Short-Term Therapies for Treatment of Acute and Advanced Heart Failure—Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline?
Abstract
:1. Introduction
2. A Systematic Analysis of the Past 20 Years
3. Also the Recent Clinical Trials have Disappointed
4. Levosimendan—A Rare Case
5. Even the Established Drugs May Not “Work.”
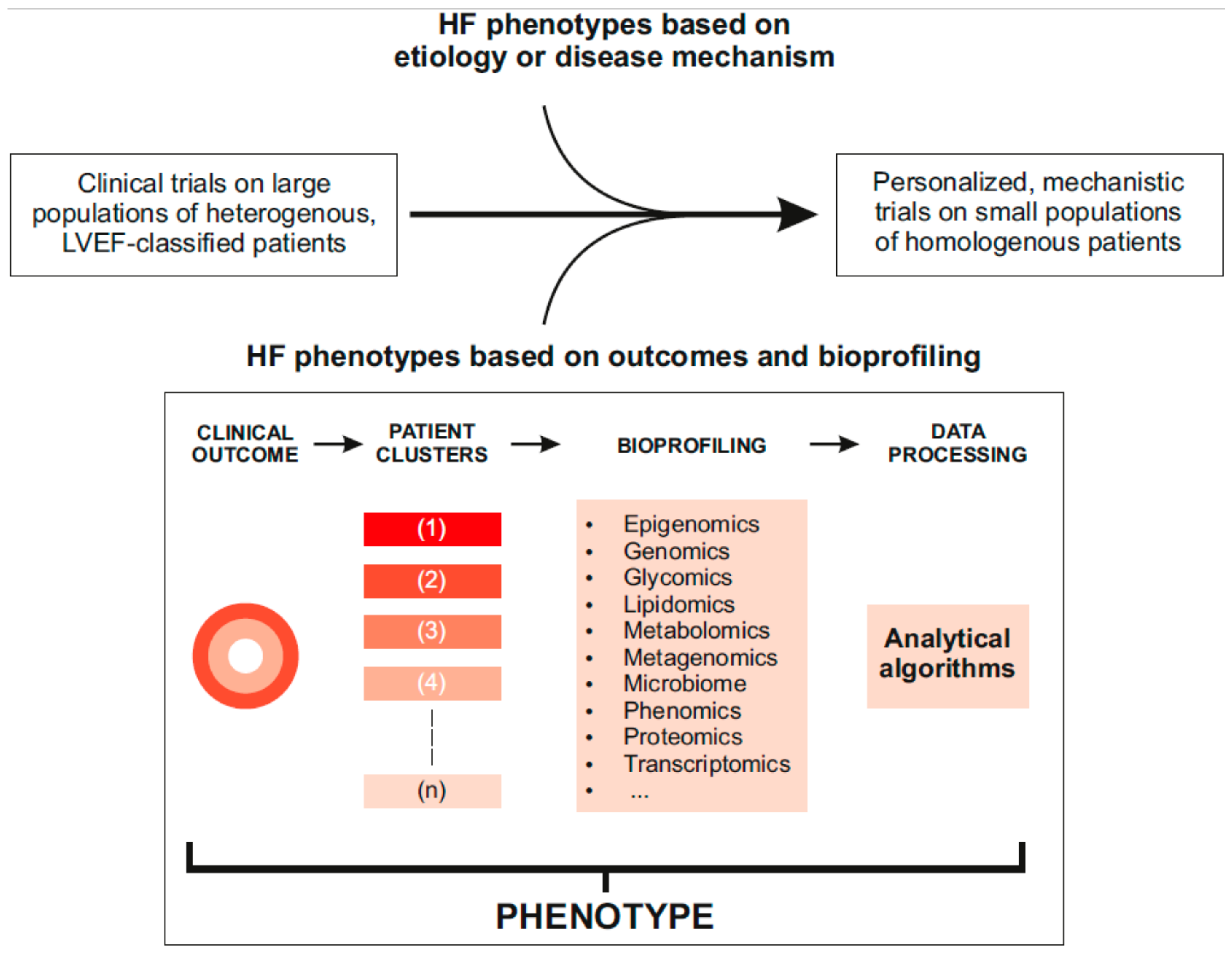
6. Where Next and How to Get There?
- Bidirectional transitions of LVEF due to disease treatment and progression
- Endothelial dysfunction, cardiomyocyte dysfunction and cardiomyocyte injury
- Systolic and diastolic left ventricular dysfunction
- Left atrial dysfunction
- Myocardial fibrosis
- Skeletal myopathy
- Heart failure serum markers
- Neurohumoral activation
7. Invasive versus Non-Invasive Monitoring
8. Will the Data Revolution Break the Logjam?
9. Trials Design—Time for a Change?
10. Some Views for the Future
11. Implications for Drug Development
12. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Machaj, F.; Dembowska, E.; Rosik, J.; Szostak, B.; Mazurek-Mochol, M.; Pawlik, A. New therapies for the treatment of heart failure: A summary of recent accomplishments. Clin. Risk Manag. 2019, 15, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hamo, C.E.; Butler, J.; Gheorghiade, M.; Chioncel, O. The bumpy road to drug development for acute heart failure. Eur. Heart J. Suppl. 2016, 18, G19–G32. [Google Scholar] [CrossRef] [Green Version]
- Tamargo, J.; Caballero, R.; Delpón, E. New drugs in preclinical and early stage clinical development in the treatment of heart failure. Expert Opin. Investig. Drugs 2019, 28, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Rame, J.E. Introduction to topical collection on updates in advanced heart failure. Curr. Heart Fail. Rep. 2019. [Google Scholar] [CrossRef]
- Pollesello, P. Drug discovery and development for acute heart failure drugs: Are expectations too high? Int. J. Cardiol. 2014, 172, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Farmakis, D.; Simitsis, P.; Bistola, V.; Triposkiadis, F.; Ikonomidis, I.; Katsanos, S.; Bakosis, G.; Hatziagelaki, E.; Lekakis, J.; Mebazaa, A.; et al. Acute heart failure with mid-range left ventricular ejection fraction: Clinical profile, in-hospital management, and short-term outcome. Clin. Res. Cardiol. 2017, 106, 359–368. [Google Scholar] [CrossRef]
- Packer, M.; Colucci, W.; Fisher, L.; Massie, B.M.; Teerlink, J.R.; Young, J.; Padley, R.J.; Thakkar, R.; Delgado-Herrera, L.; Salon, J.; et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013, 1, 103–111. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R.; Felker, G.M.; Greenberg, B.H.; Filippatos, G.; Ponikowski, P.; Teichman, S.L.; Unemori, E.; Voors, A.A.; Weatherley, B.D.; et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur. J. Heart Fail. 2010, 12, 1130–1139. [Google Scholar] [CrossRef]
- Haikala, H.; Pollesello, P. Calcium sensitivity enhancers. IDrugs 2000, 3, 1199–1205. [Google Scholar]
