intervention. J. Am. Coll. Cardiol. 2023, 82, 2167–2176. [Google Scholar] [CrossRef]
- Reith, S.; Battermann, S.; Jaskolka, A.; Lehmacher, W.; Hoffmann, R.; Marx, N.; Burgmaier, M. Relationship between optical coherence tomography derived intraluminal and intramural criteria and haemodynamic relevance as determined by fractional flow reserve in intermediate coronary stenoses of patients with type 2 diabetes. Heart 2013, 99, 700–707. [Google Scholar] [CrossRef]
- Tomaniak, M.; Ochijewicz, D.; Kołtowski, Ł.; Rdzanek, A.; Pietrasik, A.; Jąkała, J.; Slezak, M.; Malinowski, K.P.; Zaleska, M.; Maksym, J.; et al. OCT-Derived Plaque Morphology and FFR-Determined Hemodynamic Relevance in Intermediate Coronary Stenoses. J. Clin. Med. 2021, 10, 2379. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Barbero, U.; Cerrato, E.; Lipinski, M.J.; Omedè, P.; Montefusco, A.; Taha, S.; Naganuma, T.; Reith, S.; Voros, S.; et al. Accuracy of intravascular ultrasound and optical coherence tomography in identifying functionally significant coronary stenosis according to vessel diameter: A meta-analysis of 2581 patients and 2807 lesions. Am. Heart J. 2015, 169, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, F.; Katagiri, Y.; Barlis, P.; Bourantas, C.; Collet, C.; Coskun, U.; Daemen, J.; Dijkstra, J.; Edelman, E.; Evans, P.; et al. Expert recommendations on the assessment of wall shear stress in human coronary arteries: Existing methodologies, technical considerations, and clinical applications. Eur. Heart J. 2019, 40, 3421–3433. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Koo, B.K. Physiology Versus Imaging-Guided Revascularization: Where Are We in 2023? JACC Asia 2023, 3, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Neleman, T.; van Zandvoort, L.J.; Forero, M.N.T.; Masdjedi, K.; Ligthart, J.M.; Witberg, K.T.; Groenland, F.T.; Cummins, P.; Lenzen, M.J.; Boersma, E.; et al. FFR-Guided PCI Optimization Directed by High-Definition IVUS versus Standard of Care: The FFR REACT Trial. JACC Cardiovasc. Interv. 2022, 15, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.S.; Pasos, J.I.F.; Solé, J.M.; Hussain, B.; Kumar, S.; Garg, M.; Chiarito, M.; Calderón, A.T.; Sorolla-Romero, J.A.; Pinto, M.E.; et al. Fractional flow reserve use in coronary artery revascularization: A systematic review and meta-analysis. iScience 2023, 26, 107245. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Ino, Y.; Mintz, G.S.; Shiono, Y.; Shimamura, K.; Takahata, M.; Terada, K.; Higashioka, D.; Emori, H.; Wada, T.; et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.D.; Arbab-Zadeh, A.; Soeda, T.; Fuster, V.; Jang, I.-K. Optical Coherence Tomography of Plaque Vulnerability and Rupture: JACC Focus Seminar Part 1/3. J. Am. Coll. Cardiol. 2021, 78, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Gallone, G.; Bellettini, M.; Gatti, M.; Tore, D.; Bruno, F.; Scudeler, L.; Cusenza, V.; Lanfranchi, A.; Angelini, A.; De Filippo, O.; et al. Coronary Plaque Characteristics Associated With Major Adverse Cardiovascular Events in Atherosclerotic Patients and Lesions: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2023, 16, 1584–1604. [Google Scholar] [CrossRef]
- Park, S.-J.; Ahn, J.-M.; Kang, D.-Y.; Yun, S.-C.; Ahn, Y.-K.; Kim, W.-J.; Nam, C.-W.; Nam, J.-O.; Chae, I.-H.; Shiomi, H.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024. [Google Scholar] [CrossRef]
- Bellino, M.; Silverio, A.; Esposito, L.; Cancro, F.P.; Ferruzzi, G.J.; Di Maio, M.; Rispoli, A.; Vassallo, M.G.; Di Muro, F.M.; Galasso, G.; et al. Moving toward Precision Medicine in Acute Coronary Syndromes: A Multimodal Assessment of Non-Culprit Lesions. J. Clin. Med. 2023, 12, 4550. [Google Scholar] [CrossRef] [PubMed]
- Seung, J.; Choo, E.H.; Choi, I.J.; Lim, S.; Moon, D.; Lee, K.Y.; Oh, G.C.; Hwang, B.-H.; Choi, Y.S.; Park, H.J.; et al. Intracoronary Imaging-Guided Revascularization for Non-Culprit Lesions in Patients with ST-Elevation Myocardial Infarction and Multivessel Disease. J. Cardiovasc. Interv. 2023, 2, 161–169. [Google Scholar] [CrossRef]
