A Novel Mutation in the Stalk Domain of KIF5A Causes a Slowly Progressive Atypical Motor Syndrome
Abstract
1. Introduction
2. Case Presentation
2.1. Evaluation at Onset
2.2. Evaluation after Three Years from Onset
2.3. Evaluation after Four Years from Onset
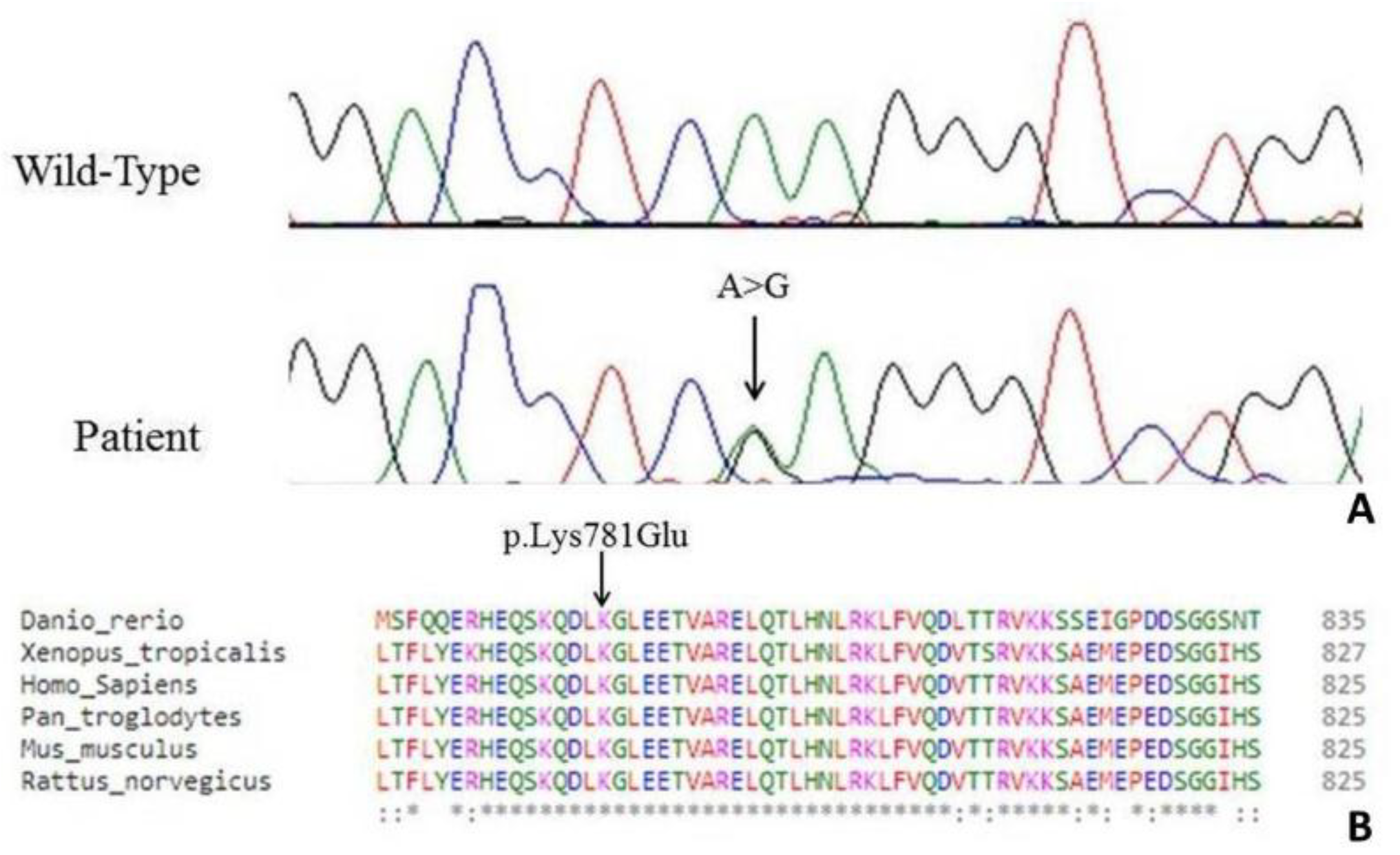
3. Genetic Analysis
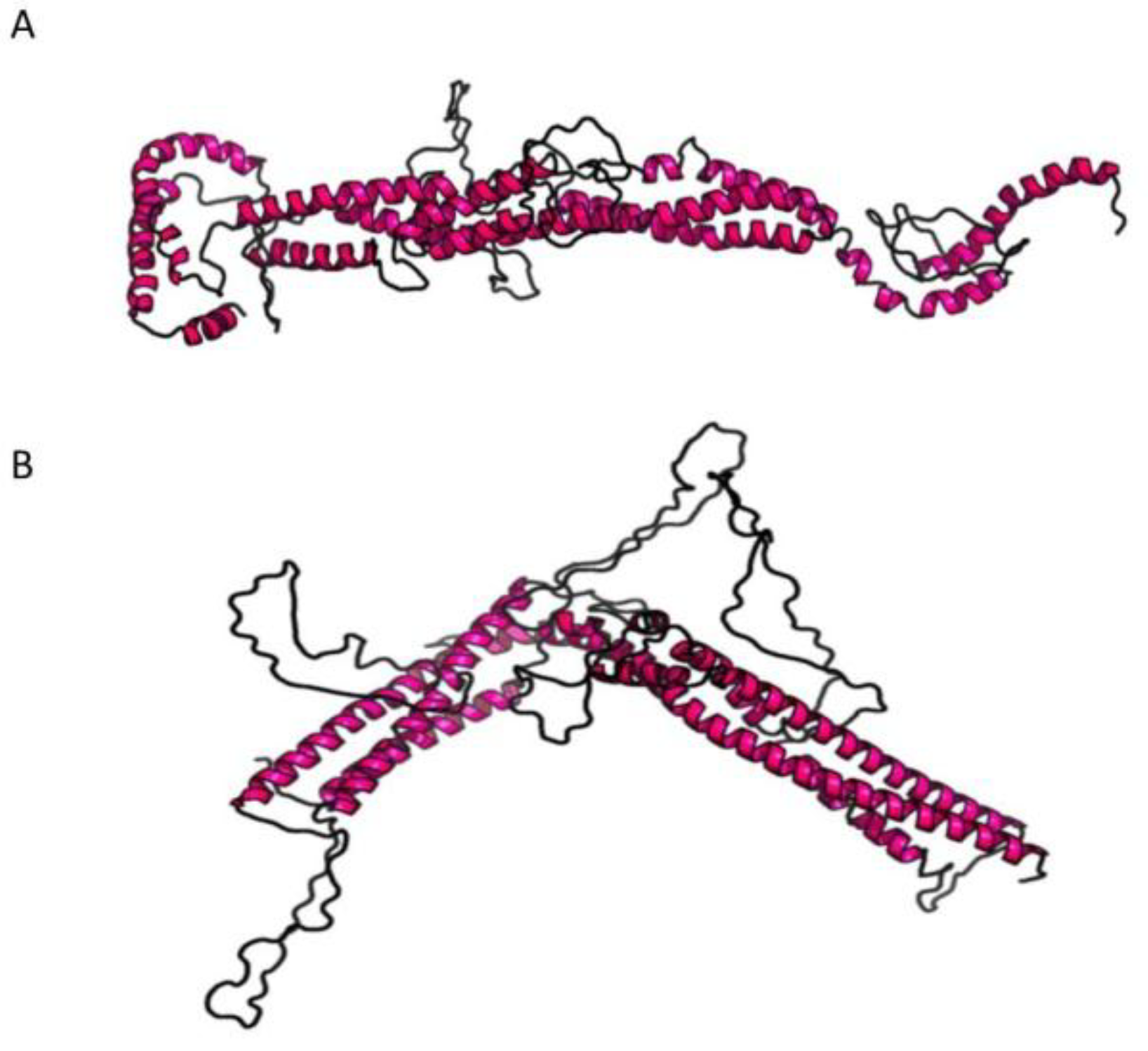
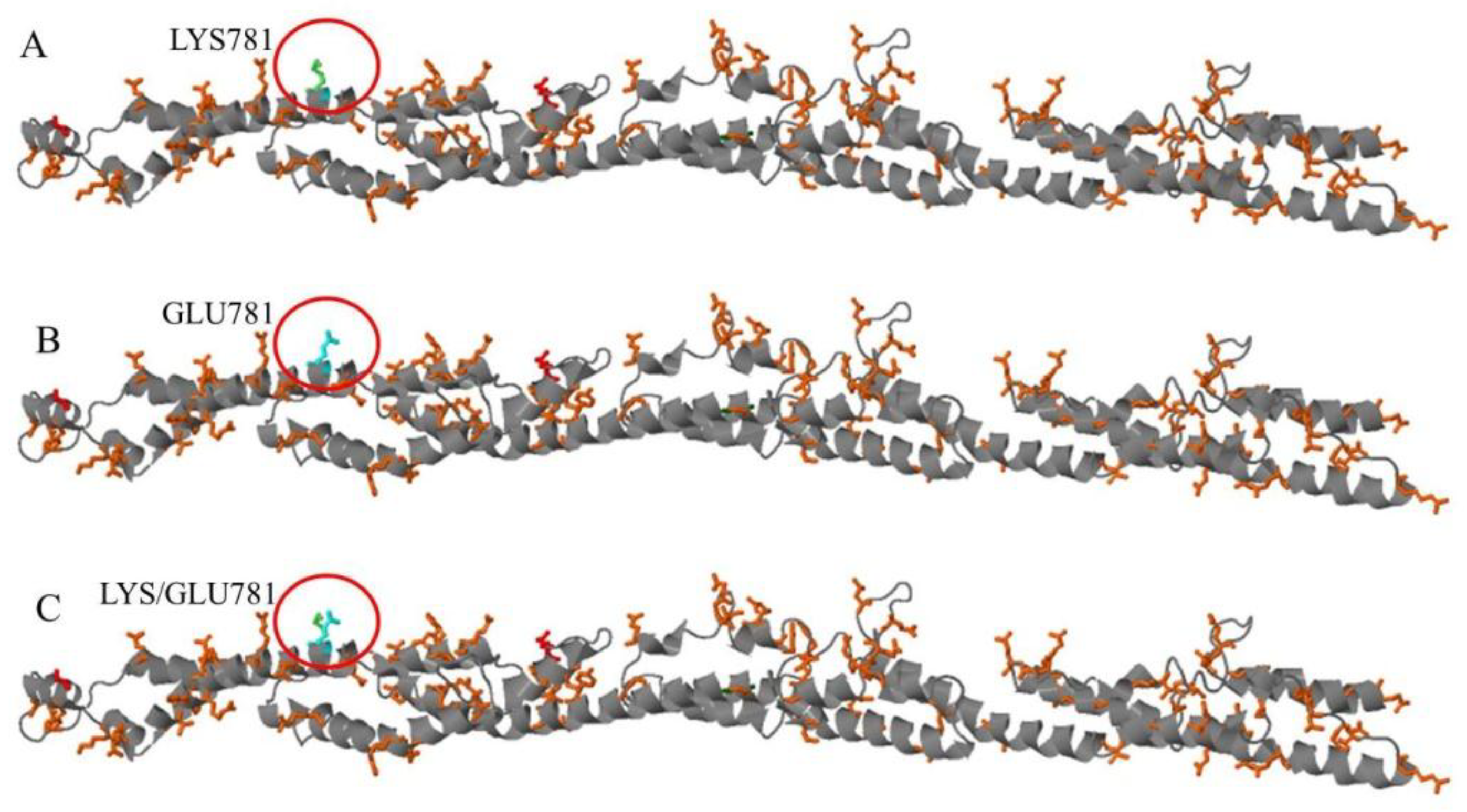
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Goldstein, L.S. Kinesin molecular motors: Transport pathways, receptors, and human disease. Proc. Natl. Acad. Sci. USA 2001, 98, 6999–7003. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Okada, Y.; Hirokawa, N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Hancock, W.O.; Howard, J. Processivity of the motor protein kinesin requires two heads. J. Cell Biol. 1998, 140, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Goizet, C.; Boukhris, A.; Mundwiller, E.; Tallaksen, C.; Forlani, S.; Toutain, A.; Carriere, N.; Paquis, V.; Depienne, C.; Durr, A.; et al. Complicated forms of autosomal dominant hereditary spastic paraplegia are frequent in SPG10. Hum. Mutat. 2009, 30, E376–E385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Laurá, M.; Hersheson, J.; Horga, A.; Jaunmuktane, Z.; Brandner, S.; Pittman, A.; Hughes, D.; Polke, J.M.; Sweeney, M.G.; Proukakis, C. Extended phenotypic spectrum of KIF5A mutations: From spastic paraplegia to axonal neuropathy. Neurology 2014, 83, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Vale, R.D.; Fletterick, R.J. The design plan of kinesin motors. Annu. Rev. Cell Dev. Biol. 1997, 13, 745–777. [Google Scholar] [CrossRef] [PubMed]
- Cuchanski, M.; Baldwin, K.J. Mutation in KIF5A c.610C>T causing hereditary spastic paraplegia with axonal sensorimotor neuropathy. Case Rep. Neurol. 2018, 10, 165–168. [Google Scholar] [CrossRef]