- Malik, F.I.; Hartman, J.J.; Elias, K.A.; Morgan, B.P.; Rodriguez, H.; Brejc, K.; Anderson, R.L.; Sueoka, S.H.; Lee, K.H.; Finer, J.T.; et al. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science 2011, 331, 1439–1443. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Felker, G.M.; McMurray, J.J.V.; Ponikowski, P.; Metra, M.; Filippatos, G.S.; Ezekowitz, J.A.; Dickstein, K.; Cleland, J.G.F.; Kim, J.B.; et al. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure. J. Am. Coll. Cardiol. 2016, 67, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Teerlink, J.R.; Senior, R.; Nifontov, E.M.; Mc Murray, J.J.; Lang, C.C.; Tsyrlin, V.A.; Greenberg, B.H.; Mayet, J.; Francis, D.P.; et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet 2011, 378, 676–683. [Google Scholar] [CrossRef]
- Gouda, P.; Ezekowitz, J.A. Update on the diagnosis and management of acute heart failure. Curr. Opin. Cardiol. 2019, 34, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F.; et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Voors, A.A.; et al. Effects of serelaxin in patients with acute heart failure. N. Engl. J. Med. 2019, 381, 716–726. [Google Scholar] [CrossRef]
- Wang, T.S.; Hellkamp, A.S.; Patel, C.B.; Ezekowitz, J.A.; Fonarow, G.C.; Hernandez, A.F. Representativeness of RELAX-AHF clinical trial population in acute heart failure. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 259–268. [Google Scholar] [CrossRef]
- Maggioni, A.P.; López-Sendón, J.; Nielsen, O.W.; Hallén, J.; Aalamian-Mattheis, M.; Wang, Y.; Ertl, G. Efficacy and safety of serelaxin when added to standard of care in patients with acute heart failure: Results from a PROBE study, RELAX-AHF-EU. Eur. J. Heart Fail. 2019, 21, 322–333. [Google Scholar] [CrossRef]
- Packer, M.; O’Connor, C.; McMurray, J.J.V.; Wittes, J.; Abraham, W.T.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Holcomb, R.; Krum, H.; et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N. Engl. J. Med. 2017, 376, 1956–1964. [Google Scholar] [CrossRef]
- Khan, H.; Metra, M.; Blair, J.E.A.; Vogel, M.; Harinstein, M.E.; Filippatos, G.S.; Sabbah, H.N.; Porchet, H.; Valentini, G.; Gheorghiade, M. Istaroxime, a first in class new chemical entity exhibiting SERCA-2 activation and Na–K-ATPase inhibition: A new promising treatment for acute heart failure syndromes? Heart Fail. Rev. 2009, 14, 277–287. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Blair, J.E.A.; Filippatos, G.S.; Macarie, C.; Ruzyllo, W.; Korewicki, J.; Bubenek-Turconi, S.I.; Ceracchi, M.; Bianchetti, M.; Carminati, P.; et al. Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent. J. Am. Coll. Cardiol. 2008, 51, 2276–2285. [Google Scholar] [CrossRef]
- NCT00616161, U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT00616161 (accessed on 6 September 2019).
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov (accessed on 1 September 2019).
- Papp, Z.; Édes, I.; Fruhwald, S.; De Hert, SG.; Salmenperä, M.; Leppikangas, H.; Mebazaa, A.; Landoni, G.; Grossini, E.; Caimmi, P.; et al. Levosimendan: Molecular mechanisms and clinical implications: Consensus of experts on the mechanisms of action of levosimendan. Int. J. Cardiol. 2012, 159, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Alvarez, J.; Gal, T.B.; Brito, D.; Fedele, F.; Fonseca, C.; Gordon, A.C.; Gotsman, I.; Grossini, E.; Guarracino, F.; et al. Levosimendan beyond inotropy and acute heart failure: Evidence of pleiotropic effects on the heart and other organs: An expert panel position paper. Int. J. Cardiol. 