Effects of Jaw Periosteal Cells on Dendritic Cell Maturation
Abstract
:1. Introduction
2. Materials and Methods
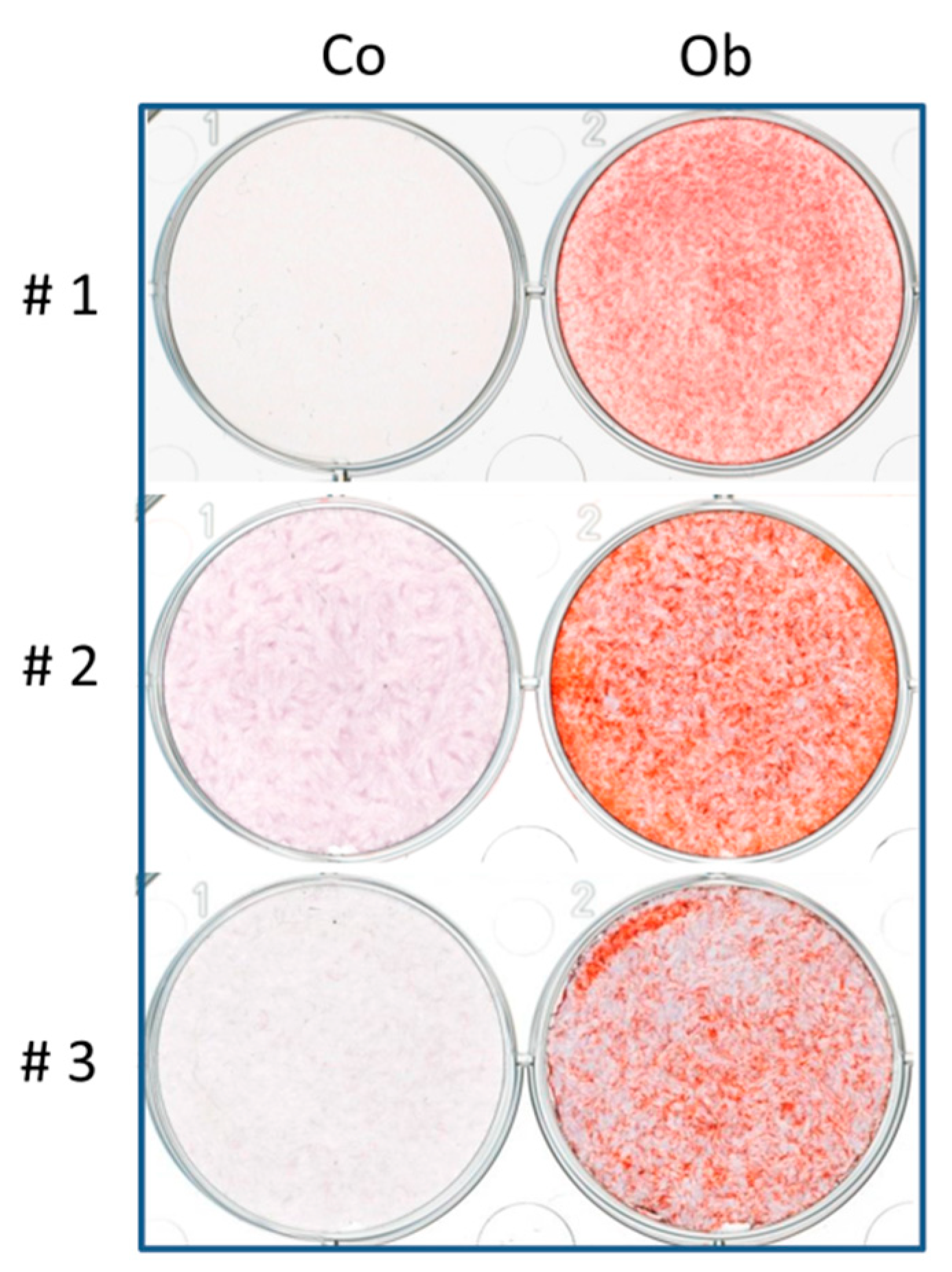
2.1. Isolation and Culture of JPCs
