A Systematic Literature Review of Predictors of Erythropoiesis-Stimulating Agent Failure in Lower-Risk Myelodysplastic Syndromes
Abstract
1. Introduction
2. Materials and Methods
3. Results
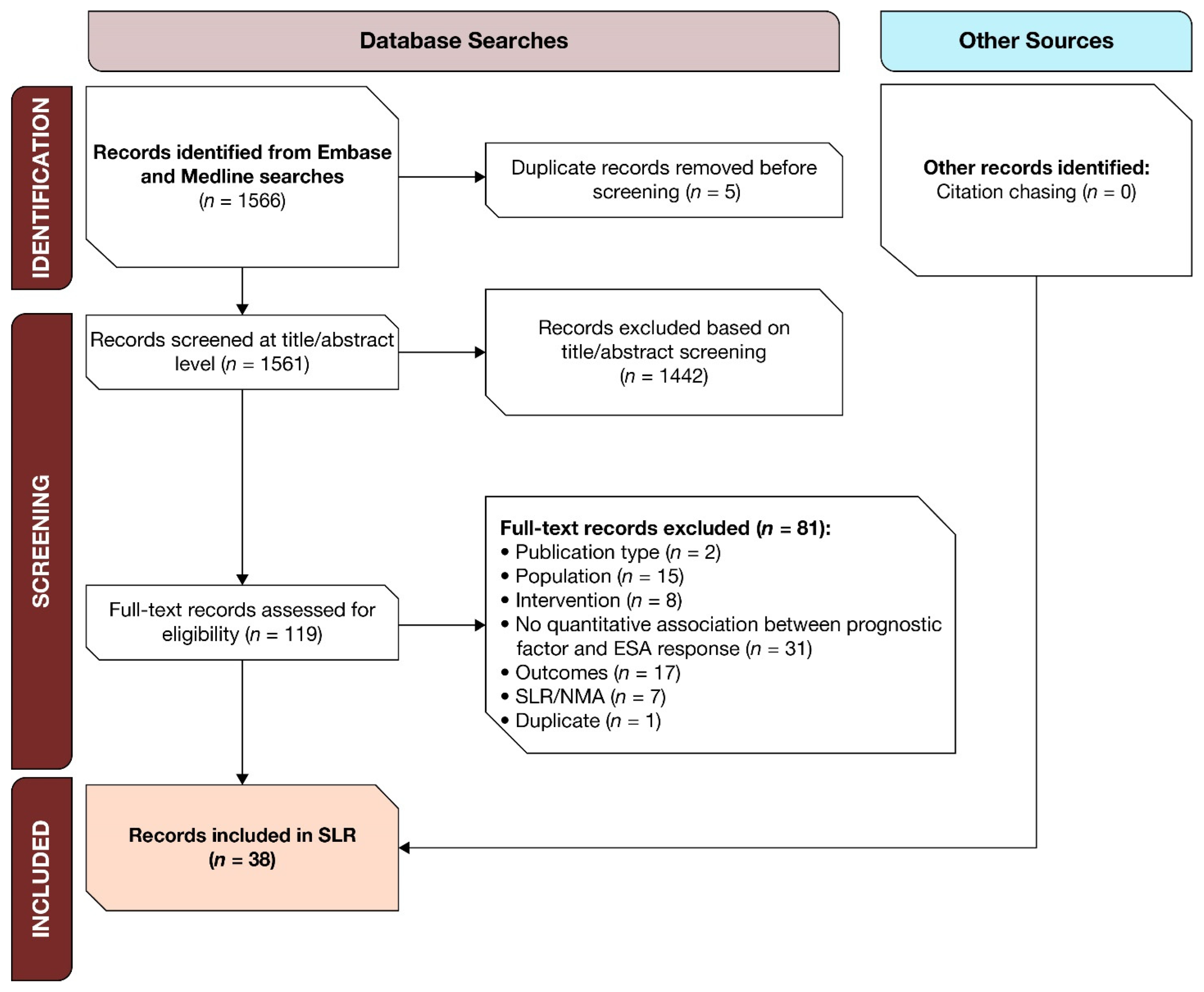
3.1. SLR
3.2. Age
3.3. Bone Marrow Blasts
3.4. Serum Ferritin Levels
3.5. Hemoglobin Levels
3.6. IPSS Risk Status
3.7. Karyotype Status
3.8. Serum EPO Levels
3.9. Transfusion Dependence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sekeres, M.A. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J. Natl. Compr. Canc. Netw. 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Does, M.; Raza, A.; Mayne, S.T. Myelodysplastic syndromes: Incidence and survival in the United States. Cancer 2007, 109, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, J.; Schoonen, W.M.; Strupp, C.; Gattermann, N.; Aul, C.; Haas, R.; Germing, U. Incidence and prevalence of myelodysplastic syndromes: Data from the Düsseldorf MDS-registry. Leuk. Res. 2011, 35, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019, 34, 1–15. [Google Scholar] [CrossRef]
- Dao, K.T. Myelodysplastic syndromes: Updates and nuances. Med. Clin. N. Am. 2017, 101, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Malcovati, L.; Germing, U.; Kuendgen, A.; Della Porta, M.G.; Pascutto, C.; Invernizzi, R.; Giagounidis, A.; Hildebrandt, B.; Bernasconi, P.; Knipp, S.; et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J. Clin. Oncol. 2007, 25, 3503–3510. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Papaermmanuil, E. Molecular International Prognostic Scoring System for myelodysplastic syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.M.; Leitch, H.A.; van de Loosdrecht, A.A.; Bonadies, N. Management of patients with lower-risk myelodysplastic syndromes. Blood Cancer J. 2022, 12, 166. [Google Scholar] [CrossRef]
- Platzbecker, U. Treatment of MDS. Blood 2019, 133, 1096–1107. [Google Scholar] [CrossRef]
- Platzbecker, U.; Fenaux, P.; Ades, L.; Giagounidis, A.; Santini, V.; van de Loosdrecht, A.A.; Bowen, D.; de Witte, T.; Garcia-Manero, G.; Hellström-Lindberg, E.; et al. Proposals for revised IWG 2018 hematological response criteria in patients with MDS included in clinical trials. Blood 2019, 133, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Löwenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, O.; Passet, M.; Santini, V.; Platzbecker, U.; Andrieu, V.; Zini, G.; Beyne-Rauzy, O.; Guerci, A.; Masala, E.; Balleari, E.; et al. Are somatic mutations predictive of response to erythropoiesis stimulating agents in lower risk myelodysplastic syndromes? Haematologica 2016, 101, e280–e283. [Google Scholar] [CrossRef] [PubMed]
- Kubasch, A.S.; Platzbecker, U. Setting fire to ESA and EMA resistance: New targeted treatment options in lower risk myelodysplastic syndromes. Int. J. Mol. Sci. 2019, 20, 3853. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Berraquero, M.L.; Alfonso-Piérola, A. Current therapy of the patients with MDS: Walking towards personalized therapy. J. Clin. Med. 2021, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Santini, V.; Spiriti, M.A.A.; Giagounidis, A.; Schlag, R.; Radinoff, A.; Gercheva-Kyuchukova, L.; Anagnostopoulos, A.; Oliva, E.N.; Symeonidis, A.; et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-α in anemic patients with low-risk MDS. Leukemia 2018, 32, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). PRISMA Elaboration and Explanation. Available online: http://prisma-statement.org/PRISMAStatement/PRISMAEandE.aspx (accessed on 7 March 2022).
