Helicobacter Pylori Serology in Relation to Hepatitis C Virus Infection and IL28B Single Nucleotide Polymorphism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Determination of HCV Status
2.3. Determination of HCV IL28B SNP
2.4. Determination of Liver Cirrhosis
2.5. Determination of H. pylori Status
2.6. Comparison of the Data to the Previously Reported Cohorts
2.7. Statistical Analysis
3. Results
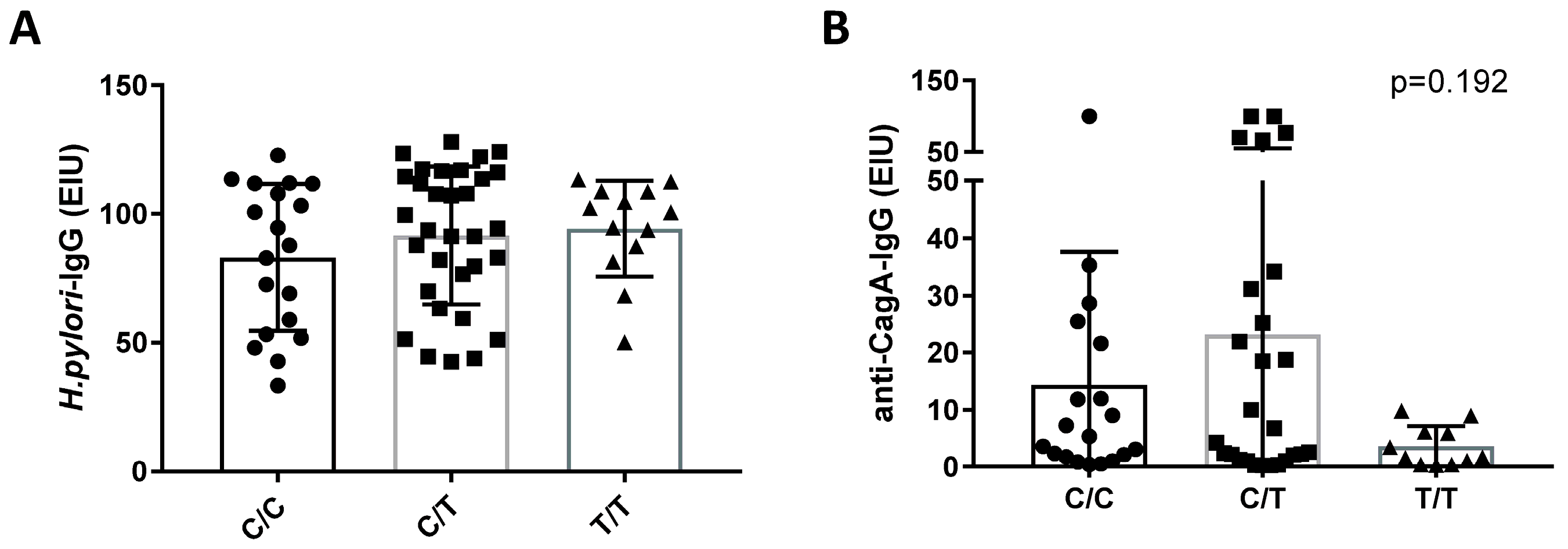
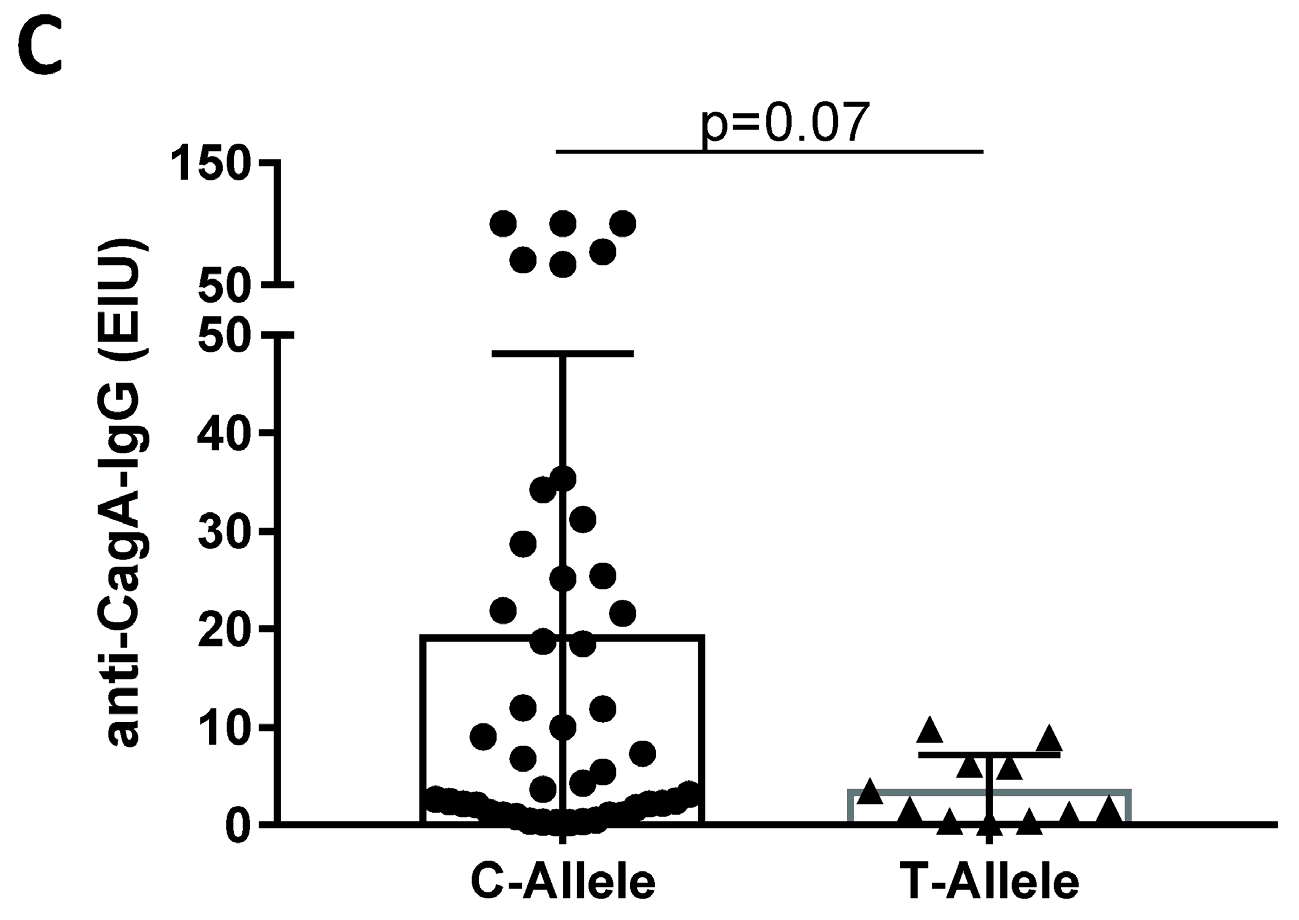
H. pylori Infection and IL28B Genotype
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.P.; Klenerman, P.; Dusheiko, G.M. Hepatitis C. Lancet 2015, 385, 1124–1135. [Google Scholar] [CrossRef]
- Poethko-Müller, C.; Zimmermann, R.; Hamouda, O.; Faber, M.; Stark, K.; Ross, R.S.; Thamm, M. Die Seroepidemiologie der Hepatitis A, B und C in Deutschland. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Vermehren, J.; Schlosser, B.; Domke, D.; Elanjimattom, S.; Müller, C.; Hintereder, G.; Hensel-Wiegel, K.; Tauber, R.; Berger, A. High prevalence of anti-HCV antibodies in two metropolitan emergency departments in Germany: A prospective screening analysis of 28,809 patients. PLoS ONE 2012, 7, e41206. [Google Scholar] [CrossRef] [PubMed]
- Au, T.H.; Destache, C.J.; Vivekanandan, R. Hepatitis C therapy: Looking toward interferon-sparing regimens. J. Am. Pharm. Assoc. 2015, 55, e72–e86. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C.; Berg, T.; Ross, R.; Schirmacher, P.; Wedemeyer, H.; Neumann, U.; Schmidt, H.H.; Spengler, U.; Wirth, S.; Kessler, H.H.; et al. Update der S 3-Leitlinie Prophylaxe, Diagnostik und Therapie der Hepatitis-C-Virus(HCV)-Infektion, AWMF-Register-Nr.: 021/012. Prophylaxis, diagnosis and therapy of Hepatitis C virus (HCV) infection: The German guidelines on the management of HCV infection. Z. Gastroenterol. 2010, 48, 289–351. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.D.; Tong, X.; Moorman, A.C.; Ly, K.N.; Rupp, L.; Xu, F.; Gordon, S.C.; Holmberg, S.D. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J. Hepatol. 2015, 63, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Shaib, Y.H.; El-Serag, H.B.; Nooka, A.K.; Thomas, M.; Brown, T.D.; Patt, Y.Z.; Hassan, M.M. