The Association between Nonalcoholic Fatty Liver Disease and CT-Measured Skeletal Muscle Mass
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Clinical and Laboratory Assessments
2.3. Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, I.H. Epidemiologic and methodologic problems in determining nutritional-status of older persons. Proceedings of a conference held in Albuquerque, New Mexico, October 19–21, 1988. Am. J. Clin. Nutr. 1989, 50, 1121–1235. [Google Scholar]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J. Cardiometab. Syndr. 2007, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic obesity: Prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Arango-Lopera, V.E.; Arroyo, P.; Gutierrez-Robledo, L.M.; Perez-Zepeda, M.U.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lim, S.; Choi, S.H.; Kim, K.M.; Yoon, J.W.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenia: An independent predictor of mortality in community-dwelling older Korean men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.A.; Younossi, Z.M.; Gramlich, T.; Boparai, N.; Liu, Y.C.; McCullough, A.J. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef]
- Clark, J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006, 40, 5–10. [Google Scholar]
- Erickson, S.K. Nonalcoholic fatty liver disease. J. Lipid Res. 2009, 50, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovo, C.V.; Fernandes, S.A.; Buss, C.; de Mattos, A.A. Sarcopenia and non-alcoholic fatty liver disease: Is there a relationship? A systematic review. World J. Hepatol. 2017, 9, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Kahn, S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Abbatecola, A.M.; Paolisso, G.; Fattoretti, P.; Evans, W.J.; Fiore, V.; Dicioccio, L.; Lattanzio, F. Discovering pathways of sarcopenia in older adults: A role for insulin resistance on mitochondria dysfunction. J. Nutr. Health Aging 2011, 15, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (adult