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, version 6.0; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: https://training.cochrane.org/handbook/current (accessed on 7 March 2022).
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the myelodysplastic syndromes. Br. J. Haematol. 1982, 51, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Cochrane. Risk of Bias Assessment in Prognostic Studies. Available online: https://methods.cochrane.org/prognosis/tools (accessed on 11 May 2022).
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef]
- Stein, R.S.; Abels, R.I.; Krantz, S.B. Pharmacologic doses of recombinant human erythropoietin in the treatment of myelodysplastic syndromes. Blood 1991, 78, 1658–1663. [Google Scholar] [CrossRef]
- Remacha, A.F.; Arrizabalaga, B.; Villegas, A.; Manteiga, R.; Calvo, T.; Julia, A.; Fernandez Fuertes, I.; Gonzalez, F.A.; Font, L.; Junca, J.; et al. Erythropoietin plus granulocyte colony-stimulating factor in the treatment of myelodysplastic syndromes. Identification of a subgroup of responders. The Spanish Erythropathology Group. Haematologica 1999, 84, 1058–1064. [Google Scholar]
- Mannone, L.; Gardin, C.; Quarre, M.C.; Bernard, J.F.; Vassilieff, D.; Ades, L.; Park, S.; Vaultier, S.; Hamza, F.; Beyne-rauzy, M.O.; et al. High-dose darbepoetin alpha in the treatment of anaemia of lower risk myelodysplastic syndrome results of a phase II study. Br. J. Haematol. 2006, 133, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Latagliata, R.; Oliva, E.N.; Volpicelli, P.; Carmosino, I.; Breccia, M.; Vincelli, I.; Alati, C.; Napoleone, L.; Vozella, F.; Nobile, F.; et al. Twice-weekly high-dose rHuEpo for the treatment of anemia in patients with low-risk myelodysplastic syndromes. Acta Haematol. 2008, 120, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Isnard, F.; Najman, A.; Jaar, B.; Fenaux, P.; Baillou, C.; Khoury, E.; Labopin, M.; Laporte, J.P.; Woler, M.; Gorin, N.C.; et al. Efficacy of recombinant human erythropoietin in the treatment of refractory anemias without excess of blasts in myelodysplastic syndromes. Leuk. Lymphoma 1994, 12, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Matera, R.; Minervini, M.M.; Checchia-de Ambrosio, C.; Bodenizza, C.; Falcone, A.; Carotenuto, M. Low serum levels of tumor necrosis factor and interleukin-1 beta in myelodysplastic syndromes responsive to recombinant erythropoietin. Haematologica 1994, 79, 265–268. [Google Scholar] [PubMed]
- Stasi, R.; Pagano, A.; Terzoli, E.; Amadori, S. Recombinant human granulocyte-macrophage colony-stimulating factor plus erythropoietin for the treatment of cytopenias in patients with myelodysplastic syndromes. Br. J. Haematol. 1999, 105, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Brunetti, M.; Terzoli, E.; Amadori, S. Sustained response to recombinant human erythropoietin and intermittent all-trans retinoic acid in patients with myelodysplastic syndromes. Blood 2002, 99, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Abruzzese, E.; Lanzetta, G.; Terzoli, E.; Amadori, S. Darbepoetin alfa for the treatment of anemic patients with low- and intermediate-1-risk myelodysplastic syndromes. Ann. Oncol. 2005, 16, 1921–1927. [Google Scholar] [CrossRef]
- Gotlib, J.; Lavori, P.; Quesada, S.; Stein, R.S.; Shahnia, S.; Greenberg, P.L. A Phase II intra-patient dose-escalation trial of weight-based darbepoetin alfa with or without granulocyte-colony stimulating factor in myelodysplastic syndromes. Am. J. Hematol. 2009, 84, 15–20. [Google Scholar] [CrossRef]
- Villegas, A.; Arrizabalaga, B.; Fernández-Lago, C.; Castro, M.; Mayans, J.R.; González-Porras, J.R.; Duarte, R.F.; Remacha, A.F.; Luño, E.; Gasquet, J.A. Darbepoetin alfa for anemia in patients with low or intermediate-1 risk myelodysplastic syndromes and positive predictive factors of response. Curr. Med. Res. Opin. 2011, 27, 951–960. [Google Scholar] [CrossRef]
- Park, S.; Kosmider, O.; Maloisel, F.; Drenou, B.; Chapuis, N.; Lefebvre, T.; Karim, Z.; Puy, H.; Alary, A.S.; Ducamp, S.; et al. Dyserythropoiesis evaluated by the RED score and hepcidin:ferritin ratio predicts response to erythropoietin in lower-risk myelodysplastic syndromes. Haematologica 2019, 104, 497–504. [Google Scholar] [CrossRef]
- Hanamoto, H.; Morita, Y.; Ichikawa, M.; Nannya, Y.; Shibayama, H.; Maeda, Y.; Hata, T.; Miyamoto, T.; Kawabata, H.; Takeuchi, K.; et al. ASXL1 mutations predict a poor response to darbepoetin alfa in anemic patients with low-risk MDS: A multicenter, phase II study. Blood 2020, 136 (Suppl. S1), 28–29. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Alves, R.; Baldeiras, I.; Jorge, J.; Marques, B.; Paiva, A.; Oliveiros, B.; Cortesão, E.; Nascimento Costa, J.M.; Sarmento-Ribeiro, A.B. Oxidative stress parameters can predict the response to erythropoiesis-stimulating agents in myelodysplastic syndrome patients. Front. Cell Dev. Biol. 2021, 9, 701328. [Google Scholar] [CrossRef]
