Molecular, Population, and Clinical Aspects of Lipoprotein(a): A Bridge Too Far?
Abstract
:1. Introduction
2. History and Renaissance
3. Molecular Aspects
3.1. Physical Chemistry
3.2. Genetics
3.3. Biology
4. Epidemiological and Observational Studies
4.1. Cohorts
4.2. Cross National Study
4.3. Statin-Treated Patients
4.4. Type 2 Diabetes Patients
5. Genetic Studies
5.1. Genome-Wide Association
5.2. Mendelian Randomisation
6. Analysis of Lp(a) Levels
6.1. Reference Values
6.2. Bioanalytical Issues
7. Pathobiology: Atherosclerotic Cardiovascular Disease and Calcific Aortic Valve Disease
7.1. Risk Factor Status
7.2. Atherosclerotic Cardiovascular Disease (ASCVD)
7.3. Calcific Aortic Valve Disease (CAVD)
7.4. Thrombosis and Fibrinolysis
8. Clinical Aspects
8.1. Clinical Practice Guidelines
8.2. Who to Test?
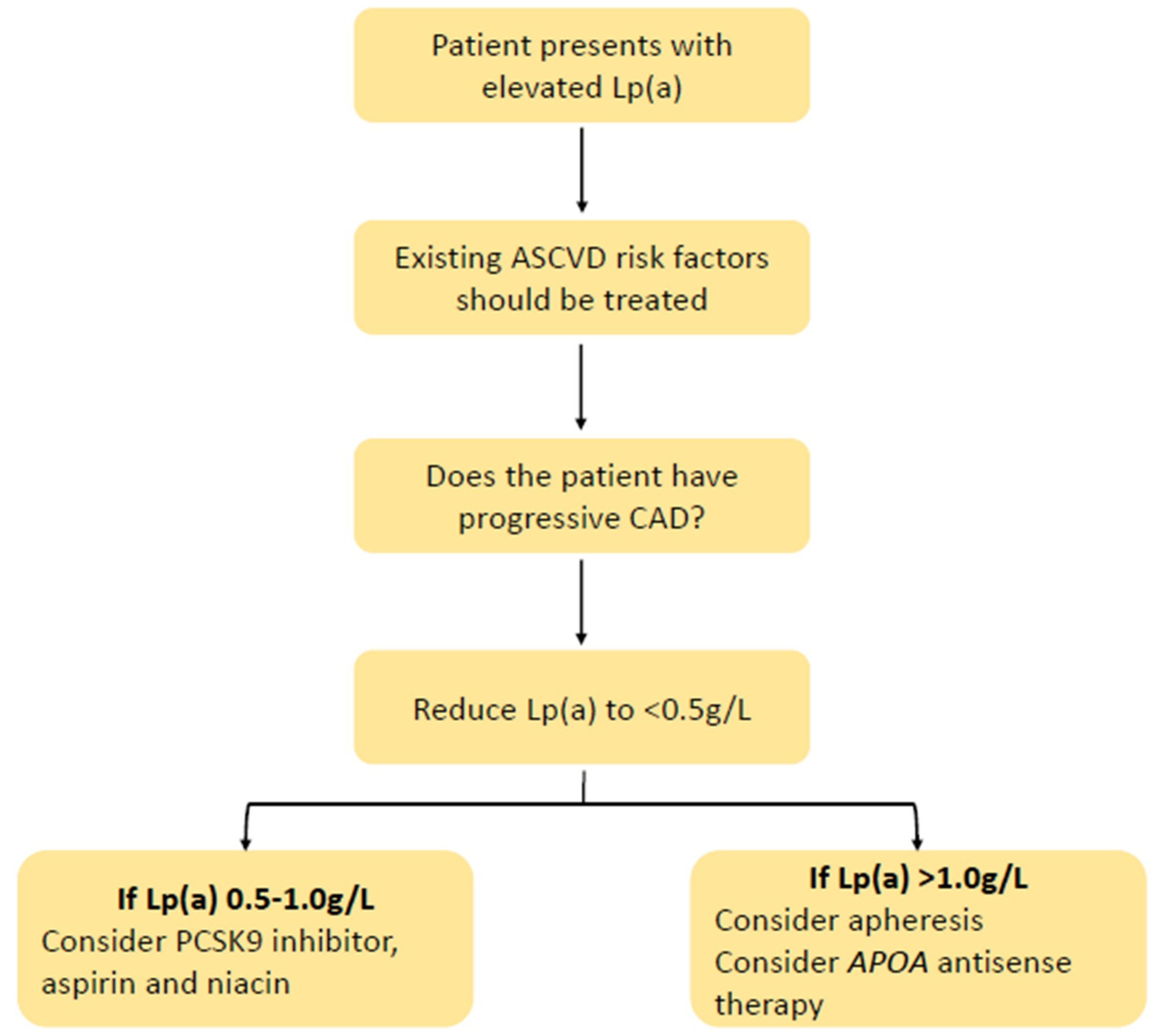
8.3. How to Treat?
8.4. What is New in Therapy?
9. Future Challenges: Resarch Questions
10. Summary and Conclusions: A Bridge Too Far for Prime-Time Clinical Practice?
Author Contributions
Conflicts of Interest
References
- Berg, K. A New Serum Type System in Man—The Lp System. Acta Pathol. Microbiol. Scand. 1963, 59, 369–382. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.W.; Tomlinson, J.E.; Kuang, W.J.; Eaton, D.L.; Chen, E.Y.; Fless, G.M.; Scanu, A.M.; Lawn, R.M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 1987, 330, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Boffa, M.B.; Koschinsky, M.L. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nature reviews. Cardiology 2019, 16, 305–318. [Google Scholar]
- Kostner, K.M.; Kostner, G.M. Lipoprotein (a): A historical appraisal. J. Lipid Res. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Weisel, J.W.; Nagaswami, C.; Woodhead, J.L.; Higazi, A.A.; Cain, W.J.; Marcovina, S.M.; Koschinsky, M.L.; Cines, D.B.; Bdeir, K. The structure of lipoprotein(a) and ligand-induced conformational changes. Biochemistry 2001, 40, 10424–10435. [Google Scholar] [CrossRef]
- Tsimikas, S.A. Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Maranhao, R.C.; Carvalho, P.O.; Strunz, C.C.; Pileggi, F. Lipoprotein (a): Structure, pathophysiology and clinical implications. Arq. Bras. Cardiol. 2014, 103, 76–84. [Google Scholar] [CrossRef]
- Kostner, K.M.; Kostner, G.M.; Wierzbicki, A.S. Is Lp(a) ready for prime time use in the clinic? A pros-and-cons debate. Atherosclerosis 2018, 274, 16–22. [Google Scholar] [CrossRef]
- Scipione, C.A.; Koschinsky, M.L.; Boffa, M.B. Lipoprotein(a) in clinical practice: New perspectives from basic and translational science. Crit. Rev. Clin. Lab. Sci. 2018, 55, 33–54. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Nelson, A.J. The time for lipoprotein(a) based intervention has arrived: Where will the light shine? J. Thorac. Dis. 2019, 11, S433–S436. [Google Scholar] [CrossRef]
- Kronenberg, F. Prediction of cardiovascular risk by Lp(a) concentrations or genetic variants within the LPA gene region. Clin. Res. Cardiol. Suppl. 2019, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Jimenez, D.; Christian, J.C.; Fabsitz, R.R.; Reed, T.; Carmelli, D.; Castelli, W.P.; Ordovas, J.M.; Wilson, P.W.; Schaefer, E.J. The NHLBI Twin Study: Heritability of apolipoprotein A-I, B, and low density lipoprotein subclasses and concordance for lipoprotein(a). Atherosclerosis 1991, 91, 97–106. [Google Scholar] [CrossRef]
