Endurant Stent Graft for Treatment of Abdominal Aortic Aneurysm Inside and Outside of the Instructions for Use for the Proximal Neck: A 14-Year, Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Baseline
3.2. Periprocedural Data
3.3. Outcomes
4. Discussion
- -
- Out-of-IFU EVAR carries a higher risk of procedure-related reintervention and endoleak and requires a more rigorous follow-up protocol;
- -
- Out-of-IFU EVAR does not carry higher aortic-related mortality;
- -
- Group 2 reintervention rate is fairly low, suggesting that out-of-IFU EVAR may be used with extreme caution in selected cases;
- -
- The Endurant stent graft proves to be reliable and is currently standing the test of time in the majority of our patients;
- -
- A larger aneurysm carries a higher risk of endoleak;
- -
- Some diseases or medications may have a role in influencing sac-related outcomes.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg 1991, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Siribumrungwong, B.; Kurita, J.; Ueda, T.; Yasui, D.; Takahashi, K.-I.; Sasaki, T.; Miyagi, Y.; Sakamoto, S.-I.; Ishii, Y.; Morota, T.; et al. Outcomes of abdominal aortic aneurysm repairs: Endovascular vs. open surgical repairs. Asian J. Surg. 2022, 45, 346–352. [Google Scholar] [CrossRef]
- Salata, K.; Hussain, M.A.; De Mestral, C.; Greco, E.; Aljabri, B.A.; Mamdani, M.; Forbes, T.L.; Bhatt, D.L.; Verma, S.; Al-Omran, M. Comparison of Outcomes in Elective Endovascular Aortic Repair vs Open Surgical Repair of Abdominal Aortic Aneurysms. JAMA Netw. Open 2019, 2, e196578. [Google Scholar] [CrossRef]
- Prinssen, M.; Verhoeven, E.L.G.; Buth, J.; Cuypers, P.W.M.; van Sambeek, M.R.H.M.; Balm, R.; Buskens, E.; Grobbee, D.E.; Blankensteijn, J.D.; Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. A Randomized Trial Comparing Conventional and Endovascular Repair of Abdominal Aortic Aneurysms. N. Engl. J. Med. 2004, 351, 1607–1618. [Google Scholar] [CrossRef]
- Pratesi, C.; Esposito, D.; Apostolou, D.; Attisani, L.; Bellosta, R.; Benedetto, F.; Blangetti, I.; Bonardelli, S.; Casini, A.; Fargion, A.T.; et al. Guidelines on the management of abdominal aortic aneurysms: Updates from the Italian Society of Vascular and Endovascular Surgery (SICVE). J. Cardiovasc. Surg. 2022, 63, 328–352. [Google Scholar] [CrossRef]
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.E.; van Keulen, J.W.; Rantner, B.; et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41 (Suppl. S1), S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Stokmans, R.A.; Teijink, J.A.W.; Forbes, T.L.; Böckler, D.; Peeters, P.J.; Riambau, V.; Hayes, P.D.; van Sambeek, M.R.H.M. Early results from the ENGAGE registry: Real-world performance of the Endurant Stent Graft for endovascular AAA repair in 1262 patients. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 369–375. [Google Scholar] [CrossRef]
- Endurant Stent Graft Proves Efficacious and Durable in ENGAGE 10-Year Data. Available online: https://vascularnews.com/endurant-stent-graft-proves-efficacious-and-durable-in-engage-10-year-data/ (accessed on 22 December 2023).
