Treatment and Prevention of Bone Metastases from Breast Cancer: A Comprehensive Review of Evidence for Clinical Practice
Abstract
:1. Introduction
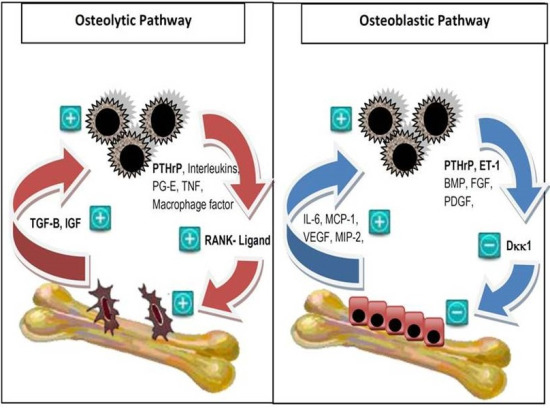
2. Pathophysiology of Bone Metastases
3. Treatment of Bone Metastases from Breast Cancer
3.1. Integration of Local and Systemic Therapy

3.2. Prevention of Skeletal-Related Events
3.2.1. Bisphosphonates
| Class | Simple bisphosphonate | Nitrogen-containing bisphosphonate (N-BP) | ||||
|---|---|---|---|---|---|---|
| Structure |  |  |  |  |  |  |
| Generic Name | Etidronate | Clodronate | Pamidronate | Alendronate | Ibandronate | Zoledronic acid |
| Product Name | Didronel® | Bonefos® | Aredia® | Fosamax® | Bondronat® | Zometa® |
| Relative Potency | 1 | 10 | 100 | 1000 | 10,000 | 100,000 |
| Possible dosing in MBC | Not indicated | PO 1600–3200 mg daily in single/divided dose | IV 90 mg once every 3–4 weeks | Not indicated | PO 50 mg daily IV 6 mg monthly | IV 4 mg once every 3–4 weeks |

| When to start? | Which bisphosphonate? | When to stop? | |
|---|---|---|---|
| ASCO Guidelines 2011 [30] | Breast cancer + radiographic evidence of bone destruction:
|
| Until evidence of substantial decline in patient’s general performance status |
| International Expert Panel Guidelines 2008 [32] | Breast cancer + first sign of radiographic evidence of bone metastases, even if patient is asymptomatic |
Nitrogen-Bisphosphonate
| Continue beyond 2 years but always based on individual risk assessment; should not discontinue treatment once SRE occurs |
3.2.2. Denosumab

- Zoledronic acid is the most potent and effective bisphosphonate in preventing SREs. Standard dose is given IV 4 mg every 3–4 weeks for 2 years and to continue if performance status remains adequate;
- Denosumab given 120 mg SC every 4 weeks, has superior efficacy over zoledronic acid in preventing SREs;
- Calcium and vitamin D supplementation could prevent treatment related hypocalcemia;
- While ONJ is rare at 2% or less, invasive dental procedures should be avoided during bisphosphonate or denosumab therapy;
- Bisphosphonates do not improve survival in women with metastatic breast cancer.
3.3. Bone Pain and Quality of Life
3.3.1. Bisphosphonates and Denosumab
3.3.2. External Beam Radiotherapy
3.3.3. Radiopharmaceuticals
3.4. Spinal Cord Compression
3.5. Systemic Endocrine and Chemotherapy
3.6. Novel Agents and Future Directions
- Bisphosphonates and denosumab improve pain in women with bone metastases from breast cancer;
- Bisphosphonates may improve quality of life, as was demonstrated with IV ibandronate;
- 8 Gy single fraction external beam radiotherapy is an effective means of palliation for bone pain;
- Combined surgery and radiotherapy for spinal cord compression is superior to radiotherapy alone in terms of functional outcomes;
- Optimal treatment of bone metastases involves integration of bone-targeted agents with local and systemic therapy and supportive care through a multidisciplinary team.