- Rydzanicz, M.; Jagła, M.; Kosinska, J.; Tomasik, T.; Sobczak, A.; Pollak, A.; Herman-Sucharska, I.; Walczak, A.; Kwinta, P.; Płoski, R. KIF5A de novo mutation associated with myoclonic seizures and neonatal onset progressive leukoencephalopathy. Clin. Genet. 2017, 91, 769–773. [Google Scholar] [CrossRef]
- Brenner, D.; Yilmaz, R.; Müller, K.; Grehl, T.; Petri, S.; Meyer, T.; Grosskreutz, J.; Weydt, P.; Ruf, W.; Neuwirth, C.; et al. Hot-spot KIF5A mutations cause familial ALS. Brain 2018, 141, 688–697. [Google Scholar] [CrossRef]
- Nicolas, A.; Kenna, K.P.; Renton, A.E.; Ticozzi, N.; Faghri, F.; Chia, R.; Dominov, J.A.; Kenna, B.J.; Nalls, M.A.; Keagle, P.; et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 2018, 97, 1268–1283. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Q.; Shen, D.; Tai, H.; Liu, S.; Wang, Z.; Shi, J.; Fu, H.; Wu, S.; Ding, Q.; et al. Mutation analysis of KIF5A in Chinese amyotrophic lateral sclerosis patients. Neurobiol. Aging 2018. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinformat. 2013, 43, 11101–11133. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kull, F.J.; Sablin, E.P.; Lau, R.; Fletterick, R.J.; Vale, R.D. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 1996, 380, 550–555. [Google Scholar] [CrossRef]
- Crimella, C.; Baschirotto, C.; Arnoldi, A.; Tonelli, A.; Tenderini, E.; Airoldi, G.; Martinuzzi, A.; Trabacca, A.; Losito, L.; Scarlato, M.; et al. Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot-Marie-Tooth type 2. Clin. Genet. 2012, 82, 157–164. [Google Scholar] [CrossRef]