2016, 222, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mebazaa, A.; Parissis, J.; Porcher, R.; Gayat, E.; Nikolaou, M.; Boas, F.V.; Delgado, J.F.; Follath, F. Short-term survival by treatment among patients hospitalized with acute heart failure: The global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011, 37, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Pollesello, P.; Parissis, J.; Kivikko, M.; Harjola, V.-P. Levosimendan meta-analyses: Is there a pattern in the effect on mortality? Int. J. Cardiol. 2016, 209, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenfuss, G.; Pieske, B.; Castell, M.; Kretschmann, B.; Maier, L.S.; Just, H. Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation 1998, 98, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Pollesello, P.; Papp, Z. Inotropes and inodilators for acute heart failure: Sarcomere active drugs in focus. J. Cardiovasc. Pharmacol. 2014, 64, 199–208. [Google Scholar] [CrossRef]
- Pollesello, P.; Papp, Z.; Papp, J.G. Calcium sensitizers: What have we learned over the last 25 years? Int. J. Cardiol. 2016, 203, 543–548. [Google Scholar] [CrossRef]
- Mebazaa, A.; Nieminen, M.S.; Filippatos, G.S.; Cleland, J.G.; Salon, J.E.; Thakkar, R.; Padley, R.J.; Huang, B.; Cohen-Solal, A. Levosimendan vs. dobutamine: Outcomes for acute heart failure patients on β-blockers in SURVIVE. Eur. J. Heart Fail. 2009, 11, 304–311. [Google Scholar] [CrossRef]
- Bergh, C.-H.; Andersson, B.; Dahlström, U.; Forfang, K.; Kivikko, M.; Sarapohja, T.; Ullman, B.; Wikström, G. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur. J. Heart Fail. 2010, 12, 404–410. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Altenberger, J.; Parissis, J.T.; Ulmer, H.; Poelzl, G.; LevoRep Investigators. Rationale and design of the multicentre randomized trial investigating the efficacy and safety of pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep study). Eur. J. Heart Fail. 2010, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Comín-Colet, J.; Manito, N.; Segovia-Cubero, J.; Delgado, J.; García Pinilla, J.M.; Almenar, L.; Crespo-Leiro, M.G.; Sionis, A.; Blasco, T.; Pascual-Figal, D.; et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: The LION-HEART multicentre randomised trial: Levosimendan in advanced HF: The LION-HEART trial. Eur. J. Heart Fail. 2018, 20, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.J.; LAICA Study Investigators. Efficacy and security of intermittent repeated levosimendan administration in patients with advanced heart failure: A randomized, double-blind, placebo controlled multicenter trial: LAICA study. In Proceedings of the European Society of Cardiology—Heart Failure Association Congress, Florence, Italy, 21 May 2016. [Google Scholar]
- Silvetti, S.; Nieminen, M.S. Repeated or intermittent levosimendan treatment in advanced heart failure: An updated meta-analysis. Int. J. Cardiol. 2016, 202, 138–143. [Google Scholar] [CrossRef]
- Silvetti, S.; Belletti, A.; Fontana, A.; Pollesello, P. Rehospitalization after intermittent levosimendan treatment in advanced heart failure patients: A meta-analysis of randomized trials: Repeated levosimendan in AdHF and rehospitalization. ESC Heart Fail. 2017, 4, 595–604. [Google Scholar] [CrossRef]