- Oliveira-Pinto, J.; Oliveira, N.; Bastos-Gonçalves, F.; Hoeks, S.; Rijn, M.J.V.A.; Raa, S.T.; Mansilha, A.; Verhagen, H.J. Long-term results of outside ‘instructions for use’ EVAR. J. Cardiovasc. Surg. 2017, 58, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, T.; Iwahashi, T.; Kamiya, K.; Kano, M.; Maruno, K.; Fujiyoshi, T.; Iwahori, A.; Suzuki, S.; Kawago, K.; Takahashi, S.; et al. Clinical and Morphological Outcomes in Endovascular Aortic Repair of Abdominal Aortic Aneurysm Using GORE C3 EXCLUDER: Comparison between Patients Treated within and Outside Instructions for Use. Ann. Vasc. Surg. 2019, 59, 54–62. [Google Scholar] [CrossRef]
- Georgiadis, G.S.; Schoretsanitis, N.; Argyriou, C.; Nikolopoulos, E.; Kapoulas, K.; Georgakarakos, E.I.; Ktenidis, K.; Lazarides, M.K. Long-term outcomes of the Endurant endograft in patients undergoing endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2023, 78, 668–678.e14. [Google Scholar] [CrossRef] [PubMed]
- Ferrel, B.; Patel, S.; Castillo, A.; Gryn, O.; Franko, J.; Chew, D. The Effect of Abdominal Aortic Aneurysm Size on Endoleak, Secondary Intervention and Overall Survival Following Endovascular Aortic Aneurysm Repair. Vasc. Endovasc. Surg. 2021, 55, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Forbes, T.L.; Harris, J.R.; Lawlor, D.K.; Derose, G. Midterm results of the Zenith endograft in relation to neck length. Ann. Vasc. Surg. 2010, 24, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Jim, J.; Sanchez, L.A.; Rubin, B.G.; Criado, F.J.; Fajardo, A.; Geraghty, P.J. A 5-Year Evaluation Using the Talent Endovascular Graft for Endovascular Aneurysm Repair in Short Aortic Necks. Ann. Vasc. Surg. 2010, 24, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, F.; İşcan, H.Z.; Türkçü, M.A.; Mavioğlu, H.L.; Ünal, E.U. Predictive Parameters of Type 1A Endoleak for Elective Endovascular Aortic Repair: A Single-Center Experience. Ann. Vasc. Surg. 2023, 98, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Greenberg, R.K.; Mastracci, T.M.; Eagleton, M.J.; Hernandez, A.V. Patients with chronic obstructive pulmonary disease have shorter survival but superior endovascular outcomes after endovascular aneurysm repair. J. Vasc. Surg. 2012, 56, 911–919.e2. [Google Scholar] [CrossRef] [PubMed]
- Vedani, S.M.; Petitprez, S.; Weinz, E.; Corpataux, J.-M.; Déglise, S.; Deslarzes-Dubuis, C.; Côté, E.; Ricco, J.-B.; Saucy, F. Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair. J. Clin. Med. 2022, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Pasqui, E.; de Donato, G.; Molino, C.; Leil, M.A.; Anzaldi, M.G.; Galzerano, G.; Palasciano, G. Residual Aneurysmal Sac Shrinkage Post-Endovascular Aneurysm Repair: The Role of Preoperative Inflammatory Markers. Biomedicines 2023, 11, 1920. [Google Scholar] [CrossRef]
- Kertai, M.D.; Boersma, E.; Westerhout, C.M.; van Domburg, R.; Klein, J.; Bax, J.J.; van Urk, H.; Poldermans, D. Association between long-term statin use and mortality after successful abdominal aortic aneurysm surgery. Am. J. Med. 2004, 116, 96–103. [Google Scholar] [CrossRef]
- Shuai, T.; Kan, Y.; Si, Y.; Fu, W. High-risk factors related to the occurrence and development of abdominal aortic aneurysm. J. Interv. Med. 2020, 3, 80–82. [Google Scholar] [CrossRef] [PubMed]




| Overall (795) | Group 1 (in IFU, 650) | Group 2 (Out of the IFU, 145) | p-Value (<0.05) | |
|---|---|---|---|---|
| Age (years; SD) | 74 ± 8 | 74 ± 8 | 75 ± 8 | 0.190 |
| Female sex | 63 (7.9%) | 50 (7.7%) | 13 (9%) | 0.442 |
| Diabetes | 138 (17.4%) | 114 (17.6%) | 24 (16.5%) | 0.798 |
| Arterial hypertension | 636 (80%) | 524 (80.7%) | 112 (77.2%) | 0.372 |
| Dyslipidemia | 426 (53.6%) | 354 (54.5%) | 72 (49.6%) | 0.319 |
| Cardiac disease | 335 (42.2%) | 275 (42.4%) | 60 (41.7%) | 0.921 |
| Previous myocardial infarction | 244 (30.6%) | 203 (31.1%) | 41 (28.3%) | 0.542 |