treatment panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. (1985) 1998, 85, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T.; Lee, H.R.; Lee, Y.J.; Linton, J.A.; Shim, J.Y. Relationship between employment status and obesity in a Korean elderly population, based on the 2007-2009 Korean National Health and Nutrition Examination Survey (KNHANES). Arch. Gerontol. Geriatr. 2013, 57, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Ryoo, J.H.; Oh, C.M.; Choi, J.M.; Kang, J.G.; Lee, J.H.; Chung, J.Y.; Jung, J.Y. Effect of overweight and obesity (defined by Asian-Specific Cutoff Criteria) on Left ventricular diastolic function and structure in a general Korean population. Circ. J. 2016, 80, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. (1985) 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Correa-de-Araujo, R.; Harris-Love, M.O.; Miljkovic, I.; Fragala, M.S.; Anthony, B.W.; Manini, T.M. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: A symposium report. Front. Physiol. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Xiao, Q. The common mechanisms of sarcopenia and NAFLD. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Peters, K.W.; Shardell, M.D.; McLean, R.R.; Dam, T.T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kim, K.M.; Kim, J.H.; Moon, J.H.; Choi, S.H.; Lim, S.; Lim, J.Y.; Kim, K.W.; Park, K.S.; Jang, H.C. Predictive values of the new sarcopenia index by the foundation for the national institutes of health sarcopenia project for mortality among older Korean adults. PLoS ONE 2016, 11, e0166344. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.C.; Wu, L.W.; Chen, W.L.; Liaw, F.Y.; Chang, Y.W.; Kao, T.W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): The importance of sarcopenia definition. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Lubrano, C.; Sergi, G.; Coin, A.; Gnessi, L.; Mariani, S.; Lenzi, A.; Donini, L.M. Sarcopenic obesity and metabolic syndrome in adult caucasian subjects. J. Nutr. Health Aging 2016, 20, 958–963. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, S.; Glynn, E.L.; Timmerman, K.L.; Rasmussen, B.B.; Volpi, E. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: Evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 2009, 52, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Pedone, C.; Incalzi, R.A.; Pahor, M. ACE-inhibition and physical function: Results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J. Am. Med. Dir. Assoc. 2010, 11, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchetta, I.; Angelico, F.; Del Ben, M.; Baroni, M.G.; Pozzilli, P.; Morini, S.; Cavallo, M.G. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.A.; Cho, H.; Eun, C.R.; Yoo, H.J.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Park, M.H.; Han, C.; et al. Association between visceral obesity and sarcopenia and vitamin D deficiency in older Koreans: The Ansan Geriatric Study. J. Am. Geriatr. Soc. 2012, 60, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Panjawatanan, P.; Thongprayoon, C.; Jaruvongvanich, V.; Ungprasert, P. Sarcopenia and risk of nonalcoholic fatty liver disease: A meta-analysis. Saudi J. Gastroenterol. 2018, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
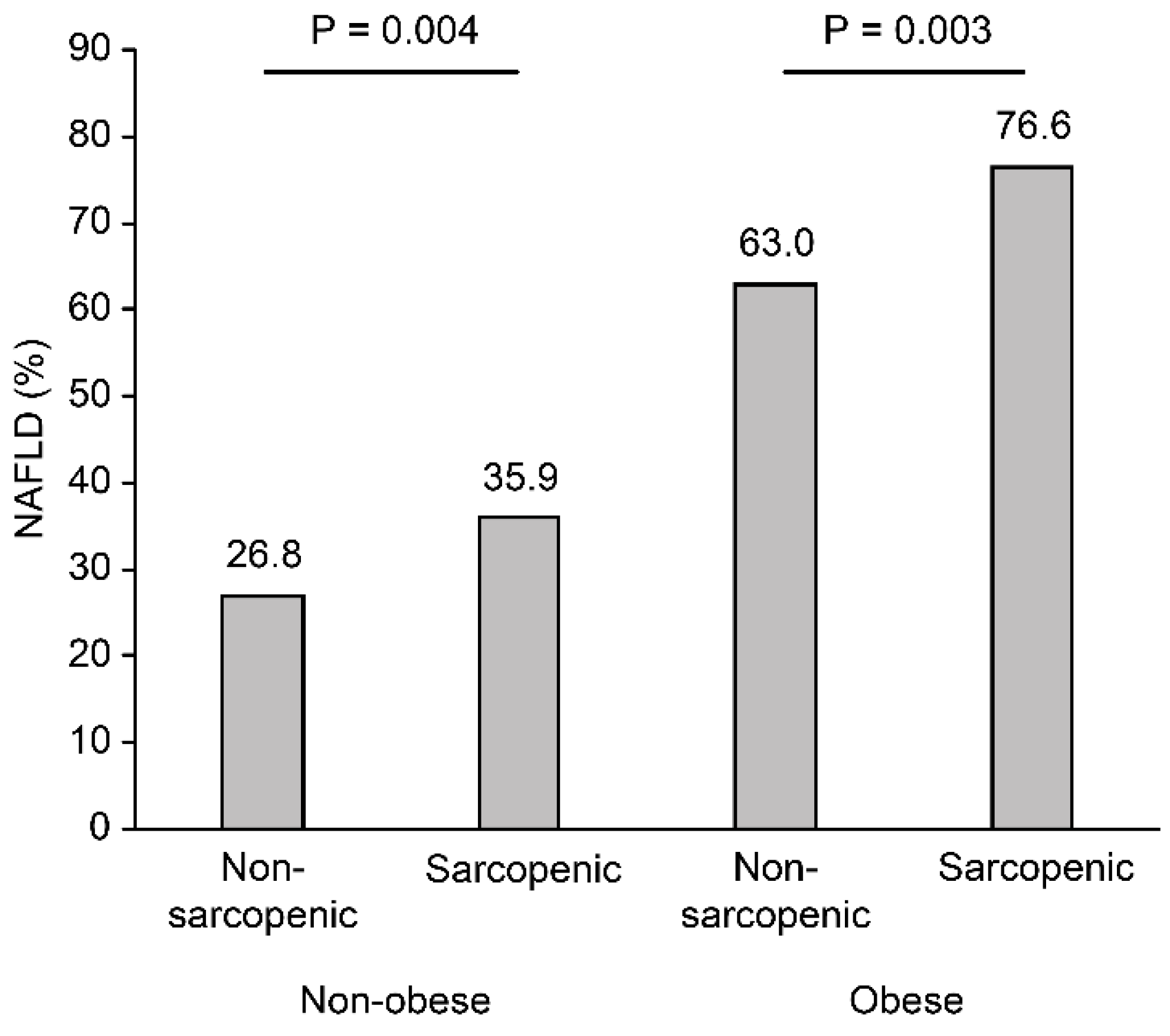
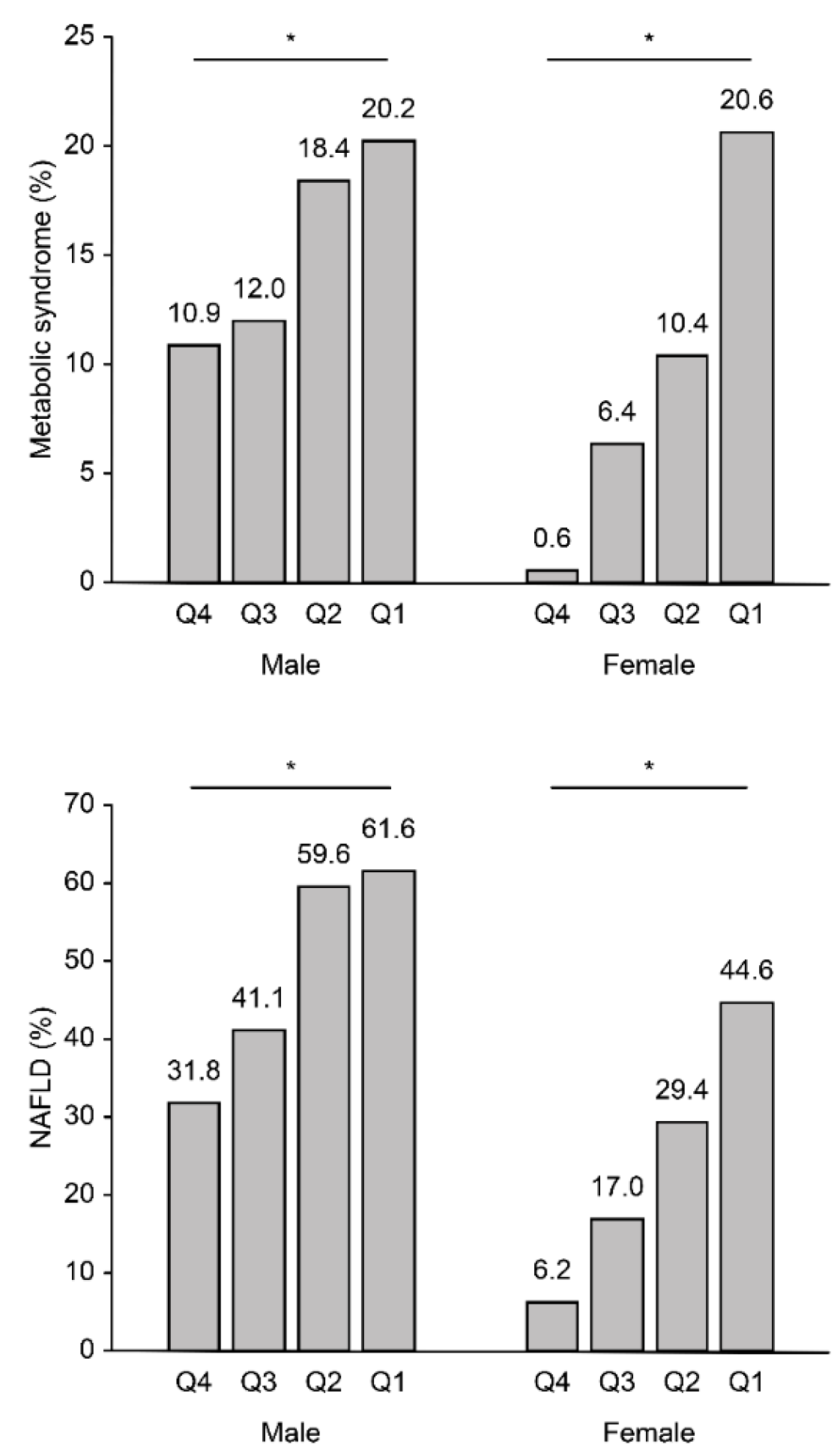
| Variable | Nonobese (n = 1341) | Obese (n = 487) | ||||
|---|---|---|---|---|---|---|
| Nonsarcopenic (n = 1071) | Sarcopenic (n = 270) | p Value | Nonsarcopenic (n = 303) | Sarcopenic (n = 184) | p Value | |
| Age, years | 54.0 ± 9.6 | 58.2 ± 8.9 | <0.001 | 53.5 ± 9.0 | 58.1 ± 9.2 | <0.001 |
| Male, % | 610 (57.0) | 91 (33.7) | <0.001 | 285 (94.1) | 135 (73.4) | <0.001 |
| Smoking status | <0.001 | <0.001 | ||||
| Never | 604 (56.8) | 213 (80.1) | 80 (26.5) | 84 (46.2) | ||
| Ex-smoker | 343 (32.2) | 42 (15.8) | 164 (54.3) | 84 (46.2) | ||
| Current smoker | 117 (11.0) | 11 (4.1) | 58 (19.2) | 14 (7.7) | ||