- Oliva, F.; Perna, E.; Marini, M.; Nassiacos, D.; Cirò, A.; Malfatto, G.; Morandi, F.; Caico, I.; Perna, G.; Meloni, S.; et al. Scheduled intermittent inotropes for ambulatory advanced heart failure. The RELEVANT-HF multicentre collaboration. Int. J. Cardiol. 2018, 272, 255–259. [Google Scholar] [CrossRef]
- Müller, C.E. GALACTIC—Goal-Directed AfterLoad Reduction in Acute Congestive Cardiac Decompensation. ESC Congress 2019, Paris, France. Hot Line Session 3. Available online: https://esc365.escardio.org/Congress/ESC-CONGRESS-2019/Hot-Line-Session-3/202174-galactic-goal-directed-afterload-reduction-in-acute-congestive-cardiac-decompensation-a-randomized-controlled-trial#video (accessed on 3 October 2019).
- McCullough, P.A. How trialists and pharmaceutical sponsors have failed us by thinking that acute heart failure Is a 48-hour illness. Am. J. Cardiol. 2017, 120, 505–508. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Butler, J.; Abboud, F.M.; Armstrong, P.W.; Adamopoulos, S.; Atherton, J.J.; Backs, J.; Bauersachs, J.; Burkhoff, D.; Bonow, R.O.; et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur. Heart. J. 2019, 40, 2155–2163. [Google Scholar] [CrossRef]
- Severino, P.; Mariani, M.V.; Fedele, F. Futility in cardiology: The need for a change in perspectives. Eur. J. Heart Fail. 2019. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Adamo, F.; Severino, P. Heart failure with preserved, mid-range, and reduced ejection fraction: The misleading definition of the new guidelines. Cardiol. Rev. 2017, 25, 4–5. [Google Scholar] [CrossRef]
- Fedele, F.; Severino, P.; Calcagno, S.; Mancone, M. Heart failure: TNM-like classification. J. Am. Coll. Cardiol. 2014, 63, 1959–1960. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Bakker, J.; Cecconi, M.; Hajjar, L.; Liu, D.W.; Lobo, S.; Monnet, X.; Morelli, A.; Myatra, S.N.; Perel, A.; et al. Alternatives to the Swan–Ganz catheter. Intensive Care Med. 2018, 44, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, P.P. Machine learning in cardiovascular medicine: Are we there yet? Heart 2018, 104, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Sim, I. Mobile Devices and Health. N. Engl. J. Med. 2019, 381, 956–968. [Google Scholar] [CrossRef]
- Eurlings, C.G.M.J.; Boyne, J.J.; de Boer, R.A.; Brunner-La Rocca, H.P. Telemedicine in heart failure—more than nice to have? Neth. Heart J. 2019, 27, 5–15. [Google Scholar] [CrossRef]
- Bondar, G.; Togashi, R.; Cadeiras, M.; Schaenman, J.; Cheng, R.K.; Masukawa, L.; Hai, J.; Bao, T.-M.; Chu, D.; Chang, E.; et al. Association between preoperative peripheral blood mononuclear cell gene expression profiles, early postoperative organ function recovery potential and long-term survival in advanced heart failure patients undergoing mechanical circulatory support. PLoS ONE 2017, 12, e0189420. [Google Scholar] [CrossRef]
- Deng, M.C. A peripheral blood transcriptome biomarker test to diagnose functional recovery potential in advanced heart failure. Biomark. Med. 2018, 12, 619–635. [Google Scholar] [CrossRef] [Green Version]
- Krittanawong, C.; Namath, A.; Lanfear, D.E.; Tang, W.H.W. Practical pharmacogenomic approaches to heart failure therapeutics. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 60. [Google Scholar] [CrossRef]