- Ebbing, B.; Mann, K.; Starosta, A.; Jaud, J.; Schöls, L.; Schüle, R.; Woehlke, G. Effect of spastic paraplegia mutations in KIF5A kinesin on transport activity. Hum. Mol. Genet. 2008, 17, 1245–1252. [Google Scholar] [CrossRef]
- Jennings, S.; Chenevert, M.; Liu, L.; Mottamal, M.; Wojcik, E.J.; Huckaba, T.M. Characterization of kinesin switch I mutations that cause hereditary spastic paraplegia. PLoS ONE 2017, 12, e0180353. [Google Scholar] [CrossRef]
- Lo Giudice, M.; Neri, M.; Falco, M.; Sturnio, M.; Calzolari, E.; Di Benedetto, D.; Fichera, M. A missense mutation in the coiled-coil domain of the KIF5A gene and late-onset hereditary spastic paraplegia. Arch. Neurol. 2006, 63, 284–287. [Google Scholar] [CrossRef]
- Reid, E.; Kloos, M.; Ashley-Koch, A.; Hughes, L.; Bevan, S.; Svenson, I.K.; Graham, F.L.; Gaskell, P.C.; Dearlove, A.; Pericak-Vance, M.A.; et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am. J. Hum. Genet. 2002, 71, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.A.; Ma, S.; Hedera, P. Mutation in KIF5A can also cause adult-onset hereditary spastic paraplegia. Neurogenetics 2006, 7, 47–50. [Google Scholar] [CrossRef]
- Collongues, N.; Depienne, C.; Boehm, N.; Echaniz-Laguna, A.; Samama, B.; Dürr, A.; Stevanin, G.; Leguern, E.; Brice, A.; Labauge, P.; et al. Novel SPG10 mutation associated with dysautonomia, spinal cord atrophy, and skin biopsy abnormality. Eur. J. Neurol. 2013, 20, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Fichera, M.; Lo Giudice, M.; Falco, M.; Sturnio, M.; Amata, S.; Calabrese, O.; Bigoni, S.; Calzolari, E.; Neri, M. Evidence of kinesin heavy chain (KIF5A) involvement in pure hereditary spastic paraplegia. Neurology 2004, 63, 1108–1110. [Google Scholar] [CrossRef] [PubMed]