- Austin, M.A.; Sandholzer, C.; Selby, J.V.; Newman, B.; Krauss, R.M.; Utermann, G. Lipoprotein(a) in women twins: Heritability and relationship to apolipoprotein(a) phenotypes. Am. J. Hum. Genet. 1992, 51, 829–840. [Google Scholar] [PubMed]
- Kronenberg, F. Human Genetics and the Causal Role of Lipoprotein(a) for Various Diseases. Cardiovasc. Drugs Ther. 2016, 30, 87–100. [Google Scholar] [CrossRef]
- Hung, M.Y.; Tsimikas, S. What is the ultimate test that lowering lipoprotein(a) is beneficial for cardiovascular disease and aortic stenosis? Curr. Opin. Lipidol. 2014, 25, 423–430. [Google Scholar] [CrossRef]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein (a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef]
- Coassin, S.; Schönherr, S.; Weissensteiner, H.; Erhart, G.; Forer, L.; Losso, J.L.; Lamina, C.; Haun, M.; Utermann, G.; Paulweber, B. A comprehensive map of single-base polymorphisms in the hypervariable LPA kringle IV type 2 copy number variation region. J. Lipid Res. 2019, 60, 186–199. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein (a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J. Lipid Res. 2016, 57, 1953–1975. [Google Scholar] [CrossRef]
- Moriarty, P.M.; Varvel, S.A.; Gordts, P.L.; McConnell, J.P.; Tsimikas, S. Lipoprotein(a) Mass Levels Increase Significantly According to APOE Genotype: An Analysis of 431 239 Patients. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 580–588. [Google Scholar] [CrossRef]
- Kritharides, L.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Kamstrup, P.R.; Afzal, S. Effect of APOE epsilon Genotype on Lipoprotein(a) and the Associated Risk of Myocardial Infarction and Aortic Valve Stenosis. J. Clin. Endocrinol. Metab. 2017, 102, 3390–3399. [Google Scholar] [CrossRef]
- Laschkolnig, A.; Kollerits, B.; Lamina, C.; Meisinger, C.; Rantner, B.; Stadler, M.; Peters, A.; Koenig, W.; Stöckl, A.; Dähnhardt, D.; et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovascular Research 2014, 103, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.L.; Boffa, M.B.; Sahebkar, A.; Koschinsky, M.L.; Watts, G.F. The renaissance of lipoprotein(a): Brave new world for preventive cardiology? Prog. Lipid Res. 2017, 68, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Kostner, K.M.; Marz, W.; Kostner, G.M. When should we measure lipoprotein (a)? Eur. Heart J. 2013, 34, 3268–3276. [Google Scholar] [CrossRef]
- Kronenberg, F.; Kuen, E.; Ritz, E.; Junker, R.; König, P.; Kraatz, G.; Lhotta, K.; Mann, J.F.; Müller, G.A.; Neyer, U. Lipoprotein(a) serum concentrations and apolipoprotein(a) phenotypes in mild and moderate renal failure. J. Am. Soc. Nephrol. 2000, 11, 105–115. [Google Scholar]
- Tsimikas, S. Potential Causality and Emerging Medical Therapies for Lipoprotein(a) and Its Associated Oxidized Phospholipids in Calcific Aortic Valve Stenosis. Circ. Res. 2019, 124, 405–415. [Google Scholar] [CrossRef]
- Nestel, P. Lipoprotein(a) Removal Still a Mystery. J. Am. Heart Assoc. 2019, 8, e011903. [Google Scholar] [CrossRef]
- McCormick, S.P.A.; Schneider, W.J. Lipoprotein(a) catabolism: A case of multiple receptors. Pathology 2019, 51, 155–164. [Google Scholar] [CrossRef]