4. Prevention of Bone Metastases in Advanced Breast Cancer without Skeletal Involvement
- Current evidence do not support the use of bisphosphonates to prevent bone metastases in women with advanced breast cancer without bone metastasis.
5. Prevention of Bone Metastases in Early Breast Cancer
5.1. Preclinical and Translational Evidence
5.2. Adjuvant Bisphosphonate Trials
5.2.1. Oral Clodronate and Ibandronate
5.2.2. Zoledronic Acid
5.2.3. Meta-Analyses
5.3. Future Directions
5.3.1. Individual Patient Data Meta-Analysis
5.3.2. Denosumab
5.3.3. Biomarkers
- Current evidence do not support the routine use of adjuvant bisphosphonates in unselected women with early breast cancer;
- The incidence of ONJ in the adjuvant setting is very rare, in the order of 0.52%;
- Adjuvant bisphosphonates may provide survival benefit in the subgroup of women with low estrogen bone microenvironment, either through natural menopause or ovarian suppression;
- Individual patient data meta-analysis will be required to definitively address this question.
6. Conclusions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA: Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef]
- Coleman, R.E. Adjuvant bisphosphonates in breast cancer: Are we witnessing the emergence of a new therapeutic strategy? Eur. J. Cancer 2009, 45, 1909–1915. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243–6249. [Google Scholar] [CrossRef]
- Colleoni, M.; O’Neill, A.; Goldhirsch, A.; Gelber, R.D.; Bonetti, M.; Thurlimann, B.; Price, K.N.; Castiglione-Gertsch, M.; Coates, A.S.; Lindtner, J.; et al. Identifying breast cancer patients at high risk for bone metastases. J. Clin. Oncol. 2000, 18, 3925–3935. [Google Scholar]
- Wei, B.; Wang, J.; Bourne, P.; Yang, Q.; Hicks, D.; Bu, H.; Tang, P. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum. Pathol. 2008, 39, 1809–1815. [Google Scholar] [CrossRef]
- Dawood, S.; Broglio, K.; Gonzalez-Angulo, A.M.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J. Clin. Oncol. 2008, 26, 4891–4898. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef]
- Briasoulis, E.; Karavasilis, V.; Kostadima, L.; Ignatiadis, M.; Fountzilas, G.; Pavlidis, N. Metastatic breast carcinoma confined to bone: Portrait of a clinical entity. Cancer 2004, 101, 1524–1528. [Google Scholar] [CrossRef]
- Domchek, S.M.; Younger, J.; Finkelstein, D.M.; Seiden, M.V. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer 2000, 89, 363–368. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef]
- Harvey, H.A. Issues concerning the role of chemotherapy and hormonal therapy of bone metastases from breast carcinoma. Cancer 1997, 80, 1646–1651. [Google Scholar]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Wong, M.H.; Pavlakis, N. Optimal management of bone metastases in breast cancer patients. Breast Cancer Targets Ther. 2011, 3, 35–60. [Google Scholar] [CrossRef]
- Guise, T. Examining the metastatic niche: Targeting the microenvironment. Semin. Oncol. 2010, 37, 2–14. [Google Scholar] [CrossRef]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12, 6213–6216. [Google Scholar] [CrossRef]
- Clines, G.A.; Guise, T.A. Molecular mechanisms and treatment of bone metastasis. Expert Rev. Mol. Med. 2008, 10. [Google Scholar] [CrossRef]
- Body, J.J. Breast cancer: Bisphosphonate therapy for metastatic bone disease. Clin. Cancer Res. 2006, 12, 6258–6263. [Google Scholar] [CrossRef]