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A hospital-based case? control study. Am. J. Gastroenterol. 2007, 102, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K.; Tack, J.; Kuipers, E.E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Link, A.; Selgrad, M. Helicobacter pylori: Perspectives and time trends. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [PubMed]
- Wex, T.; Venerito, M.; Kreutzer, J.; Götze, T.; Kandulski, A.; Malfertheiner, P. Serological prevalence of Helicobacter pylori infection in Saxony-Anhalt, Germany, in 2010. Clin. Vaccine Immunol. 2011, 18, 2109–2112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuipers, E.J. Helicobacter pylori and the risk and management of associated diseases: Gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment. Pharmacol. Ther. 1997, 11, 71–88. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Parsonnet, J.; Van Doorn, L.J.; Franceschi, S. Helicobacter species in cancers of the gallbladder and extrahepatic biliary tract. Br. J. Cancer 2009, 100, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.Y.; Xin, Y.N.; Chen, A.J.; Dong, Q.J.; Qiang, X.; Li, N.; Zheng, M.H.; Guan, H.S. Association between the presence of H pylori in the liver and hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2008, 14, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Anver, M.R.; Haines, D.C.; Benveniste, R.E. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am. J. Pathol. 1994, 145, 959–968. [Google Scholar] [PubMed]
- Wang, J.; Li, W.-T.; Zheng, Y.-X.; Zhao, S.-S.; Li, N.; Huang, Y. The Association between Helicobacter pylori infection and chronic hepatitis C: A meta-analysis and trial sequential analysis. Gastroenterol. Res. Pract. 2016, 2016, 8780695. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Nelson, D.R.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. An update on treatment of Genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Aygen, B.; Korkmaz, K.; Yıldız, O.; Zararsız, G.; Canatan, H. Characterization of the interleukin-28B gene rs12979860 C/T polymorphism in Turkish chronic hepatitis C patients and healthy individuals. Balkan Med. J. 2015, 32, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L.; Thio, C.L.; Martin, M.P.; Qi, Y.; Ge, D.; O’hUigin, C.; Kidd, J.; Kidd, K.; Khakoo, S.I.; Alexander, G.; et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009, 461, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Falleti, E.; Bitetto, D.; Fabris, C.; Cussigh, A.; Fornasiere, E.; Cmet, S.; Fumolo, E.; Bignulin, S.; Fontanini, E.; Cerutti, A.; et al. Role of interleukin 28B rs12979860 C/T polymorphism on the histological outcome of chronic hepatitis C: Relationship with gender and viral genotype. J. Clin. Immunol. 2011, 31, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Thompson, A.J.; Critelli, R.; Crosignani, A.; Rossi, S.; De Lisi, S.; Cariani, E.; Zermiani, P.; Vaira, V.; Boccaccio, V.; et al. Interferon lambda-3 is not associated with clinical outcome in patients with HCV-induced compensated cirrhosis: A long-term cohort study. Antivir. Res. 2015, 113, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Tameshkel, F.S.; Niya, M.H.; Sohrabi, M.; Panahi, M.; Zamani, F.; Imanzade, F.; Rakhshani, N. Polymophism of IL-28B gene (rs12979860) in HCV genotype 1 patients treated by pegylated interferon and ribavirin. Iran. J. Pathol. 2016, 11, 216–221. [Google Scholar]
- Aziz, H.; Raza, A.; Ali, K.; Khattak, J.Z.K.; Irfan, J.; Gill, M.L. Polymorphism of the IL28B gene (rs8099917, rs12979860) and virological response of Pakistani hepatitis C virus Genotype 3 patients to pegylated interferon therapy. Int. J. Infect. Dis. 2015, 30, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C.; Susser, S.; Doehring, A.; Lange, C.M.; Müller, T.; Schlecker, C.; Herrmann, E.; Lötsch, J.; Berg, T. Importance of IL28B gene polymorphisms in hepatitis C virus Genotype 2 and 3 infected patients. J. Hepatol. 2011, 54, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Khubaib, B.; Idrees, M.; Fatima, Z.; Akram, M.; Afzal, S.; Amin, I.; Shahid, M.; Wasim, M. Evaluation of three techniques for detection of IL28B SNP: A prognostic tool for HCV treatment outcome. J. Dig. Dis. 2017, 18, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ziol, M.; Handra-Luca, A.; Kettaneh, A.; Christidis, C.; Mal, F.; Kazemi, F.; de Lédinghen, V.; Marcellin, P.; Dhumeaux, D.; Trinchet, J.C.; et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005, 41, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zarski, J.P.; Sturm, N.; Guechot, J.; Paris, A.; Zafrani, E.S.; Asselah, T.; Boisson, R.C.; Bosson, J.L.; Guyader, D.; Renversez, J.C.; et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: The ANRS HCEP-23 study. J. Hepatol. 