- Hellström-Lindberg, E.; Negrin, R.; Stein, R.; Krantz, S.; Lindberg, G.; Vardiman, J.; Ost, A.; Greenberg, P. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: Proposal for a predictive model. Br. J. Haematol. 1997, 99, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kelaidi, C.; Sapena, R.; Vassilieff, D.; Beyne-Rauzy, O.; Coiteux, V.; Vey, N.; Ravoet, C.; Cheze, S.; Rose, C.; et al. Early introduction of ESA in low risk MDS patients may delay the need for RBC transfusion: A retrospective analysis on 112 patients. Leuk. Res. 2010, 34, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Azzara, A.; Carulli, G.; Galimberti, S.; Barate, C.; Fazzi, R.; Cervetti, G.; Petrini, M. High-dose (40,000 IU twice/week) alpha recombinant human erythropoietin as single agent in low/intermediate risk myelodysplastic syndromes: A retrospective investigation on 133 patients treated in a single institution. Am. J. Hematol. 2011, 86, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Tatarelli, C.; Piccioni, A.L.; Maurillo, L.; Naso, V.; Battistini, R.; D’Andrea, M.; Criscuolo, M.; Nobile, C.; Villiva, N.; Mancini, S.; et al. Recombinant human erythropoietin in very elderly patients with myelodysplastic syndromes: Results from a retrospective study. Ann. Hematol. 2014, 93, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Castelli, R.; Deliliers, G.L.; Colombo, R.; Moreo, G.; Gallipoli, P.; Pantaleo, G. Biosimilar epoetin in elderly patients with low-risk myelodysplastic syndromes improves anemia, quality of life, and brain function. Ann. Hematol. 2014, 93, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Buccisano, F.; Piccioni, A.L.; Nobile, C.; Criscuolo, M.; Niscola, P.; Tatarelli, C.; Fianchi, L.; Villiva, N.; Neri, B.; Carmosino, I.; et al. Real-life use of erythropoiesis-stimulating agents in myelodysplastic syndromes: A "Gruppo Romano Mielodisplasie (GROM)" multicenter study. Ann. Hematol. 2016, 95, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Buckstein, R.; Balleari, E.; Wells, R.; Santini, V.; Sanna, A.; Salvetti, C.; Crisa, E.; Allione, B.; Danise, P.; Finelli, C.; et al. ITACA: A new validated international erythropoietic stimulating agent-response score that further refines the predictive power of previous scoring systems. Am. J. Hematol. 2017, 92, 1037–1046. [Google Scholar] [CrossRef]
- Houston, B.L.; Jayakar, J.; Wells, R.A.; Lenis, M.; Zhang, L.; Zhu, N.; Leitch, H.A.; Nevill, T.J.; Yee, K.W.L.; Leber, B.; et al. A predictive model of response to erythropoietin stimulating agents in myelodysplastic syndrome: From the Canadian MDS patient registry. Ann. Hematol. 2017, 96, 2025–2029. [Google Scholar] [CrossRef]
- Moura, A.T.G.; Duarte, F.B.; Barbosa, M.C.; Santos, T.; Lemes, R.P.G. Prolonged response to recombinant human erythropoietin treatment in patients with myelodysplastic syndrome at a single referral centre in Brazil. Clinics 2019, 74, e771. [Google Scholar] [CrossRef] [PubMed]
- Antelo, G.B.; Coltro, G.; Mangaonkar, A.A.; Lasho, T.; Finke, C.; Carr, R.M.; Gangat, N.; Binder, M.; Al-Kali, A.; Elliott, M.A.; et al. Response to erythropoiesis stimulating agents in patients with WHO-defined myelodysplastic syndrome/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T). Blood 2019, 134 (Suppl. S1), 4182. [Google Scholar] [CrossRef]
- Muniz, J.P.; Yellapragada, S.V.; Rivero, G.A. Evaluating mean corpuscular volume as predictor for erythropoiesis stimulating agent response in elderly patients diagnosed with myelodysplasia. Blood 2019, 134 (Suppl. S1), 5420. [Google Scholar] [CrossRef]
- Balleari, E.; Filiberti, R.A.; Salvetti, C.; Allione, B.; Angelucci, E.; Bruzzone, M.; Calzamiglia, T.; Cavaliere, M.; Cavalleri, M.; Cilloni, D.; et al. Effects of different doses of erythropoietin in patients with myelodysplastic syndromes: A propensity score-matched analysis. Cancer Med. 2019, 8, 7567–7576. [Google Scholar] [CrossRef] [PubMed]
- Rosati, S.; Ansuinelli, M.; Carmosino, I.; Scalzulli, E.; Mohamed, S.; Porrazzo, M.; Bruzzese, A.; Molica, M.; Rizzo, L.; Mariggio, E.; et al. Efficacy of high-doses of alpha-erythropoietin in patients with lower risk myelodysplastic syndromes: A retrospective single center analysis. Haematologica 2019, 104 (Suppl. S2), 108. [Google Scholar]
- Boggio, F.; Del Gobbo, A.; Barella, M.; Croci, G.; Cassin, R.; Reda, G.; Pettine, L.; Bandiera, L.; Bonoldi, E.; Riva, M.; et al. CD34-positive blast count and p53 expression in bone marrow biopsies of patients with low-risk myelodysplastic syndromes: Potential predictive tools of response to erythropoietin stimulating agents. Pathobiology 2021, 88, 242–250. [Google Scholar] [CrossRef]
- Hattakitpanitchakul, S.; Kobbuaklee, S.; Wudhikarn, K.; Polprasert, C. Prediction of response to erythropoiesis stimulating agents in low-risk myelodysplastic syndromes. Asian Pac. J. Cancer Prev. 2021, 22, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Rigolin, G.M.; Porta, M.D.; Bigoni, R.; Cavazzini, F.; Ciccone, M.; Bardi, A.; Cuneo, A.; Castoldi, G. rHuEpo administration in patients with low-risk myelodysplastic syndromes: Evaluation of erythroid precursors’ response by fluorescence in situ hybridization on May-Grunwald-Giemsa-stained bone marrow samples. Br. J. Haematol. 2002, 119, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Westers, T.M.; Alhan, C.; Chamuleau, M.E.; van der Vorst, M.J.; Eeltink, C.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Aberrant immunophenotype of blasts in myelodysplastic syndromes is a clinically relevant biomarker in predicting response to growth factor treatment. Blood 2010, 115, 1779–1784. [Google Scholar] [CrossRef]