- Stewart, A.F. Clinical practice. Hypercalcemia associated with cancer. N. Engl. J. Med. 2005, 352, 373–379. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Thompson, K.; Gordon, S.; Rogers, M.J. Molecular mechanisms of action of bisphosphonates: Current status. Clin. Cancer Res. 2006, 12, 6222–6230. [Google Scholar] [CrossRef]
- Wong, M.H.; Stockler, M.R.; Pavlakis, N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2012, 2. [Google Scholar] [CrossRef]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.; Coleman, R.E.; Reitsma, D.J.; et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001, 7, 377–387. [Google Scholar]
- Rosen, L.S.; Gordon, D.H.; Dugan, W., Jr.; Major, P.; Eisenberg, P.D.; Provencher, L.; Kaminski, M.; Simeone, J.; Seaman, J.; Chen, B.L.; et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004, 100, 36–43. [Google Scholar] [CrossRef]
- Barrett-Lee, P.J.; Casbard, A.; Abraham, J.; Grieve, R.; Wheatley, D.; Simmons, P.; Coleman, R.; Hood, K.; Griffiths, G.; Murray, N. Zoledronate versus ibandronate comparative evaluation (ZICE) trial—First results of a UK NCRI 1405 patient phase III trial comparing oral ibandronate versus intravenous zoledronate in the treatment of breast cancer patients with bone metastases. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Kaminski, M.; Rosen, L.; Gordon, D.; Zheng, M.; Hei, Y.J. Zoledronic acid versus pamidronate in patients with breast cancer and multiple myeloma who are at high risk for skeletal complications. J. Clin. Oncol. 2004, 22, 857. [Google Scholar]
- Van Poznak, C.H.; Temin, S.; Yee, G.C.; Janjan, N.A.; Barlow, W.E.; Biermann, J.S.; Bosserman, L.D.; Geoghegan, C.; Hillner, B.E.; Theriault, R.L.; et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J. Clin. Oncol. 2011, 29, 1221–1227. [Google Scholar] [CrossRef]
- National Breast and Ovarian Cancer Centre. Recommendations for Use of Bisphosphonates for Advanced Breast Cancer; Cancer Australia: Surry Hills, NSW, Australia, 2011. [Google Scholar]
- Aapro, M.; Abrahamsson, P.A.; Body, J.J.; Coleman, R.E.; Colomer, R.; Costa, L.; Crinò, L.; Dirix, L.; Gnant, M.; Gralow, J.; et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann. Oncol. 2008, 19, 420–432. [Google Scholar]
- Amadori, D.; Aglietta, M.; Alessi, B.; Gianni, L.; Ibrahim, T.; Farina, G.; Gaion, F.; Bertoldo, F.; Santini, D.; Rondena, R.; et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): A phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013, 14, 663–670. [Google Scholar] [CrossRef]
- Kohno, N.; Aogi, K.; Minami, H.; Nakamura, S.; Asaga, T.; Iino, Y.; Watanabe, T.; Goessl, C.; Ohashi, Y.; Takashima, S. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: A randomized, placebo-controlled trial. J. Clin. Oncol. 2005, 23, 3314–3321. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef]
- Borromeo, G.L.; Tsao, C.E.; Darby, I.B.; Ebeling, P.R. A review of the clinical implications of bisphosphonates in dentistry. Aust. Dent. J. 2011, 56, 2–9. [Google Scholar]
- Lipton, A. Denosumab in breast cancer. Curr. Oncol. Rep. 2011, 13, 1–4. [Google Scholar] [CrossRef]
- Fizazi, K.; Lipton, A.; Mariette, X.; Body, J.J.; Rahim, Y.; Gralow, J.R.; Gao, G.; Wu, L.; Sohn, W.; Jun, S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J. Clin. Oncol. 2009, 27, 1564–1571. [Google Scholar] [CrossRef]