2.2. Isolation and Culture of PBMCs, Dendritic Cell Differentiation
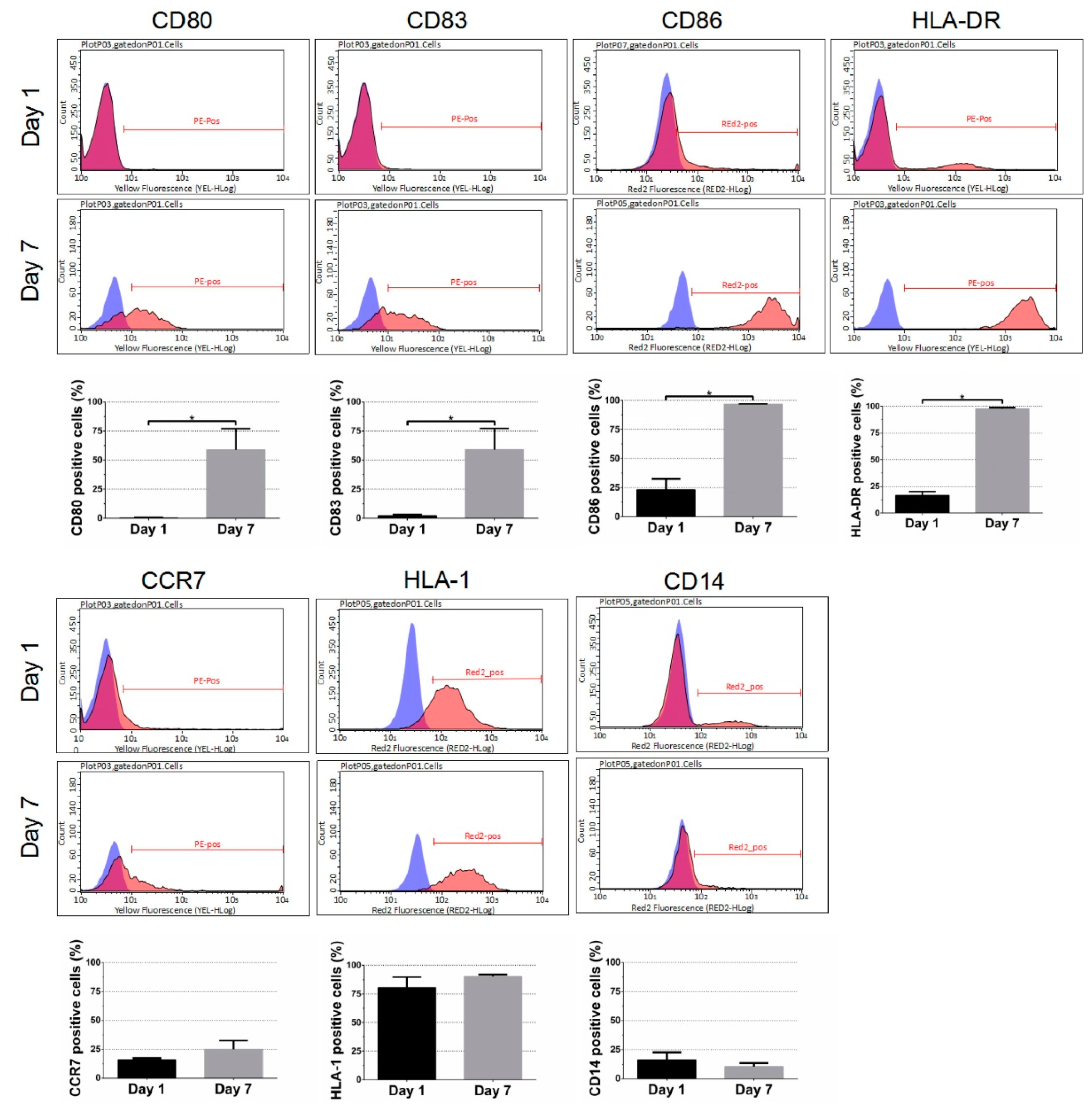
2.3. Flow Cytometric Analyses of Dendritic Marker Expression
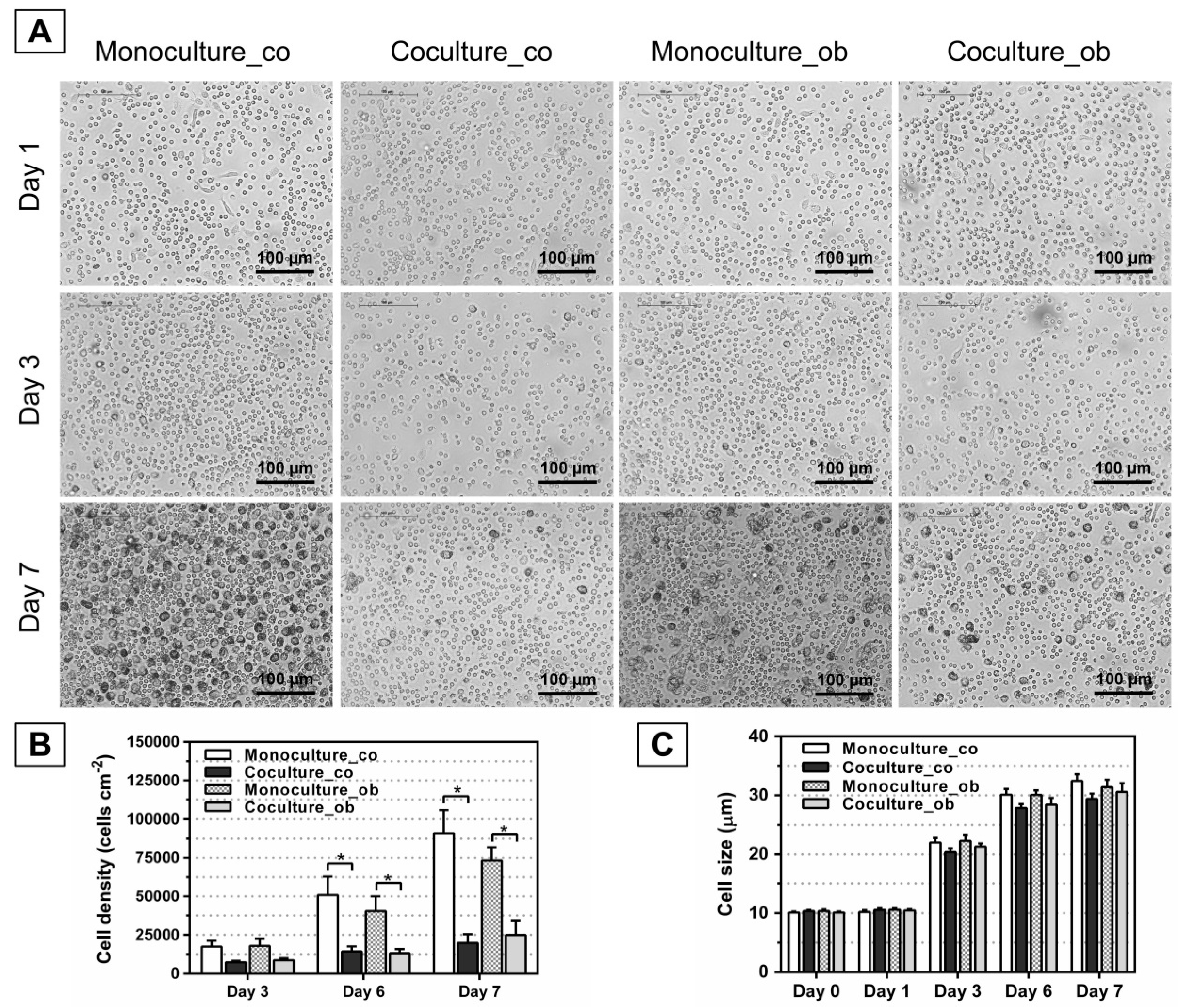
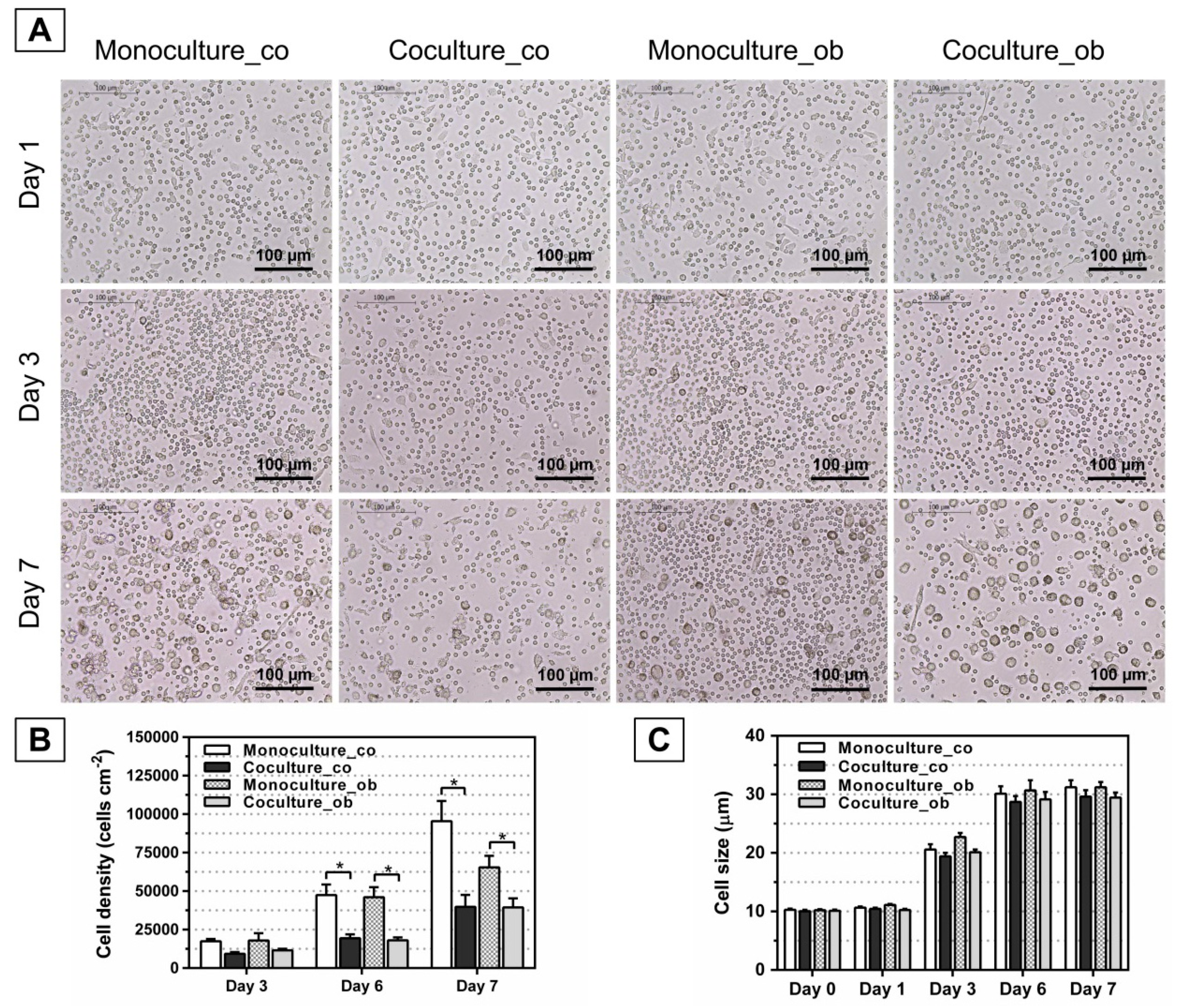
2.4. Co-Cultivation of JPCs and PBMCs
- JPC-free culture with complete DMEM/F12 medium (Monoculture_co);
- JPC-free culture with osteogenic medium (ob—complete DMEM/F12 medium containing 100 mM L-ascorbic acid 2-phosphate, 10 mM β-glycerophosphate, and 4 µM dexamethasone, Sigma-Aldrich) (Monoculture_ob);
- Co-culture with undifferentiated JPCs (Coculture_co);
- Co-culture with osteogenic differentiated JPCs (Coculture_ob).
