Multi-Detector Computed Tomography Imaging Techniques in Arterial Injuries
Abstract
:1. Introduction
2. Technique
2.1. Contrast Bolus
2.2. Imaging Phases
2.3. Image Acquisition and Reconstruction
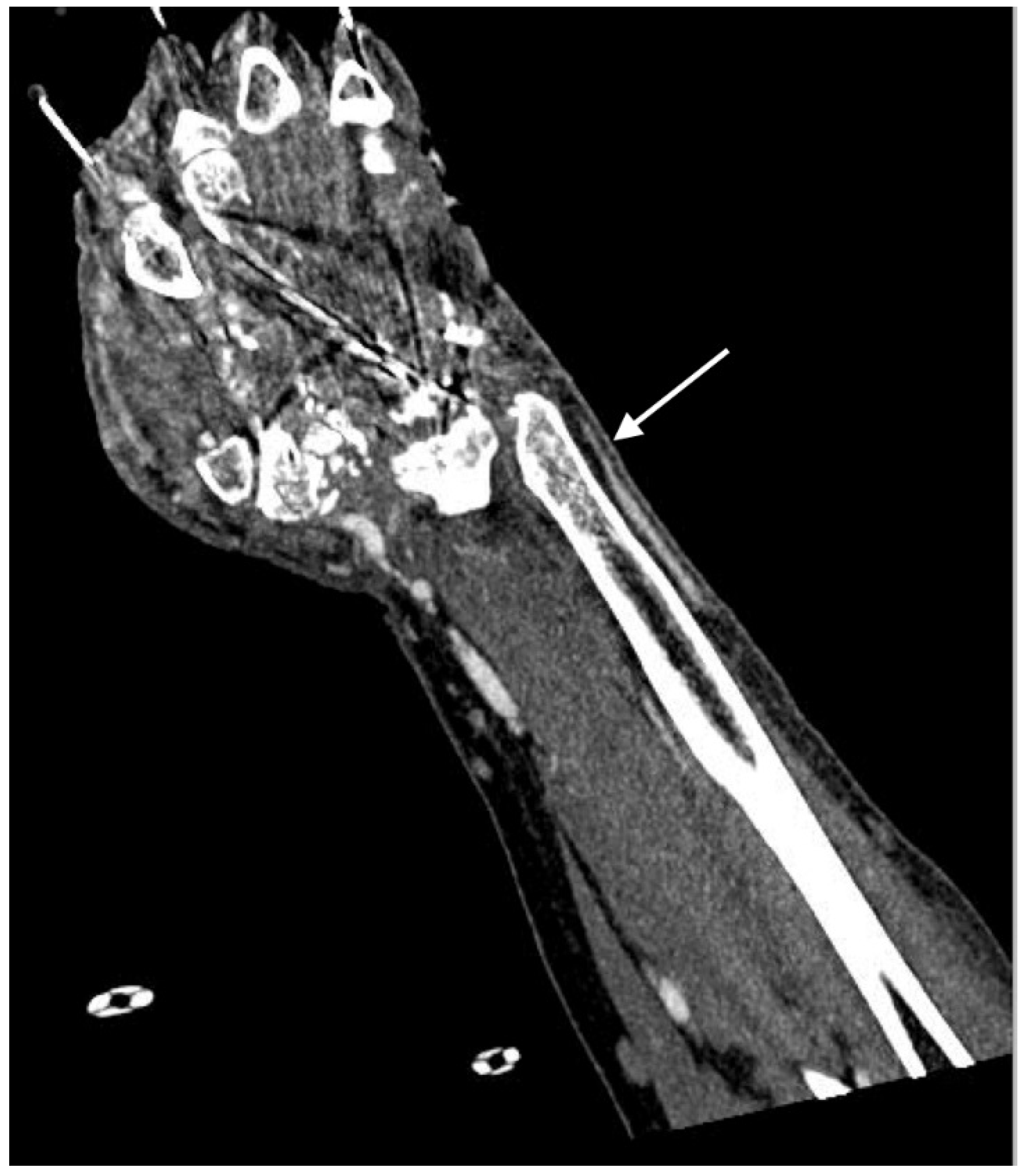
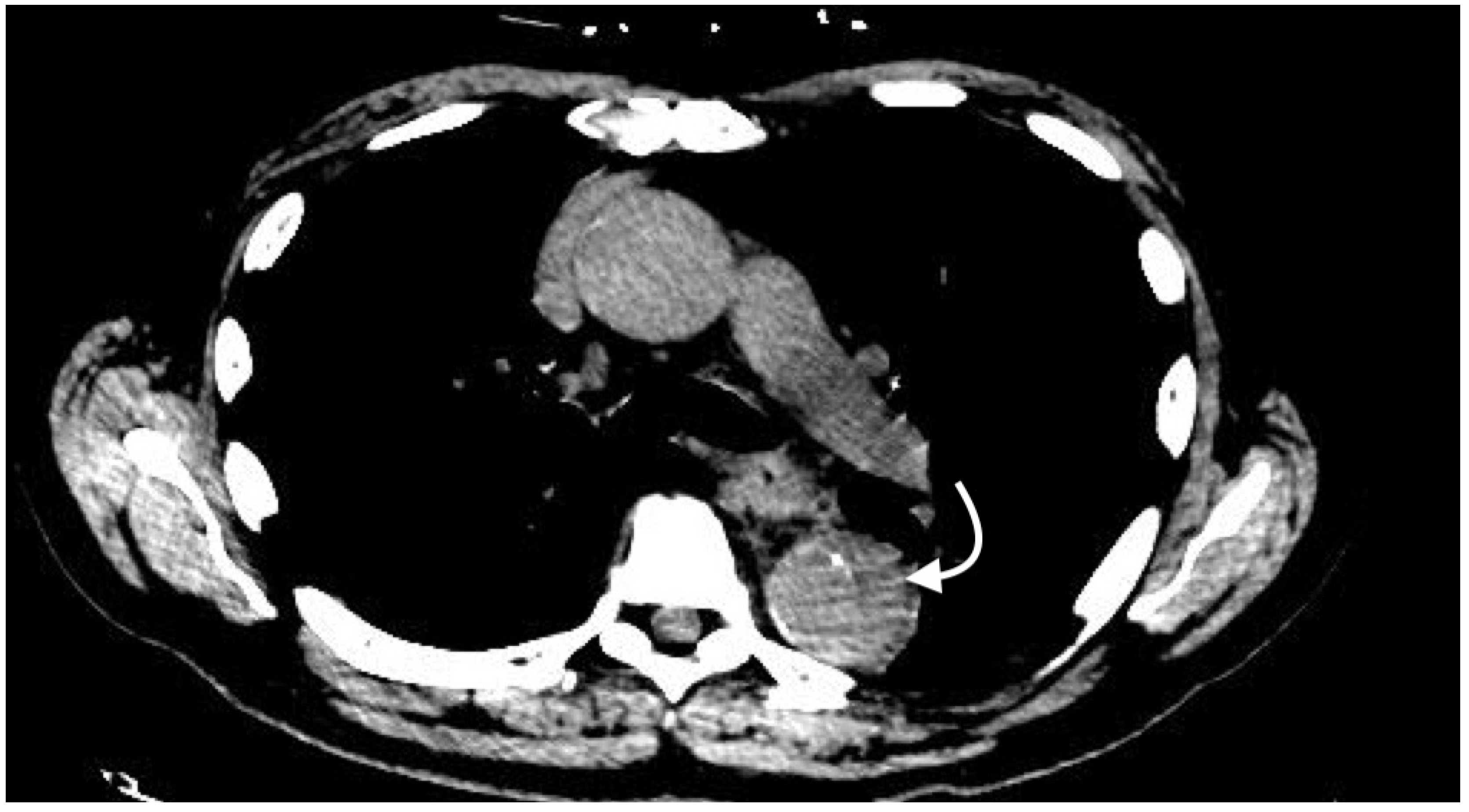
2.4. Imaging Patterns in Arterial Injury
2.4.1. Active Arterial Extravasation
2.4.2. Arterial Occlusion or Stenosis
2.4.3. Arterial Dissection
2.4.4. Intramural Hematoma
2.4.5. Pseudoaneurysm
2.4.6. Arteriovenous Fistula
2.5. Imaging Performance
2.6. Pitfalls
2.7. Future Technology
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Múnera, F.; Soto, J.A.; Palacio, D.; Velez, S.M.; Medina, E. Diagnosis of arterial injuries caused by penetrating trauma to the neck: Comparison of helical CT angiography and conventional angiography. Radiology 2000, 216, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lell, M.M.; Anders, K.; Uder, M.; Klotz, E.; Ditt, H.; Vega-Higuera, F.; Boskamp, T.; Bautz, W.A.; Tomandl, B.F. New Techniques in CT Angiography. RadioGraphics 2006, 26, S45–S62. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Kim, J.K.; Jeong, Y.Y.; Seo, J.J.; Park, J.G.; Kang, H.K. Pelvic arterial hemorrhage in patients with pelvic fractures: Detection with contrast-enhanced CT. Radiographics 2004, 24, 1591–1606. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M.; Mallouhi, A.; Tauscher, T.; Lutz, M.; Jaschke, W.R. Traumatic arterial injuries of the extremities: Initial evaluation with MDCT angiography. Am. J. Roentgenol. 2006, 186, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.D.; Manickam Kumaravel, B.; Michael, B.L. Multidetector CT Evaluation of Active Extravasation in Blunt Abdominal and Pelvic Trauma Patients. Radiographics 2008, 28, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T. Peak Contrast Enhancement in CT and MR Angiography: When Does It Occur and Why? Pharmacokinetic Study in a Porcine Model. Radiology 2003, 227, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Adibi, A.; Shahbazi, A. Automatic bolus tracking versus fixed time-delay technique in biphasic multidetector computed tomography of the abdomen. Iran. J. Radiol. 