- Schüle, R.; Kremer, B.P.; Kassubek, J.; Auer-Grumbach, M.; Kostic, V.; Klopstock, T.; Klimpe, S.; Otto, S.; Boesch, S.; van de Warrenburg, B.P.; et al. SPG10 is a rare cause of spastic paraplegia in European families. J. Neurol. Neurosurg. Psychiatry 2008, 79, 584–587. [Google Scholar] [CrossRef]
- Tessa, A.; Silvestri, G.; de Leva, M.F.; Modoni, A.; Denora, P.S.; Masciullo, M. A novel KIF5A/SPG10 mutation in spastic paraplegia associated with axonal neuropathy. J. Neurol. 2008, 255, 1090–1092. [Google Scholar] [CrossRef]
- Rinaldi, F.; Bassi, M.T.; Todeschini, A.; Rota, S.; Arnoldi, A.; Padovani, A.; Filosto, M. A novel mutation in motor domain of KIF5A associated with an HSP/axonal neuropathy phenotype. J. Clin. Neuromuscul. Dis. 2015, 16, 153–158. [Google Scholar] [CrossRef]
- Ahmeti, K.B.; Ajroud-Driss, S.; Al-Chalabi, A.; Andersen, P.M.; Armstrong, J.; Birve, A.; Blauw, H.M.; Brown, R.H.; Bruijn, L.; Chen, W.; et al. Age of onset of amyotrophic lateral sclerosis is modulated by a locus on 1p34.1. Neurobiol. Aging 2013, 34, e7–e19. [Google Scholar] [CrossRef]
- Karle, K.N.; Möckel, D.; Reid, E.; Schöls, L. Axonal transport deficit in a KIF5A–/– mouse model. Neurogenetics 2012, 13, 169–179. [Google Scholar] [CrossRef]
- Wang, L.; Brown, A. A hereditary spastic paraplegia mutation in kinesin-1A/KIF5A disrupts neurofilament transport. Mol. Neurodegener. 2010, 5, 52. [Google Scholar] [CrossRef]
- Blackstone, C.; O’Kane, C.J.; Reid, E. Hereditary spastic paraplegias: Membrane traffic and the motor pathway. Nat. Rev. Neurosci. 2011, 12, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Case, R.B.; Rice, S.; Hart, C.L.; Ly, B.; Vale, R.D. Role of the kinesin neck linker and catalytic core in microtubule-based motility. Curr. Biol. 2000, 10, 157–160. [Google Scholar] [CrossRef]