- Balleari, E.; Clavio, M.; Arboscello, E.; Bellodi, A.; Bruzzone, A.; Del Corso, L.; Lucchetti, M.V.; Miglino, M.; Passalia, C.; Pierri, I.; et al. Weekly standard doses of rh-EPO are highly effective for the treatment of anemic patients with low-intermediate 1 risk myelodysplastic syndromes. Leuk. Res. 2011, 35, 1472–1476. [Google Scholar] [CrossRef]
- Raimbault, A.; Itzykson, R.; Willems, L.; Rousseau, A.; Chapuis, N.; Mathis, S.; Clauser, S.; Radford-Weiss, I.; Bouscary, D.; Fontenay, M.; et al. The fraction of CD117/c-KIT-expressing erythroid precursors predicts ESA response in low-risk myelodysplastic syndromes. Cytometry B Clin. Cytom. 2019, 96, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.H.; Abels, R.I.; Nelson, R.A.; McCullough, D.M.; Lessin, L. The use of r-HuEpo in the treatment of anaemia related to myelodysplasia (MDS). Br. J. Haematol. 1995, 89, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Brunetti, M.; Terzoli, E.; Abruzzese, E.; Amadori, S. Once-weekly dosing of recombinant human erythropoietin alpha in patients with myelodysplastic syndromes unresponsive to conventional dosing. Ann. Oncol. 2004, 15, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Musto, P.; Lanza, F.; Balleari, E.; Grossi, A.; Falcone, A.; Sanpaolo, G.; Bodenizza, C.; Scalzulli, P.R.; La Sala, A.; Campioni, D.; et al. Darbepoetin alpha for the treatment of anaemia in low-intermediate risk myelodysplastic syndromes. Br. J. Haematol. 2005, 128, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, D.; Darbesio, A.; Giai, V.; Genuardi, M.; Dellacasa, C.M.; Sorasio, R.; Bertini, M.; Boccadoro, M. Efficacy of a combination of human recombinant erythropoietin + 13-cis-retinoic acid and dihydroxylated vitamin D3 to improve moderate to severe anaemia in low/intermediate risk myelodysplastic syndromes. Br. J. Haematol. 2009, 144, 342–349. [Google Scholar] [CrossRef]
- Frisan, E.; Pawlikowska, P.; Pierre-Eugène, C.; Viallon, V.; Gibault, L.; Park, S.; Mayeux, P.; Dreyfus, F.; Porteu, F.; Fontenay, M. p-ERK1/2 is a predictive factor of response to erythropoiesis-stimulating agents in low/int-1 myelodysplastic syndromes. Haematologica 2010, 95, 1964–1968. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Bennett, J.M.; Kantarjian, H.; Pinto, A.; Schiffer, C.A.; Nimer, S.D.; Löwenberg, B.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000, 96, 3671–3674. [Google Scholar] [CrossRef]
- Italian Cooperative Study Group for rHuEPO in Myelodysplastic Syndromes; Ferrini, P.R.; Grossi, A.; Vannicchi, A.M.; Barosi, G.; Guarnone, R.; Piva, N.; Musto, P.; Balleari, E. A randomized double-blind controlled study with subcutaneous recombinant human erythropoietin in patients with low risk myelodysplastic syndromes. Br. J. Haematol. 1998, 103, 1070–1074. [Google Scholar] [PubMed]
- Savona, M.R.; Malcovati, L.; Komrokji, R.; Tiu, R.V.; Mughal, T.I.; Orazi, A.; Kiladjian, J.J.; Padron, E.; Solary, E.; Tibes, R.; et al. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood 2015, 125, 1857–1865. [Google Scholar] [CrossRef]
- Braga Lemos, M.; Rodrigues, S.R.; Schroeder, T.; Kulasekararaj, A.G.; Matos, J.E.; Tang, D. Association between red blood cell transfusion dependence and burden in patients with myelodysplastic syndromes: A systematic literature review and meta-analysis. Eur. J. Haematol. 2021, 107, 3–23. [Google Scholar] [CrossRef]
- Park, S.; Kelaidi, C.; Meunier, M.; Casadevall, N.; Gerds, A.T.; Platzbecker, U. The prognostic value of serum erythropoietin in patients with lower-risk myelodysplastic syndromes: A review of the literature and expert opinion. Ann. Hematol. 2020, 99, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Lindberg, E.; Ahlgren, T.; Beguin, Y.; Carlsson, M.; Carneskog, J.; Dahl, I.M.; Dybedal, I.; Grimfors, G.; Kanter-Lewensohn, L.; Linder, O.; et al. Treatment of anemia in myelodysplastic syndromes with granulocyte colony-stimulating factor plus erythropoietin: Results from a randomized phase II study and long-term follow-up of 71 patients. Blood 1998, 92, 68–75. [Google Scholar] [CrossRef] [PubMed]
| Domain | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adults (≥18 years of age) with LR-MDS as defined by Very Low, Low, Int-1, or Int-risk MDS, according to IPSS [6], WHO [7], FAB [19], or author-defined criteria | Studies not evaluating patients with MDS or evaluating a mixed population with <80% eligible patients according to the PICOS Studies evaluating patients with high-risk MDS Children or adolescents (<18 years of age) |
| Interventions | ESAs | Surgery, radiotherapy, adjuvant, or neoadjuvant chemotherapy |
| Comparators | NA | NA |
| Predictors/ Prognostic Factors | Any studies examining the association between prognostic factors and ESA response, including but not restricted to the following prognostic factors: a
Correlation analyses between prognostic factors and ESA response/failure ESA failure, including but not limited to the following definitions:
| Studies making statements about associations but unsupported by quantitative analyses, such as univariate or multivariate analyses Relevant outcomes not reported Studies evaluating a mixed population, but results not reported separately for the LR-MDS population |