2014, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Van Hoe, L.; Marchal, G.; Baert, A.L.; Gryspeerdt, S.; Mertens, L. Determination of scan delay time in spiral CT-angiography: Utility of a test bolus injection. J. Comput. Assist. Tomogr. 1995, 19, 216–220. [Google Scholar] [PubMed]
- Henzler, T.; Meyer, M.; Reichert, M.; Krissak, R.; Nance, J.W.; Haneder, S.; Schoenberg, S.O.; Fink, C. Dual-energy CT angiography of the lungs: Comparison of test bolus and bolus tracking techniques for the determination of scan delay. Eur. J. Radiol. 2012, 81, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T. Test-bolus versus bolus-tracking techniques for CT angiographic timing. Radiology 2005, 236, 369–370. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.; Squirrell, C.A. Multidetector CT of Aortic Dissection: A Pictorial Review. Radiographics 2010, 30, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Fishman, E.K.; Ney, D.R.; Heath, D.G.; Corl, F.M.; Horton, K.M.; Johnson, P.T. Volume Rendering versus Maximum Intensity Projection in CT Angiography: What Works Best, When, and Why. RadioGraphics 2006, 26, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, J.L.; Anderson, S.W.; Murakami, A.M.; Pieroni, S.; Rhea, J.T.; Soto, J.A. Detection of vascular injuries in patients with blunt pelvic trauma by using 64-channel multidetector CT. Radiographics 2009, 29, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Miller-Thomas, M.M.; West, O.C.; Cohen, A.M. Diagnosing traumatic arterial injury in the extremities with CT angiography: Pearls and pitfalls. Radiographics 2005, 25 (Suppl. 1), S133–S142. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.A.; Munera, F.; Morales, C.; Lopera, J.E.; Holguin, D.; Guarin, O.; Castrillon, G.; Sanabria, A.; Garcia, G. Focal arterial injuries of the proximal extremities: Helical CT arteriography as the initial method of diagnosis. Radiology 2001, 218, 188–194. [Google Scholar] [CrossRef] [PubMed]
- LePage, M.A.; Quint, L.E.; Sonnad, S.S.; Deeb, G.M.; Williams, D.M. Aortic dissection: CT features that distinguish true lumen from false lumen. Am. J. Roentgenol. 2001, 177, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Gutschow, S.E.; Walker, C.M.; Martínez-Jiménez, S.; Rosado-de-Christenson, M.L.; Stowell, J.; Kunin, J.R. Emerging Concepts in Intramural Hematoma Imaging. RadioGraphics 2016, 36, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Alkadhi, H.; Wildermuth, S.; Desbiolles, L.; Schertler, T.; Crook, D.; Marincek, B.; Boehm, T. Vascular emergencies of the thorax after blunt and iatrogenic trauma: Multi-detector row CT and three-dimensional imaging. Radiographics 2004, 24, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.E.A.; Saad, W.E.A.; Davies, M.G.; Waldman, D.L.; Fultz, P.J.; Rubens, D.J. Pseudoaneurysms and the Role of Minimally Invasive Techniques in Their Management. RadioGraphics 2005, 25, S173–S189. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.K.; Sullivan, J.; Frentz, G. Renal trauma: Radiological studies. Comparison of urography, computed tomography, angiography, and radionuclide studies. Radiology 1985, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Robbs, J.V.; Carrim, A.A.; Kadwa, A.M.; Mars, M. Traumatic arteriovenous fistula: Experience with 202 patients. Br. J. Surg. 1994, 81, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.J.; O’Brien, D.P.; Luchette, F.A.; Choe, K.A.; Lim, E.; Davis, K.; Hurst, J.M.; Johannigman, J.A.; Frame, S.B. Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery 2000, 128, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Potzman, J.; Munera, F.; McKenney, M.; Munoz, R.; Rivas, L.; Dunham, M.; DuBose, J.; Frykberg, E.R.; Bilello, J. Multi-slice CT angiography for arterial evaluation in the injured lower extremity. J. Trauma Inj. Infect. Crit. Care 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Gavant, M.L.; Menke, P.G.; Fabian, T.; Flick, P.A.; Graney, M.J.; Gold, R.E. Blunt traumatic aortic rupture: Detection with helical CT of the chest. Radiology 1995, 197, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.C.; Krauss, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Erdahl, C.; Zeng, K.; Potts, T.; Sharafuddin, M.; Saba, O.; Wang, G.; Bai, E.-W. Adaptive bolus chasing computed tomography angiography: Control scheme and experimental results. Biomed. Signal Process. Control 2008, 3, 319–326. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adler, C.; Hangge, P.T.; Albadawi, H.; Knuttinen, M.-G.; Alzubaidi, S.J.; Naidu, S.G.; Oklu, R. Multi-Detector Computed Tomography Imaging Techniques in Arterial Injuries. J. Clin. Med. 2018, 7, 88. https://doi.org/10.3390/jcm7050088
Adler C, Hangge PT, Albadawi H, Knuttinen M-G, Alzubaidi SJ, Naidu SG, Oklu R. Multi-Detector Computed Tomography Imaging Techniques in Arterial Injuries. Journal of Clinical Medicine. 2018; 7(5):88. https://doi.org/10.3390/jcm7050088
Chicago/Turabian StyleAdler, Cameron, Patrick T. Hangge, Hassan Albadawi, M-Grace Knuttinen, Sadeer J. Alzubaidi, Sailendra G. Naidu, and Rahmi Oklu. 2018. "Multi-Detector Computed Tomography Imaging Techniques in Arterial Injuries" Journal of Clinical Medicine 7, no. 5: 88. https://doi.org/10.3390/jcm7050088
APA StyleAdler, C., Hangge, P. T., Albadawi, H., Knuttinen, M.-G., Alzubaidi, S. J., Naidu, S. G., & Oklu, R. (2018). Multi-Detector Computed Tomography Imaging Techniques in Arterial Injuries. Journal of Clinical Medicine, 7(5), 88. https://doi.org/10.3390/jcm7050088