- Stopeck, A.; Fallowfield, L.; Patrick, D.; Cleeland, C.S.; de Boer, R.H.; Steger, G.G.; Qian, Y.; Jiang, Q.; Dansey, R.D.; Chung, K. Effects of denosumab versus zoledronic acid on pain in patients with metastatic breast cancer: Results from a phase III clinical trial. J. Clin. Oncol. 2010, 28, 1024. [Google Scholar]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Goblirsch, M.J.; Zwolak, P.P.; Clohisy, D.R. Biology of bone cancer pain. Clin. Cancer Res. 2006, 12, 6231–6235. [Google Scholar] [CrossRef]
- Wong, R.; Wiffen, P.J. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst. Rev. 2002, 2. [Google Scholar] [CrossRef]
- Lipton, A.; Theriault, R.L.; Hortobagyi, G.N.; Simeone, J.; Knight, R.D.; Mellars, K.; Reitsma, D.J.; Heffernan, M.; Seaman, J.J. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: Long term follow-up of two randomized, placebo-controlled trials. Cancer 2000, 88, 1082–1090. [Google Scholar] [CrossRef]
- Diel, I.J. Effectiveness of bisphosphonates on bone pain and quality of life in breast cancer patients with metastatic bone disease: A review. Support Care Cancer 2007, 15, 1243–1249. [Google Scholar] [CrossRef]
- Clemons, M.J.; Dranitsaris, G.; Ooi, W.S.; Yogendran, G.; Sukovic, T.; Wong, B.Y.; Verma, S.; Pritchard, K.I.; Trudeau, M.; Cole, D.E. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J. Clin. Oncol. 2006, 24, 4895–4900. [Google Scholar] [CrossRef]
- Body, J.J.; Diel, I.J.; Bell, R.; Pecherstorfer, M.; Lichinitser, M.R.; Lazarev, A.F.; Tripathy, D.; Bergström, B. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 2004, 111, 306–312. [Google Scholar] [CrossRef]
- Diel, I.J.; Body, J.J.; Lichinitser, M.R.; Kreuser, E.D.; Dornoff, W.; Gorbunova, V.A.; Budde, M.; Bergström, B.; MF 4265 Study Group. Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur. J. Cancer 2004, 40, 1704–1712. [Google Scholar] [CrossRef]
- Hoskin, P.J. Bisphosphonates and radiation therapy for palliation of metastatic bone disease. Cancer Treat. Rev. 2003, 29, 321–327. [Google Scholar] [CrossRef]
- Chow, E.; Harris, K.; Fan, G.; Tsao, M.; Sze, W.M. Palliative radiotherapy trials for bone metastases: A systematic review. J. Clin. Oncol. 2007, 25, 1423–1436. [Google Scholar] [CrossRef]
- Dennis, K.; Makhani, L.; Zeng, L.; Lam, H.; Chow, E. Single fraction conventional external beam radiation therapy for bone metastases: A systematic review of randomised controlled trials. Radiother. Oncol. 2013, 106, 5–14. [Google Scholar] [CrossRef]
- Chow, E.; van der Linden, Y.; Roos, D.; Hartsell, W.F.; Hoskin, P.; Wu, J.S.Y.; Brundage, M.; Nabid, A.; Wilson, C.F.; Meyer, R.M.; et al. A randomized trial of single versus multiple fractions (Fx) for re-irradiation (RE-RT) of painful bone metastases (PBM): NCIC CTG SC.20. J. Clin. Oncol. 2013, 31, 9502. [Google Scholar]
- Lutz, S.; Berk, L.; Chang, E.; Chow, E.; Hahn, C.; Hoskin, P.; Howell, D.; Konski, A.; Kachnic, L.; Lo, S.; et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 965–976. [Google Scholar] [CrossRef]
- Baczyk, M.; Czepczynski, R.; Milecki, P.; Pisarek, M.; Oleksa, R.; Sowiński, J. 89Sr versus 153Sm-EDTMP: Comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl. Med. Commun. 2007, 28, 245–250. [Google Scholar] [CrossRef]
- Plunkett, T.A.; Smith, P.; Rubens, R.D. Risk of complications from bone metastases in breast cancer: Implications for management. Eur. J. Cancer 2000, 36, 476–482. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef]