| Chronic obstructive pulmonary disease | 477 (59.8%) | 383 (59.1%) | 94 (63%) | 0.650 |
| Chronic kidney disease | 192 (24.1%) | 157 (24.1%) | 35 (24.1%) | 0.937 |
| Ruptured aneurysm | 40 (5.1%) | 30 (5.1%) | 10 (6.6%) | 0.502 |
| Overall | Group 1 (in IFU) | Group 2 (Out of the IFU) | p-Value (<0.05) | |
|---|---|---|---|---|
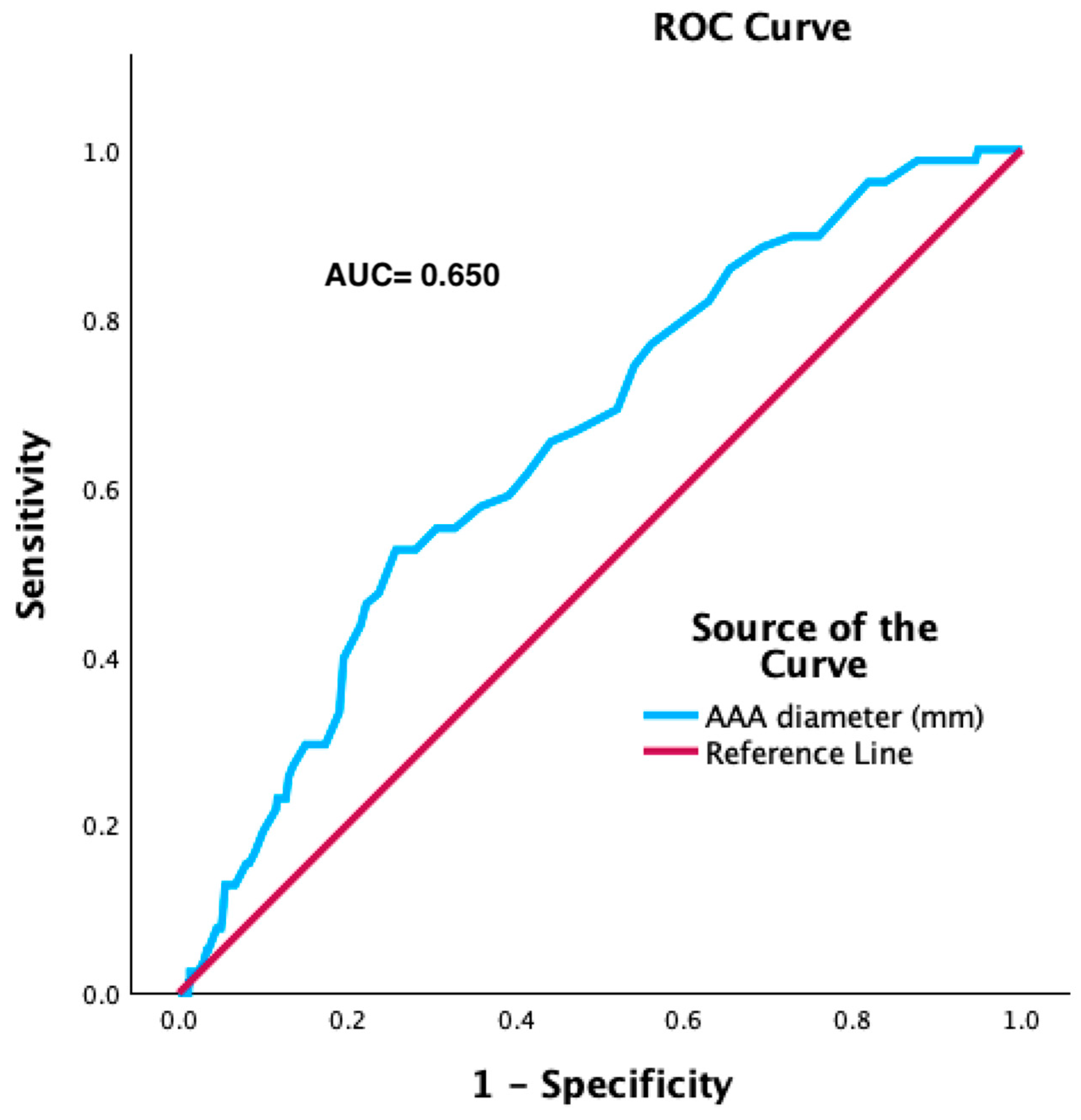
| AAA diameter (mm) | 61 (52; 68) | 57 (50; 67) | 62 (55; 74) | <0.001 * |
| Aortic bifurcation diameter (mm) | 29 (23; 28) | 29 (23; 36) | 30 (25; 43) | 0.025 * |
| Access common iliac artery diameter (mm) | 15 (12; 20) | 15 (12; 21) | 15 (12; 19) | 0.977 |
| Access external iliac artery diameter (mm) | 9 (8; 11) | 9 (8; 11) | 9 (8; 11) | 0.119 |
| Access femoral artery diameter (mm) | 10 (9; 12) | 10 (9; 12) | 10 (9; 12) | 0.572 |
| Lowest renal to aortic bifurcation length (mm) | 112 (101; 125) | 111 (101; 124) | 115 (103; 129) | 0.056 |
| Number of patent lumbar arteries | 3 (2; 4) | 3 (2; 4) | 3 (2; 4) | 0.09 |
| Overall (795) | Group 1 (in IFU, 650) | Group 2 (out of the IFU, 145) | p-Value (<0.05) | |
|---|---|---|---|---|
| Neck length | 17 (12; 25) | 19 (13; 26) | 10 (7; 17) | <0.001 * |
| Neck diameter | 25.1 ± 4.3 | 24 (22; 26) | 28 (23; 33) | <0.001 * |
| Infrarenal neck angulation | 25 (14; 42) | 22 (13; 38) | 35 (20; 62) | <0.001 * |
| Neck calcium: | ||||
| Absent | 330 (41.6%) | 280 (43.1%) | 50 (34.4%) | 0.157 |
| <90° | 380 (47.9%) | 308 (47.3%) | 72 (49.6%) | 0.640 |
| <180° | 62 (7.9%) | 48 (7.4%) | 14 (9.6%) | 0.404 |
| >180° | 20 (2.6%) | 14 (2.1%) | 6 (2.6%) | 0.138 |
| Neck thrombus: | ||||
| Absent | 418 (52.6%) | 343 (51.5%) | 75 (52.6%) | 0.756 |
| <90° | 99 (12.5%) | 75 (11.5%) | 24 (16.3%) | 0.146 |
| <180° | 156 (19.6%) | 121 (18.5%) | 35 (23.5%) | 0.194 |
| >180° | 121 (15.2%) | 109 (17%) | 12 (8.8%) | 0.019 * |
| Neck morphology | ||||
| Straight | 446 (56.1%) | 409 (62.9%) | 47 (32.6%) | <0.001 * |