2012, 56, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Langner, C.; Schirrmeister, W.; Habendorf, W.; Weigt, J.; Venerito, M.; Tammer, I.; Schlüter, D.; Schlaermann, P.; Meyer, T.F.; et al. Helicobacter pylori vacA genotype is a predominant determinant of immune response to Helicobacter pylori CagA. World J. Gastroenterol. 2017, 23, 4712–4723. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.; Hoffmann, A.; Link, A.; Schulz, C.; Wuttig, K.; Becker, E.; Heim, M.; Venerito, M. Prevalence of Helicobacter pylori infection among blood donors in Saxony-Anhalt, Germany—A region at intermediate risk for gastric cancer. Z. Gastroenterol. 2017, 55, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Bose, K.; Franck, C.; Müller, M.N.; Canbay, A.; Link, A. Perioperative therapy of oesophagogastric adenocarcinoma: Mainstay and future directions. Gastroenterol. Res. Pract. 2017, 2017, 5651903. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Axon, A.; Brenner, H. Epidemiology of Helicobacter pylori infection. Helicobacter 2016, 21, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weck, M.N.; Schöttker, B.; Rothenbacher, D.; Brenner, H. Gastric parietal cell antibodies, Helicobacter pylori infection, and chronic atrophic gastritis: Evidence from a large population-based study in Germany. Cancer Epidemiol. Biomark. Prev. 2013, 22, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Krumbiegel, P.; Richter, T.; Richter, M.; Röder, S.; Rolle-Kampczyk, U.; Herbarth, O. Helicobacter pylori prevalence in children influenced by non-specific antibiotic treatments. Cent. Eur. J. Public Health 2014, 22, 48–53. [Google Scholar] [PubMed]
- Roberts, E.A.; Yeung, L. Maternal-Infant Transmission. Hepatology 2002, 36, S106–S113. [Google Scholar] [PubMed]
- Mele, A.; Stroffolini, T.; Tosti, M.E.; Corona, R.; Santonastasi, F.; Gallo, G.; Ragni, P.; Balocchini, E.; Bernacchia, R.; Moiraghi, A. Heterosexual transmission of hepatitis C in Italy. J. Med. Virol. 1999, 57, 111–113. [Google Scholar] [CrossRef]