- Gensini, G.F.; Alderighi, C.; Rasoini, R.; Mazzanti, M.; Casolo, G. Value of telemonitoring and telemedicine in heart failure management. Card. Fail. Rev. 2017, 3, 116–121. [Google Scholar] [CrossRef]
- Hemingway, H.; Asselbergs, F.W.; Danesh, J.; Dobson, R.; Maniadakis, N.; Maggioni, A.; van Thiel, G.J.M.; Cronin, M.; Brobert, G.; Vardas, P.; et al. Big data from electronic health records for early and late translational cardiovascular research: Challenges and potential. Eur. Heart J. 2018, 39, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.; Shameer, K.; Salem Omar, A.M.; Dudley, J.T.; Sengupta, P.P. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J. Am. Coll. Cardiol. 2016, 68, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Maragatham, G.; Devi, S. LSTM model for prediction of heart failure in big data. J. Med. Syst. 2019, 43, 111. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy of Tafamidis in Patients with Transthyretin Cardimyopathy (ATTR-ACT). Available online: https://clinicaltrials.gov/ct2/show/results/NCT01994889?term=NCT01994889.&rank=1 (accessed on 11 September 2019).
- Pölzl, G.; Allipour Birgani, S.; Comín-Colet, J.; Delgado, J.F.; Fedele, F.; García-Gonzáles, M.J.; Gustafsson, F.; Masip, J.; Papp, Z.; Störk, S.; et al. Repetitive levosimendan infusions for patients with advanced Chronic heart failure in the vulnerable post-discharge period: Rationale and design of the LeoDOR Trial. Esc. Heart Fail. 2019, 6, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Pölzl, G.; Altenberger, J.; Baholli, L.; Beltrán, P.; Borbély, A.; Comin-Colet, J.; Delgado, J.F.; Fedele, F.; Fontana, A.; Fruhwald, F.; et al. Repetitive use of levosimendan in advanced heart failure: Need for stronger evidence in a field in dire need of a useful therapy. Int. J. Cardiol. 2017, 243, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.P.; Lindsell, C.J.; Pang, P.S.; Storrow, A.B.; Peacock, W.F.; Levy, P.; Rahbar, M.H.; Del Junco, D.; Gheorghiade, M.; Berry, D.A. Bayesian adaptive trial design in acute heart failure syndromes: Moving beyond the mega trial. Am. Heart J. 2012, 164, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Bunn, V. Comparison of multi-arm multi-stage design and adaptive randomization in platform clinical trials. Contemp. Clin. Trials 2017, 54, 48–59. [Google Scholar] [CrossRef]
- Wason, J.M.S.; Trippa, L. A comparison of Bayesian adaptive randomization and multi-stage designs for multi-arm clinical trials. Stat. Med. 2014, 33, 2206–2221. [Google Scholar] [CrossRef] [Green Version]
- Saville, B.R.; Berry, S.M. Efficiencies of platform clinical trials: A vision of the future. Clin. Trials 2016, 13, 358–366. [Google Scholar] [CrossRef]
- Morrell, L.; Hordern, J.; Brown, L.; Sydes, M.R.; Amos, C.L.; Kaplan, R.S.; Parmar, M.K.B.; Maughan, T.S. Mind the gap? The platform trial as a working environment. Trials 2019, 20, 297. [Google Scholar] [CrossRef]
