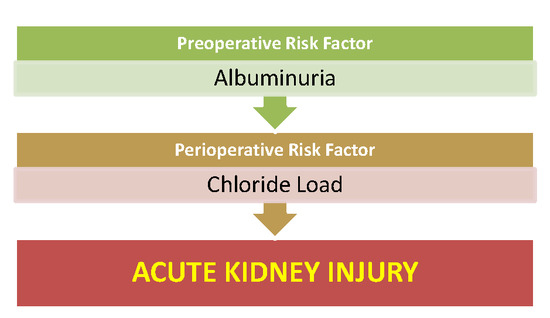
Preoperative Albuminuria and Intraoperative Chloride Load: Predictors of Acute Kidney Injury Following Major Abdominal Surgery
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Outcomes
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ishani, A.; Nelson, D.; Clothier, B.; Schult, T.; Nugent, S.; Greer, N.; Slinin, Y.; Ensrud, K.E. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch. Intern. Med. 2011, 171, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Bihorac, A.; Delano, M.J.; Schold, J.D.; Lopez, M.C.; Nathens, A.B.; Maier, R.V.; Layon, A.J.; Baker, H.V.; Moldawer, L.L. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann. Surg. 2010, 252, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A. Update on acute kidney injury after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2012, 143, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Sang, Y.; Coresh, J.; Ballew, S.; Matsushita, K.; Zolnar, M.Z.; Szabo, Z.; Kalantar-Zadeh, K.; Covesdy, C.P. Acute kidney injury after major surgery: A retrospective analysis of Veterans Health Administration data. Am. J. Kidney Dis. 2016, 67, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, E.; Ferguson, A. Perioperative acute kidney injury: Risk factors, recognition, management and outcomes. BMJ 2010, 341, c3365. [Google Scholar] [CrossRef] [PubMed]
- Bihorac, A.; Yavas, S.; Subbiah, S.; Hobson, C.E.; Schold, J.D.; Gabrielli, A.; Layon, A.J.; Segal, M.S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg. 2009, 249, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.E.; Yavas, S.; Segal, M.S.; Schold, J.D.; Tribble, C.G.; Layon, A.J.; Bihorac, A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009, 119, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.E.; Ozrazgat-Baslanti, T.; Kuxhausen, A.; Thottakara, P.; Efron, P.A.; Moore, F.A.; Moldawer, L.L.; Segal, M.S.; Bihorac, A. Cost and mortality associated with postoperative acute kidney injury. Ann. Surg. 2015, 261, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Kork, F.; Balzer, F.; Spies, C.D.; Wernecke, K.D.; Ginde, A.A.; Jankowski, J.; Eltzschik, H.K. Minor postoperative increases of creatinine are associated with higher mortality and longer hospital length of stay in surgical patients. Anesthesiology 2015, 123, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.P.; Paganini, E.P. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol. 2005, 16, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Grab, J.D.; O’Brien, S.M.; Bridges, C.R.; Gammie, J.S.; Haan, C.K.; Ferguson, T.B.; Peterson, E.D. Society of thoracic surgeons national cardiac surgery database investigators. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 2006, 114, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, 179–184. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists. New classification of physical status. Anesthesiology 1963, 24, 111. [Google Scholar]
- National kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Huen, S.C.; Parikh, C.R. Predicting acute kidney injury after cardiac surgery: A systematic review. Ann. Thorac. Surg. 2012, 93, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Quan, S.; Cheema, K.; Zarnke, K.; Quinn, R.; de Koning, L.; Dixon, E.; Pannu, N.; James, M.T. Risk prediction models for acute kidney injury following major noncardiac surgery: Systematic review. Nephrol. Dial. Transplant. 2016, 31, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kheterpal, S.; Tremper, K.K.; Englesbe, M.J.; O’Reilly, M.; Shanks, A.M.; Fetterman, D.M.; Rosenberg, A.L.; Swartz, R.D. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007, 107, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Biteker, M.; Dayan, A.; Tekkesin, A.I.; Can, M.M.; Tayci, I.; Ilhan, E.; Gülizar, S. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am. J. Surg. 2014, 207, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal Failure: Definition, outcome measures, animal models, fluid therapy and information technology needs. The second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.H.; Marcantonio, E.R.; Mangione, C.M.; Thomas, E.J.; Polanczyk, C.A.; Cook, E.F.; Sugarbaker, D.J.; Donaldson, M.C.; Poss, R.; Ho, K.K.; et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999, 100, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Joannidis, M.; Metnitz, B.; Bauer, P.; Schusterschitz, N.; Moreno, R.; Druml, W.; Metnitz, P.G. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009, 35, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Ando, H.; Fujimura, A. Early detection of drug-induced kidney injury by vanin-1. J. Pharmacol. Exp. Ther. 2012, 341, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Astor, B.C.; Bash, L.D.; Matsushita, K.; Wang, Y.; Coresh, J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J. Am. Soc. Nephrol. 2010, 21, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Dariane, C.; Coscas, R.; Boulitrop, C.; Javerliat, I.; Vilaine, E.; Goeau-Brissonierre, O.; Coggia, M.; Massy, Z.A. Acute kidney injury after open repair of intact abdominal aortic aneurysms. Ann. Vasc. Surg. 2017, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Haase-Fielitz, A.; Bellomo, R.; Devarajan, P.; Story, D.; Matalanis, G.; Dragun, D.; Haase, M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—A prospective cohort study. Crit. Care Med. 2009, 37, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, A.; Joannes-Boyau, O.; Sidobre, C.; Fleureau, C.; Bats, M.L.; Derarche, P.; Leuillet, S.; Ripoche, J.; Combe, C.; Ouattara, A. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin. J. Am. Soc. Nephrol. 2015, 10, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR estimation: From physiology to public health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jongh, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO controversies conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Andersen, L.; Christensen, T.; Hansen, T.K.; Eiskjaer, H.; Gjedsted, J.; Johnsen, S.P.; Hjortdal, V.E. Microalbuminuria is associated with high adverse event rate following cardiac surgery. Eur. J. Cardiothorac. Surg. 2011, 39, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Wu, V.; Young, G.; Lin, Y.F.; Shiao, C.C.; Wu, P.C.; Li, W.Y.; Yu, H.Y.; Hu, F.C.; Lin, J.W.; et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J. Am. Soc. Nephrol. 2010, 22, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.E.; Singhania, G.; Bihorac, A. Acute kidney injury in the surgical patient. Crit. Care Clin. 2015, 31, 705–723. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Salimi, S.; Pierre, V.; Giffuni, G.; Katzel, L.; Parsa, A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Brienza, N.; Giglio, M.T.; Marucci, M.; Fiore, T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit. Care Med. 2009, 37, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Jain, A.K.; Brunelli, S.M.; Coca, S.G.; Devereaux, P.J.; James, M.T.; Luo, J.; Molnar, A.O.; Mrkobrada, M.; Pannu, N.; et al. Association between angiotensin converting enzyme inhibitor or angiotensin receptor blocker use prior to major elective surgery and the risk of acute dialysis. BMC Nephrol. 