| Study Design | Observational cohort studies (prospective or retrospective) Cross-sectional studies Non-randomized and single-arm designs RCTs (post hoc analysis) SLRs (only to be used for bibliographic searches) | Full-text article not published in English Conference abstract published prior to 2019 Editorial, erratum, trial protocol, guideline, case report, narrative review, etc. In vitro, ex vivo, animal, or pharmacokinetic study, phase 1 trial, etc. |
| Author, Year | Study Design | Country | Data Source | No. of Patients | ESA Intervention/ Outcome |
|---|---|---|---|---|---|
| Stein et al., 1991 [22] | RCT | USA | Clinical trial records | 20 | 24% response a to high-dose rhEPO |
| Isnard et al., 1994 [26] | Single-arm trial | France | Clinical trial records | 20 | 35% response b,c to rhEPO |
| Musto et al., 1994 [27] | Single-arm trial | Italy | Clinical trial records | 26 | 15% response d to rhEPO |
| Rose et al., 1995 [55] | Multicenter, open-label, compassionate, therapeutic trial | USA | Clinical trial records | 116 | 28% response e to rhEPO |
| Hellström-Lindberg et al., 1997 [36] | Retrospective study | NR | NR | 120 | 36% response f to ESAs mixed |
| Stasi et al., 1999 [28] | Single-arm trial | Italy | Clinical trial records | 31 | 34.6% response g to G-CSF + rhEPO |
| Remacha et al., 1999 [23] | Non-randomized trial, phase 4 | Spain | Clinical trial records | 33 | 50% response h to rhEPO ± G-CSFs |
| Stasi et al., 2002 [29] | Single-arm trial | Italy | Clinical trial records | 27 | 48% response i to ATRA + rhEPO |
| Rigolin et al., 2002 [51] | Prospective study | Italy | NR | 13 | 46% response j to rhEPO |
| Stasi et al., 2004 [56] | NR | NR | NR | 48 | 27% response i rhEPO |
| Musto et al., 2005 [57] | NR | NR | NR | 37 | 40.5% response i to DPO-α |
| Stasi et al., 2005 [30] | Single-arm trial | Italy | Medical records of patients enrolled in the clinical trial | 53 | 45% response i to DPO-α |
| Mannone et al., 2006 [24] | Non-randomized trial, phase 2 | France | Eight centers of the Groupe Français des Myélodysplasies | 62 | 71% response i to DPO-α |
| Latagliata et al., 2008 [25] | Non-randomized trial, phase 2 | NR | Medical records of MDS patients from two hematological departments | 60 | 50% response i to rhEPO |
| Gotlib et al., 2009 [31] | Single-arm trial | USA | Medical records at Stanford University School of Medicine and Vanderbilt University | 24 | 67% response i to DPO-α ± G-CSF |
| Ferrero et al., 2009 [58] | NR | Italy | NR | 63 | 65% response i to 13-cis-retinoic acid, dihydroxylated vitamin D3 ± 6-thioguanine + rhEPO |
| Westers et al., 2010 [52] | Prospective study | The Netherlands | Medical records of patients at Vrije Universiteit University Medical Center | 46 | 39% response k to epoetin + G-CSF |
| Park et al., 2010 [37] | Retrospective study | France, Belgium | Medical records at 25 French and Belgian centers of the Groupe Francophone des Myélodysplasies | 112 | 63.1% response k to epoetin-α/β or DPO |
| Frisan et al., 2010 [59] | NR | France | NR | 127 | 57.4% response c,k to epoetin-α/β or DPO-α ± G-CSF |
| Villegas et al., 2011 [32] | Single-arm trial | Spain | Clinical trial records | 44 | 72.7% response i to DPO-α ± filgrastim |
| Azzara et al., 2011 [38] | Retrospective study | Italy | Medical records | 133 | 59% response k to rhEPO |
| Balleari et al., 2011 [53] | Prospective study | Italy | NR | 55 | 65.5% response k to rhEPO |
| Tatarelli et al., 2014 [39] | Retrospective study | Italy | GROM database | 93 | 63.4% response k to epoetin-α/β |
| Castelli et al., 2014 [40] | Retrospective study | NR | NR | 24 | 66.7% response k to biosimilar epoetin-α |
| Buccisano et al., 2016 [41] | Retrospective study | Italy | Medical records of MDS patients recruited in 11 hematological centers (five university hospitals and six community-based hospitals) located in the metropolitan area of Rome, Italy | 543 | 59.5% response c,k to EPO-α/β |
| Buckstein et al., 2017 [42] | Retrospective study | Canada, Italy | MDS-CAN, FISiM, and GROM | 996 | Overall response rate k 59% to EPO or DPO |
| Houston et al., 2017 [43] | Retrospective study | Canada | MDS-CAN registry | 208 | 47% response k to EPO or DPO |
| Park et al., 2019 [33] | Single-arm trial | France | Medical records of lower-risk MDS patients receiving treatment at Assistance Publique-Hôpitaux de Paris through the Unité de Recherche Clinique Paris Descartes Necker Cochin | 70 | 48% response k to epoetin-Z |
| Raimbault et al., 2019 [54] | Prospective study | France | NR | 144 | 75% response k to EPO-α/β/Z or DPO |
| Moura et al., 2019 [44] | Retrospective study | Brazil | Hospital Universitário Walter Cantídeo, Ceará, Brazil | 36 | 80.5% l response k to epoetin-α |
| Antelo et al., 2019 [45] | Retrospective study | NR | Medical records | 47 | 46% response m to EPO-α, DPO, or EPO-α and DPO |
| Muniz et al., 2019 [46] | Retrospective study | USA | Michael E. DeBakey Houston VA Medical Center | 81 | 38.2% response n to ESAs |
| Balleari et al., 2019 [47] | Retrospective study | Italy | Nationwide dataset of FISiM (FISiM-Onlus) | 445 | 52.6% achieved HI-E k to rhEPO |
| Rosati et al., 2019 [48] | Retrospective study | Italy | NR | 193 | 53.3% response k to EPO-α |
| Hanamoto et al., 2020 [34] | Single-arm trial | NR | Clinical trial records (multicenter) | 85 | Overall response rate k 70.9% to DPO-α |