- Hague, D.; Townsend, S.; Masters, L.; Rauchenberger, M.; Van Looy, N.; Diaz-Montana, C.; Gannon, M.; James, N.; Maughan, T.; Parmar, M.K.B.; et al. Changing platforms without stopping the train: Experiences of data management and data management systems when adapting platform protocols by adding and closing comparisons. Trials 2019, 20, 294. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, F.; Bathia, R.; Letchemanan, K.; Masters, L.; Amos, C.; Bara, A.; Brown, L.; Gilson, C.; Pugh, C.; Atako, N.; et al. This is a platform alteration: A trial management perspective on the operational aspects of adaptive and platform and umbrella protocols. Trials 2019, 20, 264. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Why are physicians so confused about acute heart failure? N. Engl. J. Med. 2019, 381, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Blecker, S.; Sontag, D.; Horwitz, L.I.; Kuperman, G.; Park, H.; Reyentovich, A.; Katz, S.D. Early identification of patients with acute decompensated heart failure. J. Card. Fail. 2018, 24, 357–362. [Google Scholar] [CrossRef]
- Blecker, S.; Katz, S.D.; Horwitz, L.I.; Kuperman, G.; Park, H.; Gold, A.; Sontag, D. Comparison of approaches for heart failure case identification from electronic health record data. JAMA Cardiol. 2016, 1, 1014. [Google Scholar] [CrossRef]
- Golas, S.B.; Shibahara, T.; Agboola, S.; Otaki, H.; Sato, J.; Nakae, T.; Hisamitsu, T.; Kojima, G.; Felsted, J.; Kakarmath, S.; et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: A retrospective analysis of electronic medical records data. BMC Med. Inf. Decis. Mak. 2018, 18, 44. [Google Scholar] [CrossRef]
- Karanasiou, G.S.; Tripoliti, E.E.; Papadopoulos, T.G.; Kalatzis, F.G.; Goletsis, Y.; Naka, K.K.; Bechlioulis, A.; Errachid, A.; Fotiadis, D.I. Predicting adherence of patients with HF through machine learning techniques. Healthc. Technol. Lett. 2016, 3, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Kreusser, M.M.; Tschierschke, R.; Beckendorf, J.; Baxmann, T.; Frankenstein, L.; Dösch, A.O.; Schultz, J.-H.; Giannitsis, E.; Pleger, S.T.; Ruhparwar, A.; et al. The need for dedicated advanced heart failure units to optimize heart failure care: Impact of optimized advanced heart failure unit care on heart transplant outcome in high-risk patients: The need for dedicated AHFUs to optimize HF care. ESC Heart Fail. 2018, 5, 1108–1117. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Möckel, M.; Koehler, K.; Anker, S.D.; Vollert, J.; Moeller, V.; Koehler, M.; Gehrig, S.; Wiemer, J.C.; Haehling, S.; Koehler, F. Biomarker guidance allows a more personalized allocation of patients for remote patient management in heart failure: Results from the TIM-HF2 trial. Eur. J. Heart Fail. 2019. [Google Scholar] [CrossRef]
- Matsumura, T.; Matsui, M.; Iwata, Y.; Asakura, M.; Saito, T.; Fujimura, H.; Sakoda, S. A Pilot Study of Tranilast for Cardiomyopathy of Muscular Dystrophy. Intern. Med. 2018, 57, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrès, E.; Talha, S.; Zulfiqar, A.-A.; Hajjam, M.; Ervé, S.; Hajjam, J.; Gény, B.; Hajjam El Hassani, A. Current research and new perspectives of telemedicine in chronic heart failure: Narrative review and points of interest for the clinician. J. Clin. Med. 2018, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Eschenhagen, T.; Hamdani, N.; Heinzel, F.R.; Lyon, A.R.; Manstein, D.J.; Metzger, J.; Papp, Z.; Tocchetti, C.G.; Yilmaz, M.B.; et al. Treatments targeting inotropy. Eur. Heart J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Pollesello, P.; Haikala, H.; Végh, Á.; Sorsa, T.; Levijoki, J.; Szilágyi, S.; Édes, I.; Tóth, A.; Papp, Z.; et al. ORM-3819 promotes cardiac contractility through Ca2+ sensitization in combination with selective PDE III inhibition, a novel approach to inotropy. Eur. J. Pharm. 