- Choi, I.J.; Lim, S.; Choo, E.H.; Hwang, B.-H.; Kim, C.J.; Park, M.-W.; Lee, J.-M.; Park, C.S.; Kim, H.Y.; Yoo, K.-D.; et al. Impact of Intravascular Ultrasound on Long-Term Clinical Outcomes in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 2431–2443. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Stevens, C.; Ludman, P.; Wijeysundera, H.C.; Siudak, Z.; Sharp, A.S.; Kinnaird, T.; Mohamed, M.O.; Ahmed, J.M.; Rashid, M.; et al. Intracoronary imaging in PCI for acute coronary syndrome: Insights from British Cardiovascular Intervention Society registry. Cardiovasc. Revasc. Med. 2023, 56, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; DE Filippo, O.; Montabone, A.; Marengo, G.; Maltese, L.; Ugo, F.; Quadri, G.; Mennuni, M.; Secco, G.G.; Taglialatela, V.; et al. OCT guided vs. COmplete pci in patieNts with sT segment elevation myocArdial infarCtion and mulTivessel disease: OCT-CONTACT RCT. Minerva Cardiol. Angiol. 2023, 71, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Kim, H.; Lee, H.; Lee, J.; Cho, J.; Shin, D.; Lee, S.H.; Kim, H.K.; Choi, K.H.; Park, T.K.; et al. Long-Term Cost-Effectiveness of Fractional Flow Reserve–Based Percutaneous Coronary Intervention in Stable and Unstable Angina. JACC Adv. 2022, 1, 100145. [Google Scholar] [CrossRef]
- Hong, D.; Lee, J.; Lee, H.; Cho, J.; Guallar, E.; Choi, K.H.; Lee, S.H.; Shin, D.; Lee, J.-Y.; Lee, S.Y.; et al. RENOVATE-COMPLEX-PCI Investigators. Cost-Effectiveness of Intravascular Imaging-Guided Complex PCI: Prespecified Analysis of RENOVATE-COMPLEX-PCI Trial. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e010230. [Google Scholar] [CrossRef]
| Study | Publication Year/Enrollment Year | Country | Study Design | Patients (N) | Imaging Type | ||
|---|---|---|---|---|---|---|---|
| Total | Imaging | FFR | |||||
| Burzotta et al. [13] | 2020/2013–2019 | Italy | RCT | 350 | 174 | 176 | OCT |
| Nam et al. [19] | 2010/2006–2008 | Korea | Observational | 167 | 83 | 94 | IVUS |
| D’Ascenzo et al. [17] | 2017/2009–2015 | Italy, France | PSM | 394 | 197 | 197 | OCT |
| De La Torre et al. [14] | 2013/NA | Spain | PSM | 800 | 400 | 400 | IVUS |
| Koo et al. [16] | 2022/2016–2022 | China, Korea | RCT | 1682 | 844 | 838 | IVUS |
| Soh et al. [15] | 2023/2014–2015 | China | PSM | 266 | 133 | 133 | IVUS/OCT |
| Budrys et al. [18] | 2023/NA | Lithuania | Observational | 154 | 80 | 74 | IVUS |
| Choi et al. [20] | 2023/NA | Korea | Observational | 1878 | 1269 | 609 | IVUS |
| Study | Age- FFR/Imaging (Years ± SD) | Male- FFR/Imaging (%) | Diabetes- FFR/Imaging (%) | Previous PCI- FFR/Imaging (%) | LAD- FFR/Imaging (%) | SIHD- FFR/Imaging (%) | ACS- FFR/Imaging (%) | Multivessel Disease- FFR/Imaging (%) |
|---|---|---|---|---|---|---|---|---|
| Burzotta et al. [13] | 68 ± 10/69 ± 9 | 71.6/77.6 | 34.7/36.2 | 41.5/43.7 | 66.7/60.6 | 79/82.2 | 21/17.8 | 53.3/47.7 |
| Nam et al. [19] | 63 ± 9/62 ± 9 | 66.3/58.2 | 21.7/22.5 | 20.5/12.8 | 48.2/58.2 | 45.8/36.2 | 54.2/63.8 | 66.3/51.1 |
| D’Ascenzo et al. [17] | 64 ± 11/ 63 ± 10 | 76.6/77.7 | 24.4/19.3 | N/A | 56.9/64.5 | N/A | N/A | 16.2/21.8 |
| De La Torre et al. [14] | 65.9 ± 9.5/ 65.2 ± 10 | 74.2/74.5 | 39.8/37.5 | 23.3/19 | 57.2/57.4 | 31.5/30 | N/A | 22.5/20.2 |
| Koo et al. [16] | 65.4 ± 9.4/ 64.8 ± 9.9 | 69.7/71.4 | 32.5/33.4 | 19.7/19.3 | 62.4/61.5 | 61.9/64.5 | 30.1/28.9 | 53.1/50.9 |
| Soh et al. [15] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Budrys et al. [18] | 66.3 ± 9.2/ 66.2 ± 9 | 73/71 | 21.6/18.8 | 39.2/57.5 | 82.4/82.5 | 74.3/75 | N/A | 85.1/85 |
| Choi et al. [20] | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Improta, R.; Di Pietro, G.; Giansanti, M.; Bruno, F.; De Filippo, O.; Tocci, M.; Colantonio, R.; Sardella, G.; D’Ascenzo, F.; Mancone, M. Comparison between Imaging and Physiology in Guiding Coronary Revascularization: A Meta-Analysis. J. Clin. Med. 2024, 13, 2504. https://doi.org/10.3390/jcm13092504
Improta R, Di Pietro G, Giansanti M, Bruno F, De Filippo O, Tocci M, Colantonio R, Sardella G, D’Ascenzo F, Mancone M. Comparison between Imaging and Physiology in Guiding Coronary Revascularization: A Meta-Analysis. Journal of Clinical Medicine. 2024; 13(9):2504. https://doi.org/10.3390/jcm13092504
Chicago/Turabian StyleImprota, Riccardo, Gianluca Di Pietro, Michele Giansanti, Francesco Bruno, Ovidio De Filippo, Marco Tocci, Riccardo Colantonio, Gennaro Sardella, Fabrizio D’Ascenzo, and Massimo Mancone. 2024. "Comparison between Imaging and Physiology in Guiding Coronary Revascularization: A Meta-Analysis" Journal of Clinical Medicine 13, no. 9: 2504. https://doi.org/10.3390/jcm13092504