| Barrel (focal <3 mm enlargement) | 51 (6.3%) | 48 (7.5%) | 3 (2.2%) | 0.042 * |
| Angled (>45°) | 179 (22.5%) | 106 (16.3%) | 33 (22.5%) | <0.001 * |
| Bulge | 9 (1.3%) | 7 (1.3%) | 2 (1.3%) | 1.000 |
| Conic (>10%) | 134 (17.1%) | 100 (15.1%) | 34 (23.7%) | 0.020 * |
| Short (<10 mm) | 95 (12.1%) | 27 (4.2%) | 68 (47.1%) | <0.001 * |
| Dilated (>28 mm) | 228 (29%) | 149 (22.9%) | 79 (54.3%) | <0.001 * |
| Irregular (elliptic shape) | 23 (3%) | 17 (2.6%) | 6 (4.4%) | 0.256 |
| Overall | Group 1 (in IFU) | Group 2 (out of the IFU) | p-Value (<0.05) | |
|---|---|---|---|---|
| Length of stay (days) | 4 (3; 6) | 4 (3; 6) | 5 (4; 6) | 0.01 * |
| Procedure duration (hours) | 3.2 (2.3; 4.3) | 3.2 (2.3; 4.2) | 3.3 (2.45; 4.5) | 0.221 |
| Sac embolization | 184 (23.2%) | 149 (22.9%) | 35 (24.1%) | 0.827 |
| Access or procedural Complications | 50 (6.3%) | 35 (5.5%) | 15 (10.3%) | 0.041 * |
| Number of Endurant components | 3 (2; 3) | 2 (2; 3) | 3 (2; 3) | 0.486 |
| Bell-bottom | 224 (28.2%) | 181 (27.9%) | 43 (29.8%) | 0.824 |
| Proximal Oversize | 1.14 (1.06; 1.23) | 1.14 (1.06; 1.23) | 1.12 (1.05; 1.23) | 0.381 |
| Overall (795) | Group 1 (in IFU) (650) | Group 2 (out of the IFU) (145) | p-Value (<0.05) | |
|---|---|---|---|---|
| 30-days death | 29 (3.7%) | 20 (3.1%) | 9 (6.2%) | 0.070 |
| Follow-up time (months) | 43 ± 39 | 43 ± 40 | 40 ± 37 | 0.582 |
| Any endoleak | 82 (10.5%) | 60 (9.4%) | 22 (15.3%) | 0.037 * |
| Type 1A | 34 (4.3%) | 23 (3.5%) | 11 (7.6%) | 0.030 * |
| Type 1B | 19 (2.4%) | 13 (2%) | 6 (4.1%) | 0.129 |
| Type 2 | 30 (3.8%) | 25 (3.9%) | 5 (3.4%) | 0.523 |
| Graft thrombosis | 17 (2.1%) | 14 (2.2%) | 3 (2.1%) | 0.947 |
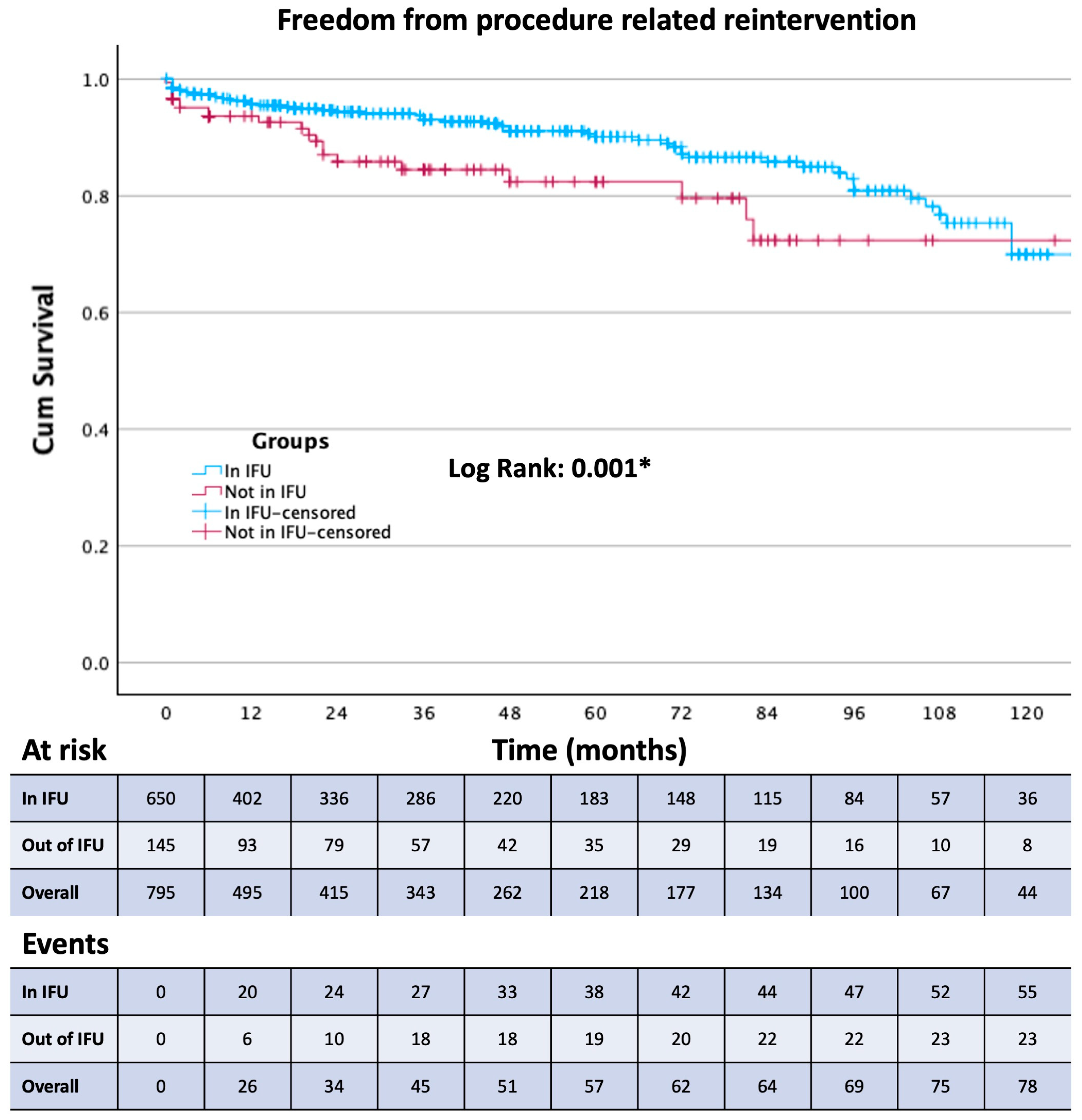
| Procedure-related reintervention | 83 (10.4%) | 59 (9.1%) | 24 (16.6%) | 0.008 * |
| Aneurysm-related mortality | 9 (1.1%) | 6 (0.9%) | 3 (2.1%) | 0.377 |
| All-cause mortality | 290 (36.5%) | 229 (35.2%) | 61 (42.1%) | 0.328 |
| Sac regression at more than one year: | N = 509 | N = 404 | N = 105 | |