- Cantin, B.; Gagnon, F.; Moorjani, S.; Després, J.P.; Lamarche, B.; Lupien, P.J.; Dagenais, G.R. Is lipoprotein(a) an independent risk factor for ischemic heart disease in men? The Quebec Cardiovascular Study. J. Am. Coll. Cardiol. 1998, 31, 519–525. [Google Scholar] [CrossRef]
- Suk Danik, J.; Rifai, N.; Buring, J.E.; Ridker, P.M. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA J. Am. Med. Assoc. 2006, 296, 1363–1370. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA J. Am. Med. Assoc. 2009, 302, 412–423. [Google Scholar]
- von Eckardstein, A.; Schulte, H.; Cullen, P.; Assmann, G. Lipoprotein(a) further increases the risk of coronary events in men with high global cardiovascular risk. J. Am. Coll. Cardiol. 2001, 37, 434–439. [Google Scholar] [CrossRef]
- Sharrett, A.R.; Ballantyne, C.M.; Coady, S.A.; Heiss, G.; Sorlie, P.D.; Catellier, D.; Patsch, W. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2001, 104, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Luc, G.; Bard, J.M.; Arveiler, D.; Ferrieres, J.; Evans, A.; Amouyel, P.; Fruchart, J.C.; Ducimetiere, P. Lipoprotein (a) as a predictor of coronary heart disease: The PRIME Study. Atherosclerosis 2002, 163, 377–384. [Google Scholar] [CrossRef]
- Ariyo, A.A.; Thach, C.; Tracy, R. Cardiovascular Health Study I. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N. Engl. J. Med. 2003, 349, 2108–2115. [Google Scholar] [CrossRef]
- Rifai, N.; Ma, J.; Sacks, F.M.; Ridker, P.M.; Hernandez, W.J.; Stampfer, M.J.; Marcovina, S.M. Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: The Physicians’ Health Study. Clin. Chem. 2004, 50, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Kamstrup, P.R.; Benn, M.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: The Copenhagen City Heart Study. Circulation 2008, 117, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Seed, M.; Hoppichler, F.; Reaveley, D.; McCarthy, S.; Thompson, G.R.; Boerwinkle, E.; Utermann, G. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N. Engl. J. Med. 1990, 322, 1494–1499. [Google Scholar] [CrossRef]
- Danesh, J.; Collins, R.; Peto, R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000, 102, 1082–1085. [Google Scholar] [CrossRef] [Green Version]
- Nestel, P.J.; Barnes, E.H.; Tonkin, A.M.; Simes, J.; Fournier, M.; White, H.D.; Colquhoun, D.M.; Blankenberg, S.; Sullivan, D.R. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2902–2908. [Google Scholar] [CrossRef] [Green Version]
- Willeit, P.; Ridker, P.M.; Nestel, P.J.; Simes, J.; Tonkin, A.M.; Pedersen, T.R.; Schwartz, G.G.; Olsson, A.G.; Colhoun, H.M.; Kronenberg, F.; et al. Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: Individual patient-data meta-analysis of statin outcome trials. Lancet 2018, 392, 1311–1320. [Google Scholar] [CrossRef] [Green Version]
- Verbeek, R.; Hoogeveen, R.M.; Langsted, A.; Stiekema, L.C.A.; Verweij, S.L.; Hovingh, G.K.; Wareham, N.J.; Khaw, K.T.; Boekholdt, S.M.; Nordestgaard, B.G.; et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur. Heart J. 2018, 39, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Rallidis, L.S.; Pavlakis, G.; Foscolou, A.; Kotakos, C.; Katsimardos, A.; Drosatos, A.; Zolindaki, M.; Panagiotakos, D.B. High levels of lipoprotein (a) and premature acute coronary syndrome. Atherosclerosis 2018, 269, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Foscolou, A.G.E.; Magriplis, E.; Naumovski, N.; Rallidis, L.; Matalas, A.-L.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D. The mediating role of Mediterranean diet on the association between Lp(a) levels and cardiovascular disease risk: A 10-year follow-up of the ATTICA study. Clin. Biochem. 2019, 60, 33–37. [Google Scholar] [CrossRef]