2016, 775, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Márton, Z.; Pataricza, J.; Pollesello, P.; Varró, A.; Papp, J.G. The Novel inodilator ORM-3819 relaxes isolated porcine coronary arteries: Role of voltage-gated potassium channel activation. J. Cardiovasc. Pharm. 2019, 74, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.B.; Brass, E.P.; Gilbert, D.N.; Bartlett, J.G.; Spellberg, B. Sustainable discovery and development of antibiotics—Is a nonprofit approach the future? N. Engl. J. Med. 2019, 381, 503–505. [Google Scholar] [CrossRef]
- Dungen, H.-D.; Petroni, R.; Correale, M.; Coiro, S.; Monitillo, F.; Triggiani, M.; Leone, M.; Antohi, E.-L.; Ishihara, S.; Sarwar, C.M.S.; et al. A new educational program in heart failure drug development: The Brescia international master program. J. Cardiovasc. Med. 2018, 19, 411–421. [Google Scholar] [CrossRef]


| Agent Name | Omecamtiv Mecarbil | Ularitide | Serelaxin | |
|---|---|---|---|---|
| Trial name | ATOMIC-AHF | TRUE-AHF | RELAX-AHF | RELAX-AHF-2 |
| Registry number | NCT01300013 | NCT01661634 | NCT00520806 | NCT01870778 |
| Sample size | 614 AHF patients | 2.157 AHF patients | 1.161 pts hospitalized for AHF | 6.600 AHF patients |
| Outcomes | • failed to meet the primary endpoint of dyspnoea improvement • increased SET | • no significant differences in primary endpoints • significant dyspnea reduction in 83% of eligible patients | • VAS AUC scale dyspnea improvement • fewer deaths at day 180 | • failed to meet primary endpoints (180-day cardiovascular death and worsening heart failure through day-5) |
| Observed adverse events | • no difference in adverse effect rate compared to placebo | • adverse effect on dyspnea in 17% of ineligible patients (prohibited intravenous medications) | • infrequent hypotensive events | • no serious adverse events |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollesello, P.; Ben Gal, T.; Bettex, D.; Cerny, V.; Comin-Colet, J.; Eremenko, A.A.; Farmakis, D.; Fedele, F.; Fonseca, C.; Harjola, V.-P.; et al. Short-Term Therapies for Treatment of Acute and Advanced Heart Failure—Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline? J. Clin. Med. 2019, 8, 1834. https://doi.org/10.3390/jcm8111834
Pollesello P, Ben Gal T, Bettex D, Cerny V, Comin-Colet J, Eremenko AA, Farmakis D, Fedele F, Fonseca C, Harjola V-P, et al. Short-Term Therapies for Treatment of Acute and Advanced Heart Failure—Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline? Journal of Clinical Medicine. 2019; 8(11):1834. https://doi.org/10.3390/jcm8111834
Chicago/Turabian StylePollesello, Piero, Tuvia Ben Gal, Dominique Bettex, Vladimir Cerny, Josep Comin-Colet, Alexandr A. Eremenko, Dimitrios Farmakis, Francesco Fedele, Cândida Fonseca, Veli-Pekka Harjola, and et al. 2019. "Short-Term Therapies for Treatment of Acute and Advanced Heart Failure—Why so Few Drugs Available in Clinical Use, Why Even Fewer in the Pipeline?" Journal of Clinical Medicine 8, no. 11: 1834. https://doi.org/10.3390/jcm8111834