2.5. RNA Isolation and Quantitative Gene Expression Analyses in PBMCs
2.6. IL-8 Protein Release in JPCs and PBMCs
2.7. Statistical Analyses
3. Results
3.1. Effect of DC Differentiation Cocktails on the Phenotype of Monocyte
3.2. Effect of JPCs on the Morphology, Number, and Size of DC
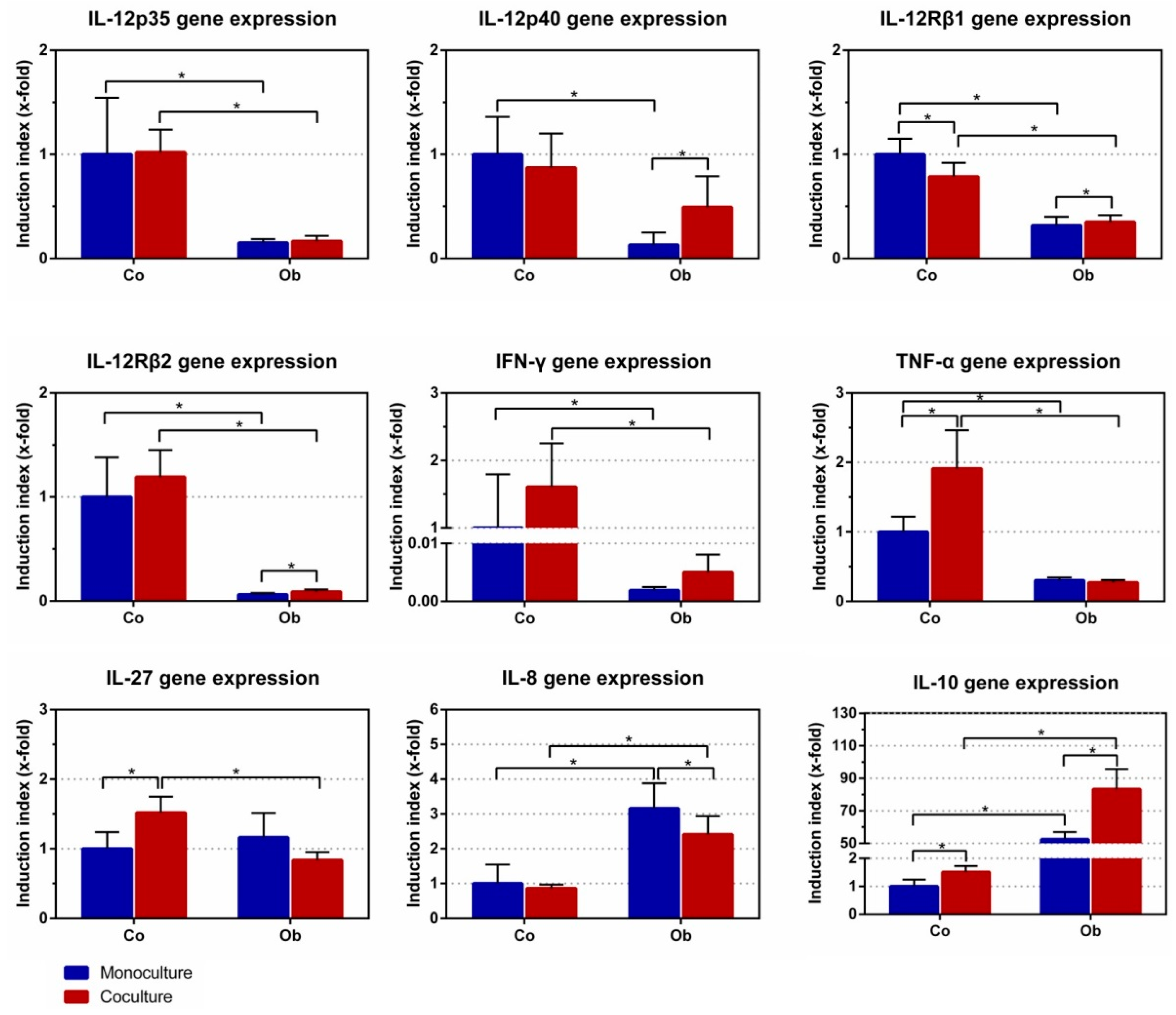
3.3. Effect of JPCs on DC Gene Expression
3.4. Effect of JPCs on IL-8 Secretion of DC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Le Blanc, K.; Davies, L.C. MSCs-cells with many sides. Cytotherapy 2018, 20, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, Q.; Bu, H.; Lin, F. Mesenchymal stem cells inhibit complement activation by secreting factor h. Stem Cells Dev. 2010, 19, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal stem cells inhibit generation and function of both cd34+-derived and monocyte-derived dendritic cells. J. Immunol. 2006, 177, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccarelli, G.; Graziano, A.; Benedetti, L.; Imbriani, M.; Romano, F.; Ferrarotti, F.; Aimetti, M.; Cusella de Angelis, G.M. Osteogenic potential of human oral-periosteal cells (PCs) isolated from different oral origin: An in vitro study. J. Cell. Physiol. 2016, 231, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: The stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Yang, C.Y.; Liu, J.W.; Brey, E.M.; Cheng, M.H. Periosteal osteogenic capacity depends on tissue source. Tissue Eng. Part A 2018. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Li, X.; Dai, J.; Cole, L.; Camacho, J.A.; Zhang, Y.; Ji, Y.; Wang, J.; Yang, X.F.; Wang, H. Immune cell subset differentiation and tissue inflammation. J. Hematol. Oncol. 2018, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Ohashi, P.S. Molecular programming of steady-state dendritic cells: Impact on autoimmunity and tumor immune surveillance. Ann. N. Y. Acad. Sci. 2013, 1284, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive cd4+ T cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar] [PubMed]
- Stobie, L.; Gurunathan, S.; Prussin, C.; Sacks, D.L.; Glaichenhaus, N.; Wu, C.Y.; Seder, R.A. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 2000, 97, 8427–8432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Rengaraju, M.; Valiante, N.M.; Chehimi, J.; Kubin, M.; Aste, M.; Chan, S.H.; Kobayashi, M.; Young, D.; Nickbarg, E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 1992, 176, 1387–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Carvajal, D.; Ling, P.; Podlaski, F.J.; Stremlo, D.L.; Familletti, P.C.; Gubler, U.; Presky, D.H.; Stern, A.S.; Gately, M.K. Mouse interleukin-12 (IL-12) p40 homodimer: A potent IL-12 antagonist. Eur. J. Immunol. 