| Boggio et al., 2021 [49] | Retrospective study | Italy | Hospital database | 96 | 67.7% response c,k to EPO-α or DPO |
| Gonçalves et al., 2021 [35] | Single-arm trial | Portugal | Clinical trial records | 66 | 55.5% l response h to ESA |
| Hattakitpanitchakul et al., 2021 [50] | Retrospective study | Thailand | Medical records at Chulalongkorn Memorial Hospital | 47 | 46.8% response c,k to ESAs |
| Author, Year | Patient Population | N | Age Median [Range] or Mean (SD) | Male, n (%) | FAB Subtype, n (%) | MDS WHO Subtype, n (%) | IPSS Risk Group, n (%) | IPSS-R Risk Group, n (%) | Karyotype, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Stein et al., 1991 [22] a | Patients with MDS receiving rhEPO | 20 | 64 [42–83] | 4/8 (50) | NR | NA | NA | NA | Normal: 3 (15) Abnormal: 1 (5) Not obtained: 4 (20) |
| Patients with MDS receiving placebo | 68 [34–87] | 5/12 (42) | NR | NA | NA | NA | Normal: 2 (10) Abnormal: 7 (35) Not obtained: 3 (15) | ||
| Isnard et al., 1994 [26] | Patients with MDS | 20 | NR | 13 (NR) | RARS: 11 (NR) RA: 9 (NR) | NA | NA | NA | Normal: 9 (NR) Abnormal: 5 (NR) |
| Musto et al., 1994 [27] | Patients with MDS | 26 | NR | NR | RAEB: 5 (19.2) RARS: 9 (34.6) RA: 17 (65.3) | NA | NA | NA | NR |
| Rose et al., 1995 [55] | Patients with MDS | 100 | 70.3 [24–95] | 66 (66) | RA: 44 (44) RARS: 40 (40) RAEB: 8 (8) RAEB-t: 2 (2) CMML: 1 (1) Not specified: 5 (5) | NA | NA | NA | NR |
| Hellström-Lindberg et al., 1997 [36] a | MDS patients | 98 | 70 [±11] | 61 (62) | RA: 30 (30.6) RARS: 31 (31.6) RAEB-1: 32 (32.6) | NA | NA | NA | Normal: 50 (51) Single anomalies: 25 (25.5) Two abnormalities: 6 (6.1) Complex karyotype (≥3 anomalies): 8 (8.1) |
| Stasi et al., 1999 [28] a | MDS patients diagnosed by FAB criteria | 31 | 67 [50–80] | 13 (42) | RA: 21 (67.7) RARS: 4 (12.9) RAEB-1: 6 (19.3) | NA | Low: 7 (22.5) Int-1: 15 (48.3) Int-2: 1 (3.2) | NR | Good: 15 (48.3) Int: 5 (16.1) Poor: 3 (9.6) |
| Remacha et al., 1999 [23] | Patients with MDS, having RA or RARS status | 32 | 68 [41–89] b | 22 (69) | RA: 9 (28.1) RARS: 23 (71.8) | NR | NR | NR | NR |
| Stasi et al., 2002 [29] a | Low- or Int-risk MDS according to IPSS criteria | 27 | 68 [52–78] | 13 (48) | RA: 19 (70.3) RARS: 3 (11.1) RAEB-1: 5 (18.5) | NA | Low: 5 (18.5) Int-1: 13 (48.1) Int-2: 1 (3.7) | NR | Good: 12 (44.4) Int: 6 (22.2) Poor: 1 (3.7) |
| Rigolin et al., 2002 [51] | MDS patients | 13 | NR | 8 (61.54) | RARS: 2 (15) RAEB: 5 (33) RA: 6 (46) | NR | NR | NR | NR |
| Stasi et al., 2004 [56] a | Low- and Int-risk MDS patients | 48 | 70 [53–81] | 26 (54.17) | NR | FAB/WHO subtype RA: 36 (75) RARS: 5 (10.4) RAEB-1: 7 (14.5) | Low: 32 (66.6) Int-1: 16 (33.3) | NR | Good: 40 (83.3) Int: 8 (16.6) |
| Musto et al., 2005 [57] a | Low-to-Int-risk MDS patients | 37 | 63.1 [39–84] | 25 (67.5) | NR | RA: 11 (29.7) RARS: 5 (13.5) RCMD-RS: 2 (5) RAEB-1: 7 (18.9) MDS with del(5q): 1 (2.7) RCMD: 12 (32.4) | Low: 16 (43.2) Int-1: 17 (45.9) | NR | NR |
| Stasi et al., 2005 [30] a | Patients with Low- and Int-1-risk MDS according to IPSS | 53 | 70 [NR] | 30 (56.6) | NR | RA: 31 (58.4) RCMD: 10 (18.8) RAEB-1: 8 (15) RARS: 3 (5.6) RCMD-RS: 1 (1.8) | Low: 29 (54.7) Int-1: 24 (45.2) | NR | Good: 47 (88.6) Int: 6 (11.3) |
| Mannone et al., 2006 [24] | Patients with anemia and MDS | 62 | 78 [45–91] | 32 (51.6) | RA: 22 (35) RAEB: 18 (29) RARS: 20 (32) CMML: 2 (3) | RA: 11 (17.7) RCMD: 8 (12.9) RARS: 18 (29) RCMD-RS: 2 (3.2) RAEB-1: 18 (29) MDS with del(5q): 3 (4.8) CMML-1: 2 (3.2) | Low: 16 (25.8) Int-1: 26 (41.9) | NR | Favorable: 41 (66.1) Int: 7 (11.2) Poor: 2 (3.2) |
| Latagliata et al., 2008 [25] a | Previously untreated MDS Low- and Int-1-risk patients | 60 | 73.1 [63.2–80.4] | 26 (43.3) | NR | RA: 19 (31.6) RARS: 3 (5) RCMD: 21 (35) RAEB-1: 11 (18.3) MDS with del(5q): 6 (10) | Low: 18 (30) Int-1: 17 (28.3) | NR | NR |
| Gotlib et al., 2009 [31] a | Low- or Int-1-risk MDS patients diagnosed according to FAB and WHO criteria | 24 | 68 [31–84] | 18 (75) | RA: 10 (41.6) RARS: 9 (37.5) CMML: 2 (8.3) RAEB: 3 (12.5) | RAEB-1: 3 (12.5) RCMD: 8 (33.3) RCMD-RS: 9 (37.5) MDS with del(5q): 2 (8.3) CMML-1: 2 (8.3) | Low: 12 (50) Int-1: 10 (41.6) Int-2: 2 (8.3) | NR | NR |
| Ferrero et al., 2009 [58] a | MDS patients. All patients unsuitable for allogeneic SCT at diagnosis because of age, comorbidities, or lack of an HLA-compatible sibling | 63 | 75 [43–90] | 38 (60.3) | NR | RA: 16 (25.3) RARS: 8 (12.6) RCMD: 18 (28.5) MDS with del(5q): 2 (3.1) RAEB-1: 16 (25.3) | Low: 12 (19) Int-1: 29 (46) | NR | Favorable: 37 (58.7) Int: 5 (7.9) Unfavorable: 2 (3.1) Undetermined: 19 (30.1) |
| Westers et al., 2010 [52] a | MDS patients diagnosed by WHO 2001 classification | 46 | 69 [40–90] | NR | NR | RARS: 18 (39.1) RCMD-RS: 26 (56.5) RAEB-1: 1 (2.1) MDS-U: 1 (2.1) | Low: 25 (54.3) Int-1: 21 (45.6) | NR | NR |
| Park et al., 2010 [37] a | Patients with de novo MDS anemia (Hb < 10 g/dL) | 112 | 75 [41–91] | 62 (55) | NR | RA: 21 (18.7) RAEB-1: 22 (19.6) RARS: 34 (30.3) RCMD: 19 (16.9) RCMD-RS: 16 (14.2) | Low: 39 (34.8) Int-1: 56 (50) | NR | Favorable: 80 (71.4) Int: 15 (13.3) |
| Frisan et al., 2010 [59] a | MDS patients diagnosed according to WHO classification, Low, or Int-1 IPSS risk | 127 | 74 [69–81] | NR | NR | RA: 37 (29.1) RCMD: 18 (14.1) RAEB-1: 36 (28.3) RARS: 26 (20.4) RCMD-RS: 10 (7.8) | Low: 67 (52.7) Int-1: 50 (39.3) | NR | Good: 101 (79.5) Int: 14 (11) Poor: 2 (1.5) |