- Roberts, S.E.; Morrison-Rees, S.; Samuel, D.G.; Thorne, K.; Akbari, A.; Williams, J.G. Review article: The prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment. Pharmacol. Ther. 2016, 43, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.; Lehours, P.; Mégraud, F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 2015, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2014: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2014. [Google Scholar]
- Stein, M.; Ruggiero, P.; Rappuoli, R.; Bagnoli, F. Helicobacter pylori CagA: From pathogenic mechanisms to its use as an anti-cancer vaccine. Front. Immunol. 2013, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Achtman, M. Helicobacter pylori: Recombination, population structure and human migrations. Int. J. Med. Microbiol. 2004, 294, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, G.I.; Olivares, A.Z.; Foo, F.Y.; Foo, S.; Neusy, A.J.; Ng, C.; Holzman, R.S.; Marmor, M.; Blaser, M.J. Seroprevalence of Helicobacter pylori in New York City populations originating in East Asia. J. Urban Health 2005, 82, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 66, 310–335. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Sala, N.; Capellá, G. Genetic susceptibility and gastric cancer risk. Int. J. Cancer 2002, 100, 249–260. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | H. pylori-Positive | H. pylori-Negative | p-Value |
|---|---|---|---|---|
| n | n (%) | n (%) | ||
| HCV-positive | 189 * | 72 (38.1%) | 117 (61.9%) | n.s. |
| Gender | ||||
| male | 105 | 39 (37.1%) | 66 (62.9%) | |
| female | 84 | 33 (39.3%) | 51 (60.7%) | |
| Age | 51.32 ± 13.4 | 47.11 ± 13.4 | 53.91 ± 13.3 | <0.001 # |
| ≤40 years | 44 | 27 (61.4%) | 17 (38.6%) | <0.001 |
| >40 years | 145 | 45 (31.0%) | 100 (69.0%) | |
| HCV genotype | 182 * | 71 (39.1%) | 111 (60.9%) | |
| 1 | 156 | 55 (35.3%) | 101 (64.7%) | |
| 2 | 4 | 1 (25%) | 3 (75%) | |
| 3 | 18 | 13 (72.2%) | 5 (27.8%) | |
| 4 | 3 | 2 (66.7%) | 1 (33.3%) | |
| 5 | 0 | 0 | 0 | |
| 6 | 1 | 0 | 1 (100%) | |
| Origin: | 176 * | |||
| Western Europe | 131 | 38 (29.0%) | 93 (71.0%) | <0.001 |
| Eastern Europe | 45 | 29 (64.4%) | 16 (35.6%) | |