2014, 15, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobson, C.; Ruchi, R.; Bihorac, A. Perioperative acute kidney injury: Risk factors and predictive strategies. Crit. Care Clin. 2017, 33, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Habib, R.H.; Zacharias, A.; Schwann, T.A.; Riordan, C.J.; Engoren, M.; Durham, S.J.; Shah, A. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: Implications on operative outcome. Crit. Care Med. 2005, 33, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.D.; Davenport, D.L.; Minion, D.J.; Sorial, E.E.; Endean, E.D.; Xenos, E.S. Blood transfusion is associated with increased morbidity and mortality after lower extremity revascularization. J. Vasc. Surg. 2010, 51, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, V.; Maheshwari, K.; Yang, D.; Mascha, E.J.; Singh, A.; Sessler, D.I.; Kurz, A. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery. A retrospective cohort analysis. Anesthesiology 2017, 126, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Hallqvist, L.; Granath, F.; Huldt, E.; Bell, M. Intraoperative hypotension is associated with acute kidney injury in noncardiac surgery. An observational study. Eur. J. Anaesthesiol. 2017, 35, 273–279. [Google Scholar]
- Futier, E.; Lefrant, J.Y.; Guinot, P.G.; Godet, T.; Lorne, E.; Cuvillon, P.; Bertran, S.; Leone, M.; Pastene, B.; Piriou, V.; et al. Effect of individualized vs. standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery. JAMA 2017, 318, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.B.; Jensen, B.L.; Skott, O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension 1998, 32, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Cox, E.F.; Francis, S.T.; Lobo, D.N. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasmalyte (R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann. Surg. 2012, 256, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.D.; Bagshaw, S.M.; Goldstein SL Scherer, L.A.; Duan, M.; Schermer, C.R.; Kellum, J.A. Major complications, mortality and resource utilization after open abdominal surgery: 0.9% Saline compared to plasma-lyte. Ann. Surg. 2012, 255, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.M.; Bellomo, R.; Hegarty, C.; Story, D.; Ho, L.; Bailey, M. Association between a chloride-liberal vs. chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012, 308, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, S.A.; Karkouti, K.; Wijeysundera, D.; Minkovich, L.; Tait, G.; Beattie, W.S. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: A propensity-matched cohort study. Anesth. Analg. 2013, 117, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, M.; Raghunathan, K.; Palusckiewicz, S.M.; Schermer, C.R.; Shaw, A.D. Meta-analysis of high-versus low-chloride content in perioperative and critical care fluid resuscitation. Br. J. Surg. 2015, 102, 24–36. [Google Scholar] [CrossRef] [PubMed]



| AKI Staging | Definition |
|---|---|
| Stage 1 | 1.5-fold increase in sCr or increase of ≥0.3 mg·dL−1 |
| Stage 2 | 2-fold increase in sCr |
| Stage 3 | 3-fold increase in sCr or sCr ≥ 4 mg·dL−1, with acute rise of ≥0.5 mg·dL−1 |
| Non-AKI Group (n = 50) | AKI Group (n = 11) | p Value | Odd’s Ratio (95% CI) | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, (years ± SD) | 66.9 ± 10 | 68.2 ± 10.4 | 0.699 | 1.01 (0.95–1.08) |
| Male gender, (n) | 33 | 8 | 0.67 | 1.02 (0.92–1.13) |
| Mean BMI, kg·m−² | 27.2 ± 4.2 | 27.7 ± 5.6 | 0.721 | 1.03 (0.89–1.19) |
| ASA Classification, (n) | ||||
| ASA II | 27 | 4 | 0.087 | 1.0 |
| ASA III | 20 | 6 | 0.076 | 3.9 (0.73–33.80) |