| Diabetes mellitus | 29 (2.7) | 6 (2.2) | 0.815 | 14 (4.6) | 10 (5.4) | 0.852 |
| Hypertension | 327 (30.7) | 103 (38.4) | 0.018 | 189 (62.6) | 111 (60.7) | 0.744 |
| Body mass index, kg/m2 | 21.9 ± 1.9 | 22.7 ± 1.5 | <0.001 | 26.6 ± 1.5 | 27.0 ± 1.8 | 0.013 |
| Waist circumference, cm | 80.6 ± 6.4 | 82.9 ± 5.3 | <0.001 | 92.3 ± 5.0 | 93.0 ± 5.6 | 0.114 |
| Systolic blood pressure, mmHg | 113.5 ± 13.5 | 116.0 ± 13.9 | 0.007 | 121.9 ± 12.8 | 122.6 ± 11.9 | 0.548 |
| Diastolic blood pressure, mmHg | 73.1 ± 10.2 | 73.6 ± 10.2 | 0.514 | 80.5 ± 10.5 | 79.0 ± 9.5 | 0.108 |
| Total cholesterol, mg/dL | 191.3 ± 33.7 | 200.0 ± 36.9 | <0.001 | 190.1 ± 36.8 | 193.2 ± 34.7 | 0.352 |
| Triglycerides, mg/dL | 95.7 ± 55.4 | 109.0 ± 65.3 | 0.002 | 143.1 ± 86.8 | 134.7 ± 88.2 | 0.308 |
| HDL cholesterol, mg/dL | 56.1 ± 13.1 | 54.0 ± 11.4 | 0.009 | 46.5 ± 10.3 | 49.5 ± 11.1 | 0.003 |
| LDL cholesterol, mg/dL | 117.2 ± 30.0 | 125.0 ± 30.6 | 0.001 | 121.6 ± 29.8 | 124.0 ± 33.9 | 0.480 |
| AST, IU/L | 22.7 ± 8.2 | 22.8 ± 7.9 | 0.841 | 25.4 ± 19.3 | 25.4 ± 8.6 | 1.000 |
| ALT, IU/L | 21.8 ± 12.4 | 22.3 ± 14.7 | 0.591 | 29.8 ± 23.0 | 30.7 ± 16.3 | 0.613 |
| GGT, IU/L | 27.1 ± 23.8 | 27.2 ± 19.1 | 0.979 | 43.4 ± 39.6 | 42.5 ± 36.1 | 0.796 |
| Fasting glucose, mg/dL | 96.0 ± 16.2 | 96.8 ± 13.2 | 0.392 | 104.0 ± 19.7 | 102.0 ± 14.2 | 0.204 |
| HbA1c, % | 5.7 ± 0.5 | 5.7 ± 0.4 | 0.472 | 5.8 ± 0.6 | 5.9 ± 0.5 | 0.330 |
| Muscle mass, cm2 | 208.3 ± 31.5 | 163.7 ± 20.3 | <0.001 | 247.1 ± 23.4 | 201.0 ± 24.5 | <0.001 |
| SMICT, cm2/(kg/m2) | 9.5 ± 1.3 | 7.2 ± 0.7 | <0.001 | 9.3 ± 0.8 | 7.4 ± 0.7 | <0.001 |
| Metabolic syndrome | 72 (6.8) | 32 (12.1) | 0.006 | 80 (26.7) | 52 (28.7) | 0.700 |
| Variable | NAFLD (−) | NAFLD (+) | p Value |
|---|---|---|---|
| SMICT | 9.10 ± 0.05 | 8.64 ± 0.05 | <0.001 |
| SMICT, adjusted model 1 | 9.10 ± 0.02 | 8.64 ± 0.03 | <0.001 |
| SMICT, adjusted model 2 | 9.11 ± 0.03 | 8.64 ± 0.03 | <0.001 |
| SMICT, adjusted model 3 | 9.11 ± 0.03 | 8.65 ± 0.03 | <0.001 |