- Hess, K.R.; Pusztai, L.; Buzdar, A.U.; Hortobagyi, G.N. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res. Treat. 2003, 78, 105–118. [Google Scholar] [CrossRef]
- Smid, M.; Wang, Y.; Zhang, Y.; Sieuwerts, A.M.; Yu, J.; Klijn, J.G.M.; Foekens, J.A.; Martens, J.W.M. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008, 68, 3108–3114. [Google Scholar] [CrossRef]
- Beslija, S.; Bonneterre, J.; Burstein, H.J.; Cocquyt, V.; Gnant, M.; Heinemann, V.; Jassem, J.; Kostler, W.J.; Krainer, M.; Menard, S.; et al. Third consensus on medical treatment of metastatic breast cancer. Ann. Oncol. 2009, 20, 1771–1785. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., III; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Petersen, M.; Pardali, E.; van der Horst, G.; Cheung, H.; van den Hoogen, C.; van der Pluijm, G.; Dijke, P. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 2010, 29, 1351–1361. [Google Scholar] [CrossRef]
- Tan, A.R.; Alexe, G.; Reiss, M. Transforming growth factor-beta signaling: Emerging stem cell target in metastatic breast cancer? Breast Cancer Res. Treat. 2009, 115, 453–495. [Google Scholar] [CrossRef]
- National Institutes of Health. A service of the US National Institutes of Health. Available online: http://clinicaltrials.gov (accessed on 20 July 2013).
- Zhang, X.H.; Wang, Q.; Gerald, W.; Hudis, C.A.; Norton, L.; Smid, M.; Foekens, J.A.; Massagué, J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009, 16, 67–78. [Google Scholar] [CrossRef]
- Hiscox, S.; Barrett-Lee, P.; Borley, A.C.; Nicholson, R.I. Combining Src inhibitors and aromatase inhibitors: A novel strategy for overcoming endocrine resistance and bone loss. Eur. J. Cancer 2010, 46, 2187–2195. [Google Scholar] [CrossRef]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef]
- Voorzanger-Rousselot, N.; Goehrig, D.; Journe, F.; Doriath, V.; Body, J.J.; Clézardin, P.; Garnero, P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br. J. Cancer 2007, 97, 964–970. [Google Scholar]
- Van Holten-Verzantvoort, A.T.; Hermans, J.; Beex, L.V.; Blijham, G.; Cleton, F.J.; van Eck-Smit, B.C.; Sleeboom, H.P.; Papapoulos, S.E. Does supportive pamidronate treatment prevent or delay the first manifestation of bone metastases in breast cancer patients? Eur. J. Cancer 1996, 32, 450–454. [Google Scholar] [CrossRef]
- Norton, L.; Massague, J. Is cancer a disease of self-seeding? Nat. Med. 2006, 12, 875–878. [Google Scholar] [CrossRef]
- Kim, M.Y.; Oskarsson, T.; Acharyya, S.; Nguyen, D.X.; Zhang, X.H.; Norton, L.; Massagué, J. Tumor self-seeding by circulating cancer cells. Cell 2009, 139, 1315–1326. [Google Scholar] [CrossRef]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef]
- Winter, M.C.; Holen, I.; Coleman, R.E. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat. Rev. 2008, 34, 453–475. [Google Scholar] [CrossRef]
- Mundy, G.R.; Yoneda, T.; Hiraga, T. Preclinical studies with zoledronic acid and other bisphosphonates: Impact on the bone microenvironment. Semin. Oncol. 2001, 28, 35–44. [Google Scholar] [CrossRef]
- Santini, D.; Martini, F.; Fratto, M.E.; Galluzzo, S.; Vincenzi, B.; Agrati, C.; Turchi, F.; Piacentini, P.; Rocci, L.; Manavalan, J.S.; et al. In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol. Immunother. 2009, 58, 31–38. [Google Scholar] [CrossRef]
- Neville-Webbe, H.L.; Rostami-Hodjegan, A.; Evans, C.A.; Coleman, R.E.; Holen, I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int. J. Cancer 2005, 113, 364–371. [Google Scholar] [CrossRef]