| Villegas et al., 2011 [32] a | Patients with Low- or Int-1-risk MDS | 44 | 74.5 [±10.6] | 24 (54.5) | RA: 14 (31.8) RARS: 27 (61.3) RAEB-1: 3 (6.8) | NR | Low: 34 (77.2) Int-1: 10 (22.7) | NR | NR |
| Azzara et al., 2011 [38] | Patients affected by Low- and Int-grade MDS | 133 | 77 [±9] | 70 (52) | NR | RA: 73 (55) RARS: 37 (28) RCMD: 8 (6) RCMD-RS: 3 (2) MDS with del(5q): 8 (6) RAEB-1: 4 (3) | Low: 83/109 (76) Int-1: 22/109 (20) Int-2: 4/109 (4) | Very Low: 73/109 (67) Low: 21/109 (19) Int: 15/109 (14) | Undetermined: 24/113 (18) Available: 109/133 (82) Normal: 82/109 (75) Abnormal: 27/109 (25) Favorable: 13/27 (48) Int: 11/27 (41) Unfavorable: 3/27 (11) |
| Balleari et al., 2011 [53] | Lower-risk MDS patients, defined by IPSS risk score ≤ 1 and no previous treatment with ESA | 55 | 77.5 [60–92] | 29 (52.7) | NR | RA: NR (32) RARS: NR (3) RCMD: NR (15) RAEB-1: NR (1) MDS with del(5q): NR (4) | NR | NR | Favorable: 51 (92.7) Int: 4 (7.3) Unfavorable: 0 (0) |
| Tatarelli et al., 2014 [39] | MDS patients ≥ 80 years of age | 93 | 82.7 [80–99.1] | 59 (63) | NR | RA: 15 (16.1) RARS: 2 (2.1) RCMD: 41 (44.1) RCMD-S: 4 (4.3) RAEB-1: 17 (18.3) RAEB-2: 9 (9.7) MDS with del(5q): 5 (5.4) | Low: 28 (45.9) Int-1: 26 (42.6) Int-2: 6 (9.8) | NR | Favorable: 52 (85.2) Int: 6 (9.8) Unfavorable: 3 (4.9) |
| Castelli et al., 2014 [40] a | Elderly patients (≥65 years of age), newly diagnosed MDS, IPSS score < 1.5 with ≥1 cytopenia. EPO fixed dose | 24 | 72 [65–84] | 14 (58.3) | NR | RA: 15 (62.5) RCMD-RS: 5 (20.8) RAEB-1: 1 (4.1) RARS: 3 (12.5) | Low: 11 (45.8) Int: 13 (54.1) | NR | Normal: 13 (54.1) Monosomy of chromosome 7: 2 (8.3) del(20q): 8 (33.3) Deletion Y chromosome: 1 (4.1) |
| Buccisano et al., 2016 [41] | MDS patients diagnosed according to WHO 2008 classification receiving ESAs at any time during disease course | 543 | 74.2 [67.8–79.5] | 304 (55.9) | NR | RA: 103 (18.9) RARS: 16 (2.9) RCMD: 219 (40.4) RCMD-RS: 17 (3.1) RAEB-1: 105 (19.3) RAEB-2: 44 (8.1) MDS with del(5q): 34 (6.3) MDS-U: 2 (0.4) | Low: 195/425 (45.9) Int-1: 184/425 (43.3) Int-2: 41/425 (9.6) | NR | NR |
| Buckstein et al., 2017 [42] | MDS patients diagnosed as per WHO 2008 classification, risk-stratified according to both IPSS and IPSS-R | 996 | 76 [69–81] | 576 (58) | NR | NR | Low: 473 (52) Int-1: 371 (41) Int-2: 62 (7) | Very Low: 176 (22) Low: 411 (52) Int: 127 (16) | Good: 735 (83) Int: 105 (12) Poor: 43 (5) |
| Houston et al., 2017 [43] | ESA-treated patients enrolled within a prospective national MDS database | 208 | 75 [67–81] | NR (61) | NR | NR | Low: NR (49.4) Int-1: NR (44.5) Int-2: NR (6.1) | Very Low: NR (18.3) Low: NR (51.2) Int: NR (23.2) | NR |
| Park et al., 2019 [33] | Lower-risk MDS patients | 70 | 78 [57–93] | 31 (44) | NR | RCMD: 22 (31.5) RARS: 14 (20) RCUD: 19 (27) NR: 4 (6) MDS with del(5q): 2 (3) MDS-U: 3 (4) CMML: 6 (8.5) | Low: 43 (61) Int: 27 (39) | Very Low: 13 (19) Low: 47 (67) Int: 9 (13) | NR |
| Raimbault et al., 2019 [54] | Lower-risk MDS patients | 66 | 78 [71–85] | 38 (58) | NR | MDS-SLD: 11 (16.7) MDS-RS-SLD: 6 (9.1) MDS-MLD/ MDS-RS-MLD: 36 (54.6) MDS-EB-1: 7 (10.6) MDS-EB-2: 0 (0) MDS with del(5q): 4 (6.1) CMML: 2 (3) | NR | Very Low: 16 (26.7) Low: 32 (53.3) Int: 9 (15) Mixed (<20% higher-risk patients): Very High: 0 (0) High: 3 (5) | NR |
| Moura et al., 2019 [44] a | Adult patients diagnosed with MDS as per minimum criteria established at 2006 Vienna Conference on MDS | 36 | 75 [45–95] | 16 (44.5) | NR | MDS-SLD: 5 (13.8) MDS-RS: 8 (22.2) MDS-MLD: 14 (38.8) MDS-EB-1: 1 (2.7) MDS-EB-2: 2 (5.5) MDS with del(5q): 4 (11.1) | Low: 18 (50) Int-1: 14 (38.8) Int-2: 1 (2.7) | Very Low: 10 (27.7) Low: 16 (44.4) Int: 5 (13.8) | Normal: 28 (77.77) Altered: 8 (22.2) Good: 32 (88.9) Int: 2 (5.55) Poor and Very Poor: 2 (5.55) |
| Antelo et al., 2019 [45] | Patients with 2016 WHO-defined MDS/MPN-RS-T | 47 | 73 [52–93] | NR (46) | NR | MDS/MPN-RS-T: 47 (100) | NR | NR | NR |
| Muniz et al., 2019 [46] | Low-risk MDS patients | 81 | NR | NR | NR | NR | Low: 81 (100) | NR | NR |
| Balleari et al., 2019 [47] | MDS patients, standard dose | 445 | 75 [39–98] | 179/341 (52.5) | NR | MDS with del(5q): 20/341 (5.9) RA: 132/341 (38.7) RARS: 38/341 (11.1) RCMD: 102/341 (29.9) RAEB-1: 33/341 (9.7) RAEB-2: 12/341 (3.5) | Low: 205/341 (60.1) Int-1: 112/341 (32.8) Int-2: 22/341 (6.5) | Very Low: 74/341 (21.7) Low: 162/341 (47.5) Int: 68/341 (19.9) | NR |
| MDS patients, high dose | 75 [30–96] | 77/104 (74.0) | NR | MDS with del(5q): 4/104 (3.9) RA: 30/104 (28.8) RARS: 17/104 (16.5) RCMD: 32/104 (31.1) RAEB-1: 15/104 (14.6) RAEB-2: 3/104 (2.9) | Low: 46/104 (44.2) Int-1: 52/104 (50.0) Int-2: 6/104 (5.8) | Very Low: 22/104 (21.2) Low: 39/104 (37.5) Int: 30/104 (28.8) | NR | ||
| Rosati et al., 2019 [48] | MDS patients | 193 | 74.9 [68.4–81] | 94 (48.7) | NR | MDS-SLD: 30 (15.5) MDS-RS-SLD: 5 (2.6) MDS-MLD: 71 (36.8) MDS-RS-MLD: 19 (9.8) MDS-EB-1: 25 (12.9) MDS-EB-2: 15 (7.8) MDS with del(5q): 24 (12.4) | Low: 42 (21.8) Int-1: 91 (47.1) Int-2: 15 (7.8) | Very Low: 23 (12) Low: 79 (41) Int: 26 (13.5) | NR |
| Hanamoto et al., 2020 [34] | DPO-α-naive, low-risk MDS (IPSS Low- or Int-1-risk) patients with anemia | 79 | 77.0 [29–90] | 52 (65.8) | NR | NR | Low: 27 (36.7) Int-1: 50 (63.3) | NR | NR |