| ≥10 mm | 46.3% | 44.6% | 54.1% | 0.178 |
| ≥5 mm | 65.9% | 64% | 73.8% | 0.148 |
| <5 mm/stable | 21.3% | 22.8% | 14.8% | 0.164 |
| Growth | 13.3% | 13.4% | 12.9% | 0.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accarino, G.; De Vuono, F.; Accarino, G.; Fornino, G.; Puca, A.E.; Fimiani, R.; Parrella, V.; Savarese, G.; Furgiuele, S.; Vecchione, C.; et al. Endurant Stent Graft for Treatment of Abdominal Aortic Aneurysm Inside and Outside of the Instructions for Use for the Proximal Neck: A 14-Year, Single-Center Experience. J. Clin. Med. 2024, 13, 2589. https://doi.org/10.3390/jcm13092589
Accarino G, De Vuono F, Accarino G, Fornino G, Puca AE, Fimiani R, Parrella V, Savarese G, Furgiuele S, Vecchione C, et al. Endurant Stent Graft for Treatment of Abdominal Aortic Aneurysm Inside and Outside of the Instructions for Use for the Proximal Neck: A 14-Year, Single-Center Experience. Journal of Clinical Medicine. 2024; 13(9):2589. https://doi.org/10.3390/jcm13092589
Chicago/Turabian StyleAccarino, Giulio, Francesco De Vuono, Giancarlo Accarino, Giovanni Fornino, Aniello Enrico Puca, Rodolfo Fimiani, Valentina Parrella, Giovanni Savarese, Sergio Furgiuele, Carmine Vecchione, and et al. 2024. "Endurant Stent Graft for Treatment of Abdominal Aortic Aneurysm Inside and Outside of the Instructions for Use for the Proximal Neck: A 14-Year, Single-Center Experience" Journal of Clinical Medicine 13, no. 9: 2589. https://doi.org/10.3390/jcm13092589
APA StyleAccarino, G., De Vuono, F., Accarino, G., Fornino, G., Puca, A. E., Fimiani, R., Parrella, V., Savarese, G., Furgiuele, S., Vecchione, C., Galasso, G., & Bracale, U. M. (2024). Endurant Stent Graft for Treatment of Abdominal Aortic Aneurysm Inside and Outside of the Instructions for Use for the Proximal Neck: A 14-Year, Single-Center Experience. Journal of Clinical Medicine, 13(9), 2589. https://doi.org/10.3390/jcm13092589