| Variable | NAFLD (−) | NAFLD (+) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |||
| No sarcopenia | 896 | 478 | 1 | 1 | 1 | |||
| Sarcopenia | 216 | 238 | 2.63 (2.08–3.34) | <0.001 | 1.61 (1.24–2.09) | <0.001 | 1.51 (1.15–1.99) | 0.003 |
| No sarcopenia | 896 | 478 | 1 | <0.001 * | 1 | <0.001 * | 1 | 0.001 * |
| Mild sarcopenia | 201 | 214 | 2.50 (1.96–3.19) | <0.001 | 1.54 (1.18–2.02) | 0.002 | 1.45 (1.09–1.92) | 0.010 |
| Severe sarcopenia | 15 | 24 | 4.88 (2.47–9.93) | <0.001 | 2.80 (1.30–6.17) | 0.009 | 2.51 (1.16–5.56) | 0.020 |
| Variable | Q4 | Q3 | Q2 | Q1 | p for Trend |
|---|---|---|---|---|---|
| Male | |||||
| Model 1 | 1 (reference) | 1.53 (1.08–2.17) | 3.28 (2.32–4.66) | 3.70 (2.60–5.31) | <0.001 |
| Model 2 | 1 (reference) | 1.18 (0.81–1.72) | 2.08 (1.42–3.06) | 1.94 (1.30–2.91) | <0.001 |
| Model 3 | 1 (reference) | 1.09 (0.74–1.61) | 1.99 (1.33–2.98) | 1.78 (1.17–2.72) | <0.001 |
| Female | |||||
| Model 1 | 1 (reference) | 3.01 (1.49–6.51) | 5.64 (2.91–11.85) | 10.25 (5.36–21.37) | <0.001 |
| Model 2 | 1 (reference) | 1.58 (0.74–3.60) | 2.29 (1.11–5.06) | 3.06 (1.48–6.75) | <0.001 |
| Model 3 | 1 (reference) | 1.61 (0.73–3.71) | 1.76 (0.83–3.98) | 2.39 (1.13–5.37) | 0.025 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, E.K.; Kang, H.Y.; Park, B.; Yang, J.I.; Kim, J.S. The Association between Nonalcoholic Fatty Liver Disease and CT-Measured Skeletal Muscle Mass. J. Clin. Med. 2018, 7, 310. https://doi.org/10.3390/jcm7100310
Choe EK, Kang HY, Park B, Yang JI, Kim JS. The Association between Nonalcoholic Fatty Liver Disease and CT-Measured Skeletal Muscle Mass. Journal of Clinical Medicine. 2018; 7(10):310. https://doi.org/10.3390/jcm7100310
Chicago/Turabian StyleChoe, Eun Kyung, Hae Yeon Kang, Boram Park, Jong In Yang, and Joo Sung Kim. 2018. "The Association between Nonalcoholic Fatty Liver Disease and CT-Measured Skeletal Muscle Mass" Journal of Clinical Medicine 7, no. 10: 310. https://doi.org/10.3390/jcm7100310