- Boffa, M.B.; Stranges, S.; Klar, N.; Moriarty, P.M.; Watts, G.F.; Koschinsky, M.L. Lipoprotein(a) and secondary prevention of atherothrombotic events: A critical appraisal. J. Clin. Lipidol. 2018, 12, 1358–1366. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Minami, Y.; Kato, A.; Katsura, A.; Sato, T.; Kakizaki, R.; Nemoto, T.; Hashimoto, T.; Fujiyoshi, K.; Meguro, K.; et al. Lipoprotein (a) level is associated with plaque vulnerability in patients with coronary artery disease: An optical coherence tomography study. Int. J. Cardiol. Heart Vasc. 2019, 24, 100382. [Google Scholar] [CrossRef]
- Kronenberg, F. Therapeutic lowering of lipoprotein(a): How much is enough? Atherosclerosis 2019, 288, 163–165. [Google Scholar] [CrossRef] [Green Version]
- Madsen, C.M.; Kamstrup, P.R.; Langsted, A.; Varbo, A.; Nordestgaard, B.G. Lp(a) (Lipoprotein[a])-Lowering by 50 mg/dL (105 nmol/L) May Be Needed to Reduce Cardiovascular Disease 20% in Secondary Prevention: A Population-Based Study. Arterioscler. Thromb. Vasc. Biol. 2019. [Google Scholar] [CrossRef]
- Langsted, A.; Kamstrup, P.R.; Nordestgaard, B.G. High lipoprotein(a) and high risk of mortality. Eur. Heart J. 2019, 40, 2760–2770. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, B.J.; Boekholdt, S.M.; Dubé, M.P.; Rhéaume, E.; Wareham, N.J.; Khaw, K.T.; Sandhu, M.S.; Tardif, J.C. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: A prospective Mendelian randomization study and replication in a case-control cohort. Circ. Cardiovasc. Genet. 2014, 7, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Vongpromek, R.; Bos, S.; Ten Kate, G.J.; Yahya, R.; Verhoeven, A.J.; de Feyter, P.J.; Kronenberg, F.; Roeters van Lennep, J.E.; Sijbrands, E.J.; Mulder, M.T. Lipoprotein(a) levels are associated with aortic valve calcification in asymptomatic patients with familial hypercholesterolaemia. J. Intern. Med. 2015, 278, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capoulade, R.; Yeang, C.; Chan, K.L.; Pibarot, P.; Tsimikas, S. Association of Mild to Moderate Aortic Valve Stenosis Progression With Higher Lipoprotein(a) and Oxidized Phospholipid Levels: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.H.; Tsimikas, S.; Pawade, T.; Kroon, J.; Jenkins, W.S.A.; Doris, M.K.; White, A.C.; Timmers, N.K.L.M.; Hjortnaes, J.; Rogers, M.A.; et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 73, 2150–2162. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Ward, N.C.; Watts, G.F. Lipid management in people with peripheral artery disease. Curr. Opin. Lipidol. 2019, 8, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Vigna, G.B.; McDermott, M.M.; Cavalieri, M.; Maraldi, C.; Lauretani, F.; Bandinelli, S.; Zuliani, G.; Guralnik, J.M.; Fellin, R.; et al. Lipoprotein(a), inflammation, and peripheral arterial disease in a community-based sample of older men and women (the InCHIANTI study). Am. J. Cardiol. 2010, 105, 1825–1830. [Google Scholar] [CrossRef] [Green Version]