| Coinfection to HCV: | 188 * | |||
| None | 158 | 53 (33.5%) | 105 (66.5%) | <0.005 |
| HBV | 22 | 15 (68.2%) | 7 (31.8%) | |
| HIV | 8 | 3 (37.5%) | 5 (62.5%) | |
| Liver status: | 189 * | |||
| no liver cirrhosis | 134 | 61 (45.5%) | 73 (54.5%) | <0.005 |
| liver cirrhosis | 55 | 11 (20.0%) | 44 (80.0%) | |
| IL28B-SNP genotype | 169 * | |||
| C/C | 45 | 19 (42.2%) | 26 (57.8%) | n.s. |
| C/T | 91 | 32 (35.2%) | 59 (64.8%) | |
| T/T | 33 | 13 (39.4%) | 20 (60.6%) |
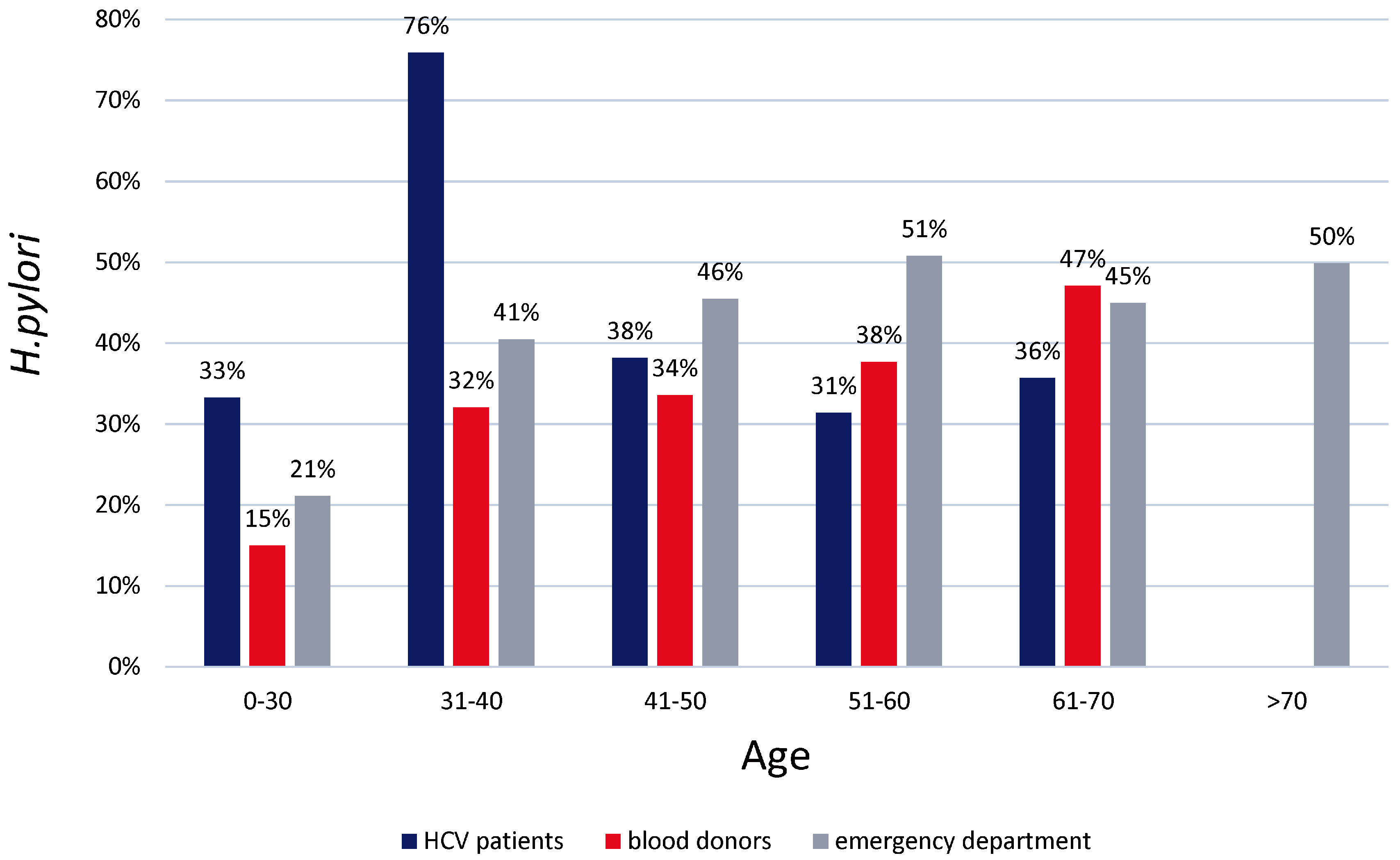
| Age in Years | No. of H. pylori-Positive Subjects/All Subjects (%) | ||
|---|---|---|---|
| Current Study | Wex et al. 2011 | Franck et al. 2017 | |
| overall | 72/189 (38.1%) | 1029/2318 (44.4%) | 149/516 (28.9%) |
| ≤30 | 5/15 (33.3%) | 61/289 (21.1%) | 15% |
| 31–40 | 22/29 (75.9%) | 75/185 (40.5%) | 32% |
| 41–50 | 13/34 (38.2%) | 122/268 (45.5%) | 34% |
| 51–60 | 22/70 (31.4%) | 167/329 (50.8%) | 38% |
| 61–70 | 10/28 (35.7%) | 167/371 (45.0%) | 47% |
| >70 | 0/13 (0%) | 437/876 (49.9%) | n.a. * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutwerk, A.; Wex, T.; Stein, K.; Langner, C.; Canbay, A.; Malfertheiner, P.; Link, A. Helicobacter Pylori Serology in Relation to Hepatitis C Virus Infection and IL28B Single Nucleotide Polymorphism. J. Clin. Med. 2018, 7, 44. https://doi.org/10.3390/jcm7030044
Gutwerk A, Wex T, Stein K, Langner C, Canbay A, Malfertheiner P, Link A. Helicobacter Pylori Serology in Relation to Hepatitis C Virus Infection and IL28B Single Nucleotide Polymorphism. Journal of Clinical Medicine. 2018; 7(3):44. https://doi.org/10.3390/jcm7030044
Chicago/Turabian StyleGutwerk, Alexander, Thomas Wex, Kerstin Stein, Cosima Langner, Ali Canbay, Peter Malfertheiner, and Alexander Link. 2018. "Helicobacter Pylori Serology in Relation to Hepatitis C Virus Infection and IL28B Single Nucleotide Polymorphism" Journal of Clinical Medicine 7, no. 3: 44. https://doi.org/10.3390/jcm7030044
APA StyleGutwerk, A., Wex, T., Stein, K., Langner, C., Canbay, A., Malfertheiner, P., & Link, A. (2018). Helicobacter Pylori Serology in Relation to Hepatitis C Virus Infection and IL28B Single Nucleotide Polymorphism. Journal of Clinical Medicine, 7(3), 44. https://doi.org/10.3390/jcm7030044