1995, 25, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Pahan, K. IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages. Mol. Immunol. 2009, 46, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinski, P.; Vieira, P.L.; Schuitemaker, J.H.N.; De Jong, E.C.; Kapsenberg, M.L. Prostaglandin e2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 2001, 97, 3466–3469. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin e2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, X.H.; Guo, X.J.; Guan, S.B.; Li, X.M.; Gu, W.; Xu, W.G. Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting galectin-1. BioMed Res. Int. 2017, 2017, 3248605. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Fujio, K.; Okamura, T.; Yamamoto, K. Interleukin-27 in T cell immunity. Int. J. Mol. Sci. 2015, 16, 2851–2863. [Google Scholar] [CrossRef] [PubMed]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.M.; Juarez, M.; Hyde, D.M.; Wu, R. Mechanism of dexamethasone-mediated interleukin-8 gene suppression in cultured airway epithelial cells. Am. J. Physiol.-Lung Cell Mol. Physiol. 2001, 280, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, J.W.; Kim, S.C.; Kang, K.H.; Lee, S.K.; Pi, S.H.; Lee, S.K.; Kim, E.C. Roles of p38 and erk map kinases in IL-8 expression in TNF-α- and dexamethasone-stimulated human periodontal ligament cells. Cytokine 2006, 35, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Toebak, M.J.; de Rooij, J.; Moed, H.; Stoof, T.J.; von Blomberg, B.M.; Bruynzeel, D.P.; Scheper, R.J.; Gibbs, S.; Rustemeyer, T. Differential suppression of dendritic cell cytokine production by anti-inflammatory drugs. Br. J. Dermatol. 2008, 158, 225–233. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Rottau, D.; Kohler, F.; Reinert, S.; Alexander, D. Effects of Jaw Periosteal Cells on Dendritic Cell Maturation. J. Clin. Med. 2018, 7, 312. https://doi.org/10.3390/jcm7100312
Dai J, Rottau D, Kohler F, Reinert S, Alexander D. Effects of Jaw Periosteal Cells on Dendritic Cell Maturation. Journal of Clinical Medicine. 2018; 7(10):312. https://doi.org/10.3390/jcm7100312
Chicago/Turabian StyleDai, Jingtao, Daniela Rottau, Franziska Kohler, Siegmar Reinert, and Dorothea Alexander. 2018. "Effects of Jaw Periosteal Cells on Dendritic Cell Maturation" Journal of Clinical Medicine 7, no. 10: 312. https://doi.org/10.3390/jcm7100312