- Gurdasani, D.; Sjouke, B.; Tsimikas, S.; Hovingh, G.K.; Luben, R.N.; Wainwright, N.W.; Pomilla, C.; Wareham, N.J.; Khaw, K.T.; Boekholdt, S.M.; et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: The EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3058–3065. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.S.; Yusuf, S.; Vuksan, V.; Devanesen, S.; Teo, K.K.; Montague, P.A.; Kelemen, L.; Yi, C.; Lonn, E.; Gerstein, H.; et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000, 356, 279–284. [Google Scholar] [CrossRef]
- Virani, S.S.; Brautbar, A.; Davis, B.C.; Nambi, V.; Hoogeveen, R.C.; Sharrett, A.R.; Coresh, J.; Mosley, T.H.; Morrisett, J.D.; Catellier, D.J.; et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2012, 125, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Waldeyer, C.; Makarova, N.; Zeller, T.; Schnabel, R.B.; Brunner, F.J.; Jørgensen, T.; Linneberg, A.; Niiranen, T.; Salomaa, V.; Jousilahti, P.; et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: Results from the BiomarCaRE consortium. Eur. Heart J. 2017, 38, 2490–2498. [Google Scholar] [CrossRef] [Green Version]
- Paré, G.; Çaku, A.; McQueen, M.; Anand, S.S.; Enas, E.; Clarke, R.; Boffa, M.B.; Koschinsky, M.; Wang, X.; Yusuf, S.; et al. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation 2019, 139, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Huang, Z.; Zhang, B.; Yu, L.; Li, L. Elevated lipoprotein (a) levels are associated with the acute myocardial infarction in patients with normal low-density lipoprotein cholesterol levels. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Steffen, B.T.; Duprez, D.; Bertoni, A.G.; Guan, W.; Tsai, M.Y. Lp(a) [Lipoprotein(a)]-Related Risk of Heart Failure Is Evident in Whites but Not in Other Racial/Ethnic Groups. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2498–2504. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.V.; Everett, B.M.; Caulfield, M.P.; Hantash, F.M.; Wohlgemuth, J.; Ridker, P.M.; Mora, S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: An analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2014, 129, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Gordts, P.L.S.M.; Nora, C.; Yeang, C.; Witztum, J.L. Statin therapy increases lipoprotein(a) levels. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Yahya, R.; Berk, K.; Verhoeven, A.; Bos, S.; van der Zee, L.; Touw, J.; Erhart, G.; Kronenberg, F.; Timman, R.; Sijbrands, E.; et al. Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis 2019, 289, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Paige, E.; Masconi, K.L.; Tsimikas, S.; Kronenberg, F.; Santer, P.; Weger, S.; Willeit, J.; Kiechl, S.; Willeit, P. Lipoprotein(a) and incident type-2 diabetes: Results from the prospective Bruneck study and a meta-analysis of published literature. Cardiovasc. Diabetol. 2017, 16, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.W.; Zhao, X.; Guo, Y.L.; Gao, Y.; Zhu, C.G.; Wu, N.Q.; Li, J.J. Elevated lipoprotein (a) levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes mellitus. Nutrition, metabolism, and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 980–986. [Google Scholar] [CrossRef]