| Boggio et al., 2021 [49] | MDS patients on EPO-α 20,000–80,000 IU/week or darbepoetin 150–300 μg/week | 96 | NR | NR | NR | NR | NR | NR | NR |
| Gonçalves et al., 2021 [35] a | MDS patients diagnosed according to WHO 2016 classification of myeloid neoplasms | 44 | 79 [47–87] | 18 (40.9) | NR | MDS-SLD: 4 (9.1) MDS-RS: 10 (22.7) MDS-MLD: 30 (68.2) MDS-EB: 0 (0) | NR | Mixed (<20% higher-risk patients): 32 (72.7) | Good: ESA-treated MDS: 22 (50) Int: ESA-treated MDS: 10 (22.7) Poor: ESA-treated MDS: 0 |
| Hattakitpanitchakul et al., 2021 [50] | Low-risk MDS (IPSS-R score ≤ 3.5) | 47 | NR | 21 (44.7) | NR | MDS-MLD: 27 (57.5) MDS-SLD: 18 (38.3) MDS-RS-SLD: 1 (2.1) MDS-EB-1: 1 (2.1) | NR | NR | NR |
| Author, Year | Prognostic Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| Age a | Bone Marrow Blasts b | Ferritin Level c | Hb Level d | IPSS Risk Status e | Karyotype Status f | Serum EPO Level g | Transfusion Dependence/Independence h | |
| Latagliata et al., 2008 [25] i | ✕ | NR | ✕ | ✓ | NR | NR | ✓ | ✓ |
| Westers et al., 2010 [52] j | NR | NR | NR | NR | NR | NR | ✓ | NR |
| Park et al., 2010 [37] i | ✕ | ✕ | ✕ | ✓ | ✕ | ✕ | ✓ | NR |
| Tatarelli et al., 2014 [39]j | NR | NR | ✓ | ✓ | NR | NR | NR | NR |
| Buccisano et al., 2016 [41] j | NR | NR | NR | NR | NR | NR | ✓ | ✓ |
| Buckstein et al., 2017 [42] j | NR | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Houston et al., 2017 [43] j | ✕ | ✓ | ✕ | ✓ | ✓ | NR | ✓ | ✓ |
| Park et al., 2019 [33] j | NR | NR | NR | NR | ✕ | NR | NR | NR |
| Raimbault et al., 2019 [54] j | NR | NR | NR | NR | NR | NR | NR | ✓ |
| Balleari et al., 2019 [47] j | NR | NR | NR | NR | NR | NR | ✕ | NR |
| Rosati et al., 2019 [48] j | NR | NR | NR | NR | NR | NR | ✓ | NR |
| Author, Year | Prognostic Factor | |||||||
|---|---|---|---|---|---|---|---|---|
| Age a | Bone Marrow Blasts b | Ferritin Level c | Hb Level d | IPSS Risk Status e | Karyotype Status f | Serum EPO Level g | Transfusion Dependence/Independence h | |
| Stein et al., 1991 [22] i | ✕ | NR | NR | NR | NR | NR | ✕ | NR |
| Isnard et al., 1994 [26] j | ✕ | ✕ | ✕ | ✓ | NR | ✕ | ✓ | ✕ |
| Musto et al., 1994 [27] k | NR | ✓ | NR | NR | NR | NR | ✓ | ✓ |
| Rose et al., 1995 [55] l | NR | NR | NR | NR | NR | NR | ✓ | NR |
| Hellström-Lindberg et al., 1997 [36] m | ✓ | ✕ | NR | ✓ | NR | NR | ✓ | NR |
| Stasi et al., 1999 [28] n | ✕ | NR | ✕ | NR | NR | NR | ✕ | NR |
| Stasi et al., 2002 [29] o | ✕ | NR | NR | ✕ | NR | NR | ✕ | NR |
| Rigolin et al., 2002 [51] p | NR | NR | NR | NR | NR | NR | ✓ | ✕ |
| Stasi et al., 2004 [56] o | ✕ | NR | NR | ✕ | NR | NR | ✕ | NR |
| Stasi et al., 2005 [30] o | ✕ | NR | NR | ✕ | ✕ | NR | ✓ | NR |
| Mannone et al., 2006 [24] o | NR | NR | NR | NR | ✕ | ✕ | ✓ | NR |
| Gotlib et al., 2009 [31] o | ✕ | NR | NR | NR | ✕ | NR | ✕ | NR |
| Ferrero et al., 2009 [50,58] o | NR | NR | NR | NR | ✕ | NR | ✕ | ✕ |
| Westers et al., 2010 [52] q | ✕ | ✕ | NR | ✓ | ✕ | NR | NR | NR |
| Frisan et al., 2010 [59] q | ✕ | ✕ | NR | ✕ | ✕ | ✕ | ✓ | ✓ |
| Azzara et al., 2011 [38] q | NR | NR | NR | NR | NR | NR | ✓ | NR |
| Balleari et al., 2011 [53] q | ✕ | NR | NR | NR | ✕ | NR | ✓ | NR |
| Tatarelli et al., 2014 [39] q | NR | NR | ✓ | ✓ | NR | NR | NR | ✓ |
| Castelli et al., 2014 [40] q | NR | NR | NR | NR | NR | NR | ✓ | NR |
| Park et al., 2019 [33] q | NR | NR | ✕ | ✕ | NR | NR | ✓ | NR |
| Raimbault et al., 2019 [54] q | NR | NR | NR | NR | NR | NR | NR | ✓ |
| Moura et al., 2019 [44] q | ✕ | ✕ | NR | NR | ✓ | ✓ | NR | ✓ |
| Antelo et al., 2019 [45] r | ✕ | ✕ | NR | ✕ | ✕ | ✕ | ✓ | NR |
| Muniz et al., 2019 [46] s | ✕ | ✕ | ✕ | ✕ | NR | NR | ✕ | ✕ |
| Balleari et al., 2019 [47] q | ✕ | ✕ | ✕ | NR | NR | NR | ✓ | ✓ |
| Rosati et al., 2019 [48] q | ✕ | NR | ✓ | ✓ | ✓ | NR | ✓ | NR |
| Boggio et al., 2021 [49] q | NR | ✕ | NR | NR | ✓ | NR | NR | ✕ |
| Hattakitpanitchakul et al., 2021 [50] o | NR | NR | ✕ | ✕ | NR | NR | ✓ | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccia, R.; Xiao, H.; von Wilamowitz-Moellendorff, C.; Raorane, R.; Deshpande, S.; Klijn, S.L.; Yucel, A. A Systematic Literature Review of Predictors of Erythropoiesis-Stimulating Agent Failure in Lower-Risk Myelodysplastic Syndromes. J. Clin. Med. 2024, 13, 2702. https://doi.org/10.3390/jcm13092702
Boccia R, Xiao H, von Wilamowitz-Moellendorff C, Raorane R, Deshpande S, Klijn SL, Yucel A. A Systematic Literature Review of Predictors of Erythropoiesis-Stimulating Agent Failure in Lower-Risk Myelodysplastic Syndromes. Journal of Clinical Medicine. 2024; 13(9):2702. https://doi.org/10.3390/jcm13092702
Chicago/Turabian StyleBoccia, Ralph, Hong Xiao, Caroline von Wilamowitz-Moellendorff, Renuka Raorane, Sohan Deshpande, Sven L. Klijn, and Aylin Yucel. 2024. "A Systematic Literature Review of Predictors of Erythropoiesis-Stimulating Agent Failure in Lower-Risk Myelodysplastic Syndromes" Journal of Clinical Medicine 13, no. 9: 2702. https://doi.org/10.3390/jcm13092702
APA StyleBoccia, R., Xiao, H., von Wilamowitz-Moellendorff, C., Raorane, R., Deshpande, S., Klijn, S. L., & Yucel, A. (2024). A Systematic Literature Review of Predictors of Erythropoiesis-Stimulating Agent Failure in Lower-Risk Myelodysplastic Syndromes. Journal of Clinical Medicine, 13(9), 2702. https://doi.org/10.3390/jcm13092702