- Hancock, W.O.; Howard, J. Kinesin’s processivity results from mechanical and chemical coordination between the ATP hydrolysis cycles of the two motor domains. Proc. Natl. Acad. Sci. USA 1999, 96, 13147–13152. [Google Scholar] [CrossRef] [PubMed]
- Kaseda, K.; Higuchi, H.; Hirose, K. Alternate fast and slow stepping of a heterodimeric kinesin molecule. Nat. Cell Biol. 2003, 5, 1079–1082. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filosto, M.; Piccinelli, S.C.; Palmieri, I.; Necchini, N.; Valente, M.; Zanella, I.; Biasiotto, G.; Lorenzo, D.D.; Cereda, C.; Padovani, A. A Novel Mutation in the Stalk Domain of KIF5A Causes a Slowly Progressive Atypical Motor Syndrome. J. Clin. Med. 2019, 8, 17. https://doi.org/10.3390/jcm8010017
Filosto M, Piccinelli SC, Palmieri I, Necchini N, Valente M, Zanella I, Biasiotto G, Lorenzo DD, Cereda C, Padovani A. A Novel Mutation in the Stalk Domain of KIF5A Causes a Slowly Progressive Atypical Motor Syndrome. Journal of Clinical Medicine. 2019; 8(1):17. https://doi.org/10.3390/jcm8010017
Chicago/Turabian StyleFilosto, Massimiliano, Stefano Cotti Piccinelli, Ilaria Palmieri, Nicola Necchini, Marialuisa Valente, Isabella Zanella, Giorgio Biasiotto, Diego Di Lorenzo, Cristina Cereda, and Alessandro Padovani. 2019. "A Novel Mutation in the Stalk Domain of KIF5A Causes a Slowly Progressive Atypical Motor Syndrome" Journal of Clinical Medicine 8, no. 1: 17. https://doi.org/10.3390/jcm8010017
APA StyleFilosto, M., Piccinelli, S. C., Palmieri, I., Necchini, N., Valente, M., Zanella, I., Biasiotto, G., Lorenzo, D. D., Cereda, C., & Padovani, A. (2019). A Novel Mutation in the Stalk Domain of KIF5A Causes a Slowly Progressive Atypical Motor Syndrome. Journal of Clinical Medicine, 8(1), 17. https://doi.org/10.3390/jcm8010017





