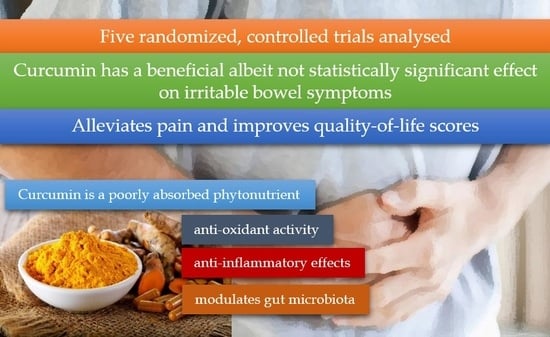
A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS)
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Akehurst, R.L.; Brazier, J.E.; Mathers, N.; O’Keefe, C.; Kaltenthaler, E.; Morgan, A.; Platts, M.; Walters, S.J. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics 2002, 20, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; De Giorgio, R.; Dothel, G.; Zecchi, L.; Bellacosa, L.; Carini, G.; Stanghellini, V.; Corinaldesi, R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr. Gastroenterol. Rep. 2011, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Thabane, M.; Kottachchi, D.T.; Marshall, J.K. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007, 26, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jalanka-Tuovinen, J.; Salojärvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.C.; de Vos, W.M. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Camilleri, M.; McKinzie, S.; Lempke, M.B.; Burton, D.D.; Thomforde, G.M.; Zinsmeister, A.R. A randomized controlled trial of a probiotic, VSL# 3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003, 17, 895–904. [Google Scholar] [PubMed]
- Sharara, A.I.; Aoun, E.; Abdul-Baki, H.; Mounzer, R.; Sidani, S.; ElHajj, I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am. J. Gastroenterol. 2006, 101, 326. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Dapoigny, M.; Scott, K.P.; Crouzet, L.; Del’homme, C.; Marquet, P.; Martin, J.C.; Pickering, G.; Ardid, D.; Eschalier, A.; et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment. Pharmacol. Ther. 2012, 35, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.; Lim, D.Y.; Yeo, W.S. Agomelatine, a novel therapeutic option for the management of irritable bowel syndrome. J. Clin. Pharm. Ther. 2018, 43, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Langmead, L.; Chitnis, M.; Rampton, D.S. Use of complementary therapies by patients with IBD may indicate psychosocial distress. Inflamm. Bowel Dis. 2002, 8, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Aggarwal, B.B. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. From exotic spice to modern drug? Cell 2007, 130, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Koh, S.S.; Chan, H.W.; Ho, C.Y. Clinical use of curcumin in depression: A meta-analysis. J. Am. Med. Dir. Assoc. 2017, 18, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Zeng, Q.; Mitchell, E.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin Enhances Neurogenesis and Cognition in Aged Rats: Implications for Transcriptional Interactions Related to Growth and Synaptic Plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef] [PubMed]
- Lubbad, A.; Oriowo, M.A.; Khan, I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol. Cell. Biochem. 2009, 322, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.; Wahl, M. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Dorn, S.D.; Kaptchuk, T.J.; Park, J.B.; Nguyen, L.T.; Canenguez, K.; Nam, B.H.; Woods, K.B.; Conboy, L.A.; Stason, W.B.; Lembo, A.J. A meta-analysis of the placebo response in complementary and alternative medicine trials of irritable bowel syndrome. Neurogastroenterol. Motil. 2007, 19, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Booth, J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: A pilot study. J. Altern. Complement. Med. 2004, 10, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Brinkhaus, B.; Hentschel, C.; Keudell, C.V.; Schindler, G.; Lindner, M.; Stützer, H.; Kohnen, R.; Willich, S.N.; Lehmacher, W.; Hahn, E.G. Herbal medicine with curcuma and fumitory in the treatment of irritable bowel syndrome: A randomized, placebo-controlled, double-blind clinical trial. Scand. J. Gastroenterol. 2005, 40, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Alt, F.; Chong, P.W.; Teng, E.; Uebelhack, R. Evaluation of Benefit and Tolerability of IQP-CL-101 (Xanthofen) in the Symptomatic Improvement of Irritable Bowel Syndrome: A Double-Blinded, Randomised, Placebo-Controlled Clinical Trial. Phytother. Res. 2017, 31, 1056–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauche, R.; Kumar, S.; Hallmann, J.; Lüdtke, R.; Rampp, T.; Dobos, G.; Langhorst, J. Efficacy and safety of ayurvedic herbs in diarrhoea-predominant irritable bowel syndrome: A randomised controlled crossover trial. Complement. Ther. Med. 2016, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Scribano, M.L.; Kohn, A.; Caporaso, N.; Festi, D.; Campanale, M.C.; Di Rienzo, T.; Guarino, M.; Taddia, M.; et al. Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. J. Gastrointestin. Liver Dis. 2016, 25, 151–157. [Google Scholar] [PubMed]