| ASA IV | 3 | 1 | 0.249 | 4.8 (0.33–70.40) |
| Comorbidities, (n) | ||||
| Hypertension | 31 | 6 | 0.738 | 1.4 (0.20–2.75) |
| CKD | 34 | 9 | 0.481 | 2.1 (0.41–10.95) |
| Stage I | 4 | 2 | 0.226 | 4 (0.42–37.78) |
| Stage II | 25 | 5 | 0.600 | 1.6 (0.28–9.26) |
| Stage III | 5 | 2 | 0.301 | 3.2 (0.35–28.94) |
| CAD | 14 | 4 | 0.717 | 1.5 (0.37–5.81) |
| CHF | 11 | 5 | 0.067 | 3.8 (0.98–14.70) |
| DM | 14 | 5 | 0.137 | 3 (0.76–11.54) |
| COPD | 19 | 6 | 0.174 | 3.1 (0.80–12.09) |
| Surgical Procedures, (n) | ||||
| Other abdominal Surgery | 35 (89.7%) | 4 (10.3%) | 0.079 | |
| Vascular surgery | 15 (68.2%) | 7 (31.8%) | 0.035 | 4.1 (1.04–16.06) |
| Non-AKI Group (n = 50) | AKI Group (n = 11) | p Value | Odd’s Ratio (95% CI) | |
|---|---|---|---|---|
| Serum Creatinine, mg·dL−1 | 0.87 (±0.23) | 1.02 (±0.21) | 0.063 | 12.6 (0.80–198.7) |
| Serum Cystatin C, mg·L−1 | 0.76 (±0.19) | 0.82 (±0.28) | 0.319 | 5.20 (0.21–126.7) |
| eGFR, mL·min−1 1.73 m−2 | 83.92 (±15.15) | 75.27 (±15.97) | 0.095 | 0.97 (0.93–1.01) |
| Urine Albumin, mg·L−1 | 24.1 (±50.7) | 325 (±729.9) | 0.042 | 1.01 (1.00–1.01) |
| Urine Creatinine, g·L−1 | 1.1 (±0.6) | 1.2 (±0.7) | 0.344 | 1.66 (0.58–4.66) |
| UACR > 30 mg·gr−1, (n) | 9 (18.0) | 6 (54.5) | 0.011 | 5.47 (1.36–21.92) |
| FeNa, % | 0.8 (±0.7) | 0.8 (±0.8) | 0.919 | 1.05 (0.40–2.75) |
| FeUrea, % | 41.4 (±12.7) | 37.2 (±10.5) | 0.303 | 0.97 (0.92–1.03) |
| Non-AKI Group (n = 50) | AKI Group (n = 11) | p Value | Odd’s Ratio (95% CI) | |
|---|---|---|---|---|
| Intraoperative Variables(mean±SD) | ||||
| Urine output (mL·kg−1·hr−1) | 2.1 (±2.0) | 1.1 (±0.8) | 0.151 | 0.37 (0.13–1.1) |
| Mean Arterial Pressure (mmHg) | 78.7 (±6.9) | 79.6 (±10.5) | 0.734 | 1.01 (0.93–1.10) |
| Vasopressor administration, (n) | 32 (64.0) | 8 (72.7) | 0.581 | 1.67 (0.86–2.65) |
| Blood loss (mL) | 479 (±508.5) | 1345.5 (±1336.7) | 0.002 | 1.06 (1.02–1.1) |
| Intraoperative Fluids(mean ± SD) | ||||
| Total crystalloids (mL) | 2441 (±1195) | 3018 (±1497) | 0.171 | 1.00 (1.00–1.00) |
| Total colloids (mL) | 865 (±354) | 1046 (±611) | 0.191 | 1.00 (1.00–1.00) |
| RBC transfusion (units) | 1.1 (±1.4) | 2.3 (±2.4) | 0.042 | 1.49 (1.03–2.01) |
| FFP transfusion (units) | 1.2 (±1.4) | 2.6 (±2.1) | 0.006 | 1.69 (1.11–2.56) |
| PLT transfusion (units) | 0.04 (±0.28) | 0.4 (±1.2) | 0.088 | 2.04 (0.73–5.71) |
| Chloride load (mEq) | 458.6 (±179.8) | 583.9 (±191.5) | 0.043 | 1.03 (1.01–1.05) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marouli, D.; Stylianou, K.; Papadakis, E.; Kroustalakis, N.; Kolyvaki, S.; Papadopoulos, G.; Ioannou, C.; Papaioannou, A.; Daphnis, E.; Georgopoulos, D.; et al. Preoperative Albuminuria and Intraoperative Chloride Load: Predictors of Acute Kidney Injury Following Major Abdominal Surgery. J. Clin. Med. 2018, 7, 431. https://doi.org/10.3390/jcm7110431
Marouli D, Stylianou K, Papadakis E, Kroustalakis N, Kolyvaki S, Papadopoulos G, Ioannou C, Papaioannou A, Daphnis E, Georgopoulos D, et al. Preoperative Albuminuria and Intraoperative Chloride Load: Predictors of Acute Kidney Injury Following Major Abdominal Surgery. Journal of Clinical Medicine. 2018; 7(11):431. https://doi.org/10.3390/jcm7110431
Chicago/Turabian StyleMarouli, Diamantina, Kostas Stylianou, Eleftherios Papadakis, Nikolaos Kroustalakis, Stavroula Kolyvaki, Georgios Papadopoulos, Christos Ioannou, Alexandra Papaioannou, Eugene Daphnis, Dimitris Georgopoulos, and et al. 2018. "Preoperative Albuminuria and Intraoperative Chloride Load: Predictors of Acute Kidney Injury Following Major Abdominal Surgery" Journal of Clinical Medicine 7, no. 11: 431. https://doi.org/10.3390/jcm7110431