- Rack, B.; Juckstock, J.; Genss, E.M.; Schoberth, A.; Schindlbeck, C.; Strobl, B.; Heinrigs, M.; Rammel, G.; Zwingers, T.; Sommer, H.; et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010, 30, 1807–1813. [Google Scholar]
- Aft, R.; Naughton, M.; Trinkaus, K.; Watson, M.; Ylagan, L.; Chavez-MacGregor, M.; Zhai, J.; Kuo, S.; Shannon, W.; Diemer, K.; et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: An open label, randomised, phase 2 trial. Lancet Oncol. 2010, 11, 421–428. [Google Scholar] [CrossRef]
- Greenberg, S.; Park, J.W.; Melisko, M.E.; Goga, A.; Moasser, M.M.; Anderson, M.; Scott, J.H.; Petrillo, L.A.; Moore, D.H.; Rugo, H.S. Effect of adjuvant zoledronic acid (ZOL) on disseminated tumor cells (DTC) in the bone marrow (BM) of women with early-stage breast cancer (ESBC): Updated results. J. Clin. Oncol. 2010, 28, 1002. [Google Scholar]
- Diel, I.J.; Solomayer, E.F.; Costa, S.D.; Gollan, C.; Goerner, R.; Wallwiener, D.; Kaufmann, M.; Bastert, G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 1998, 339, 357–363. [Google Scholar] [CrossRef]
- Diel, I.J.; Jaschke, A.; Solomayer, E.F.; Gollan, C.; Bastert, G.; Sohn, C.; Schuetz, F. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: A long-term follow-up. Ann. Oncol. 2008, 19, 2007–2011. [Google Scholar] [CrossRef]
- Powles, T.; Paterson, S.; Kanis, J.A.; McCloskey, E.; Ashley, S.; Tidy, A.; Rosenqvist, K.; Smith, I.; Ottestad, L.; Legault, S.; et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J. Clin. Oncol. 2002, 20, 3219–3224. [Google Scholar] [CrossRef]
- Powles, T.; Paterson, A.; McCloskey, E.; Schein, P.; Scheffler, B.; Tidy, A.; Ashley, S.; Smith, I.; Ottestad, L.; Kanis, J. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006, 8. [Google Scholar] [CrossRef]
- Saarto, T.; Blomqvist, C.; Virkkunen, P.; Elomaa, I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-Year results of a randomized controlled trial. J. Clin. Oncol. 2001, 19, 10–17. [Google Scholar]
- Saarto, T.; Vehmanen, L.; Virkkunen, P.; Blomqvist, C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004, 43, 650–656. [Google Scholar] [CrossRef]
- Ha, T.C.; Li, H. Meta-analysis of clodronate and breast cancer survival. Br. J. Cancer 2007, 96, 1796–1801. [Google Scholar] [CrossRef]
- Paterson, A.H.; Anderson, S.J.; Lembersky, B.C.; Fehrenbacher, L.; Falkson, C.I.; King, K.M.; Weir, L.M.; Brufsky, A.M.; Dakhil, S.; Lad, T.; et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): A multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012, 13, 734–742. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Mobus, V.; Schneeweiss, A.; Huober, J.; Thomssen, C.; Untch, M.; Jackisch, C.; Diel, I.J.; Elling, D.; Conrad, B.; et al. German adjuvant intergroup node-positive study: A phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. Am. Soc. Clin. Oncol. 2013, 31. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Schippinger, W.; Luschin-Ebengreuth, G.; Postlberger, S.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Bjelic-Radisic, V.; et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 2009, 360, 679–691. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-Month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef]