- Thompson, W.G.; Longstreth, G.F.; Drossman, D.A.; Heaton, K.W.; Irvine, E.J.; Mueller-Lissner, S.A.; Talley, N.J.; Whitehead, W.E.; Corazziari, E. Rome II: Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology, and Treatment: A Multinational Consensus, 2nd ed.; Degnon Associates: McLean, VA, USA, 2000; pp. 351–432. [Google Scholar]
- Derosa, G.; Maffioli, P.; Simental-Mendía, L.E.; Bo, S.; Sahebkar, A. Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 111, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Ng, Q.X.; Soh, A.Y.; Loke, W.; Lim, D.Y.; Yeo, W.-S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Li, J.; Wang, R.; Xie, X.; Yu, X.; Pan, J.; Xu, Y.; Zheng, L. The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: Involvement of 5-HT-dependent signaling. Metab. Brain Dis. 2015, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Yeo, W.-S. Systematic Review with Meta-analysis: The Association between Post-traumatic Stress Disorder (PTSD) and Irritable Bowel Syndrome (IBS). J. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Sundaresh, S.; Elliott, J.; Anton, P.A.; Baldi, P.; Licudine, A.; Mayer, M.; Vuong, T.; Hirano, M.; Naliboff, B.D.; et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 2009, 21, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, M.; Camilleri, M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J. Gastroenterol. 2013, 48, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Vivinus-Nebot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.; Zhao, L.X.; Lou, H.X. Curcumin alters the pharmacokinetics of warfarin and clopidogrel in Wistar rats but has no effect on anticoagulation or antiplatelet aggregation. Planta Med. 2013, 79, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Hiki, N.; Kurosaka, H.; Tatsutomi, Y.; Shimoyama, S.; Tsuji, E.; Kojima, J.; Shimizu, N.; Ono, H.; Hirooka, T.; Noguchi, C.; et al. Peppermint oil reduces gastric spasm during upper endoscopy: A randomized, double-blind, double-dummy controlled trial. Gastrointest. Endosc. 2003, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable bowel syndrome: A clinical review. JAMA 2015, 313, 949–958. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Study Design | Country | Sample Size | Curcumin Dose and Formulation | Study Duration | Diagnosis of IBS | Conclusions |
|---|---|---|---|---|---|---|---|
| Alt, 2017 [26] | Double-blind, placebo-controlled randomized trial | Germany | 99 |
| 8 weeks | Rome III | Significant improvement in IBS symptoms, compared to placebo (p < 0.001). Improvements seen as early as 4 weeks into treatment. No serious adverse events reported. |
| Bundy, 2004 [24] | Partially blinded, randomized, two-dose trial | United Kingdom | 207 |
| 8 weeks | Rome II | Significantly reduced IBS symptomatology in both treatment groups after 8 weeks (p < 0.001). No serious adverse events reported. |
| Brinkhaus, 2005 [25] | Double-blind, placebo-controlled, randomized trial | Germany | 106 |
| 18 weeks | Extensive clinical examination ruling out organic causes | Both herb-based monotherapy did not significantly improve IBS symptoms compared to placebo. |
| Lauche, 2016 [27] | Double-blind, placebo-controlled, randomized crossover trial | Germany | 32 |
| 4 weeks | Rome III | No significant difference between Ayurvedic preparation and placebo for IBS symptom severity (p = 0.26). |
| Portincasa, 2016 [28] | Double-blind, placebo-controlled, randomized trial | Italy | 121 |
| 30 days | Rome III | Significant improvement in IBS symptoms (p < 0.001) and quality of life. No serious adverse events reported. |
| Study (Author, Year) | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Alt, 2017 [26] | + | + | + | + | ? | ? |
| Bundy, 2004 [24] | + | ? | - | + | ? | - |
| Brinkhaus, 2005 [25] | ? | + | + | + | ? | ? |
| Lauche, 2016 [27] | + | - | - | + | ? | ? |
| Portincasa, 2016 [28] | - | + | + | + | ? | ? |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Yeo, W.-S. A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS). J. Clin. Med. 2018, 7, 298. https://doi.org/10.3390/jcm7100298
Ng QX, Soh AYS, Loke W, Venkatanarayanan N, Lim DY, Yeo W-S. A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS). Journal of Clinical Medicine. 2018; 7(10):298. https://doi.org/10.3390/jcm7100298
Chicago/Turabian StyleNg, Qin Xiang, Alex Yu Sen Soh, Wayren Loke, Nandini Venkatanarayanan, Donovan Yutong Lim, and Wee-Song Yeo. 2018. "A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS)" Journal of Clinical Medicine 7, no. 10: 298. https://doi.org/10.3390/jcm7100298