- Gnant, M. Zoledronic acid in the treatment of early-stage breast cancer: Is there a final verdict? Curr. Oncol. Rep. 2012, 14, 35–43. [Google Scholar] [CrossRef]
- Gnant, M. Zoledronic acid in breast cancer: Latest findings and interpretations. Ther. Adv. Med. Oncol. 2011, 3, 293–301. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Harker, W.G.; Beck, J.T.; Bosserman, L.; Vogel, C.; Seidler, C.; Jin, L.; Warsi, G.; Argonza-Aviles, E.; Hohneker, J.; et al. Final 5-year results of Z-FAST trial: Adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 2012, 118, 1192–1201. [Google Scholar] [CrossRef]
- Llombart, A.; Frassoldati, A.; Paija, O.; Sleeboom, H.P.; Jerusalem, G.; Mebis, J.; Deleu, I.; Miller, J.; Schenk, N.; Neven, P. Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-Month analysis of the E-ZO-FAST trial. Clin. Breast Cancer 2012, 12, 40–48. [Google Scholar] [CrossRef]
- Coleman, R.; de Boer, R.; Eidtmann, H.; Llombart, A.; Davidson, N.; Neven, P.; von Minckwitz, G.; Sleeboom, H.P.; Forbes, J.; Barrios, C.; et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Ann. Oncol. 2013, 24, 398–405. [Google Scholar] [CrossRef]
- Coleman, R.E.; Marshall, H.; Cameron, D.; Dodwell, D.; Burkinshaw, R.; Keane, M.; Gil, M.; Houston, S.J.; Grieve, R.J.; Barrett-Lee, P.J.; et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011, 365, 1396–1405. [Google Scholar] [CrossRef]
- Yan, T.; Yin, W.; Zhou, Q.; Zhou, L.; Jiang, Y.; Du, Y.; Shao, Z.; Lu, J. The efficacy of zoledronic acid in breast cancer adjuvant therapy: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2012, 48, 187–195. [Google Scholar]
- Valachis, A.; Polyzos, N.P.; Coleman, R.E.; Gnant, M.; Eidtmann, H.; Brufsky, A.M.; Aft, R.; Tevaarwerk, A.J.; Swenson, K.; Lind, P.; et al. Adjuvant therapy with zoledronic acid in patients with breast cancer: A systematic review and meta-analysis. Oncologist 2013, 18, 353–361. [Google Scholar] [CrossRef]
- Ellis, G.K.; Bone, H.G.; Chlebowski, R.; Paul, D.; Spadafora, S.; Smith, J.; Fan, M.; Jun, S. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J. Clin. Oncol. 2008, 26, 4875–4882. [Google Scholar] [CrossRef]
- Marguiles, A.G.; Klimberg, V.S.; Bhattacharrya, S.; Gaddy, D.; Suva, L.J. Genomics and proteomics of bone cancer. Clin. Cancer Res. 2006, 12, 6217–6221. [Google Scholar] [CrossRef]
- Schilsky, R.L. Personalized medicine in oncology: The future is now. Nat. Rev. Drug Discov. 2010, 9, 363–366. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, B.T.; Wong, M.H.; Pavlakis, N. Treatment and Prevention of Bone Metastases from Breast Cancer: A Comprehensive Review of Evidence for Clinical Practice. J. Clin. Med. 2014, 3, 1-24. https://doi.org/10.3390/jcm3010001
Li BT, Wong MH, Pavlakis N. Treatment and Prevention of Bone Metastases from Breast Cancer: A Comprehensive Review of Evidence for Clinical Practice. Journal of Clinical Medicine. 2014; 3(1):1-24. https://doi.org/10.3390/jcm3010001
Chicago/Turabian StyleLi, Bob T., Matthew H. Wong, and Nick Pavlakis. 2014. "Treatment and Prevention of Bone Metastases from Breast Cancer: A Comprehensive Review of Evidence for Clinical Practice" Journal of Clinical Medicine 3, no. 1: 1-24. https://doi.org/10.3390/jcm3010001
APA StyleLi, B. T., Wong, M. H., & Pavlakis, N. (2014). Treatment and Prevention of Bone Metastases from Breast Cancer: A Comprehensive Review of Evidence for Clinical Practice. Journal of Clinical Medicine, 3(1), 1-24. https://doi.org/10.3390/jcm3010001