- Jin, J.L.; Cao, Y.X.; Zhang, H.W.; Sun, D.; Hua, Q.; Li, Y.F.; Guo, Y.L.; Wu, N.Q.; Zhu, C.G.; Gao, Y.; et al. Lipoprotein(a) and Cardiovascular Outcomes in Patients With Coronary Artery Disease and Prediabetes or Diabetes. Diabetes Care 2019, 42, 1312–1318. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.; Sun, W.; Agarwala, A.; Virani, S.S.; Nambi, V.; Coresh, J.; Selvin, E.; Boerwinkle, E.; Jones, P.H.; Ballantyne, C.M.; et al. Lipoprotein(a) levels and risk of cardiovascular disease events in individuals with diabetes mellitus or prediabetes: The Atherosclerosis Risk in Communities study. Atherosclerosis 2019, 282, 52–56. [Google Scholar] [CrossRef]
- Werba, J.P.; Safa, O.; Gianfranceschi, G.; Michelagnoli, S.; Sirtori, C.R.; Franceschini, G. Plasma triglycerides and lipoprotein(a): Inverse relationship in a hyperlipidemic Italian population. Atherosclerosis 1993, 101, 203–211. [Google Scholar] [CrossRef]
- Consortium, C.A.D.; Deloukas, P.; Kanoni, S.; Willenborg, C.; Farrall, M.; Assimes, T.L.; Thompson, J.R.; Ingelsson, E.; Saleheen, D.; Erdmann, J.; et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013, 45, 25–33. [Google Scholar]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [Green Version]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA J. Am. Med. Assoc. 2009, 301, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Coassin, S.; Erhart, G.; Weissensteiner, H.; Eca Guimarães de Araújo, M.; Lamina, C.; Schönherr, S.; Forer, L.; Haun, M.; Losso, J.L.; Köttgen, A.; et al. A novel but frequent variant in LPA KIV-2 is associated with a pronounced Lp(a) and cardiovascular risk reduction. Eur. Heart J. 2017, 38, 1823–1831. [Google Scholar] [CrossRef]
- Noureen, A.; Fresser, F.; Utermann, G.; Schmidt, K. Sequence variation within the KIV-2 copy number polymorphism of the human LPA gene in African, Asian, and European populations. PLoS ONE 2015, 10, e0121582. [Google Scholar] [CrossRef] [Green Version]
- Saleheen, D.; Haycock, P.C.; Zhao, W.; Rasheed, A.; Taleb, A.; Imran, A.; Abbas, S.; Majeed, F.; Akhtar, S.; Qamar, N.; et al. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: A mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2017, 5, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Kamstrup, P.R.; Nordestgaard, B.G. Elevated Lipoprotein(a) Levels, LPA Risk Genotypes, and Increased Risk of Heart Failure in the General Population. JACC Heart Fail. 2016, 4, 78–87. [Google Scholar] [CrossRef]
- Lamina, C.; Kronenberg, F. Lp GC. Estimation of the Required Lipoprotein(a)-Lowering Therapeutic Effect Size for Reduction in Coronary Heart Disease Outcomes: A Mendelian Randomization Analysis. JAMA Cardiol. 2019, 4, 575–579. [Google Scholar] [CrossRef] [Green Version]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Burgess, S.; Ference, B.A.; Staley, J.R.; Freitag, D.F.; Mason, A.M.; Nielsen, S.F.; Willeit, P.; Young, R.; Surendran, P.; Karthikeyan, S.; et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018, 3, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najam, O.; Ray, K.K. Lp(a) and cardiovascular disease-Has the phoenix finally risen from the ashes? Eur. Heart J. 2019, 40, 2771–2774. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.L.; Hooper, A.J.; Burnett, J.R.; Watts, G.F. Progress in the care of common inherited atherogenic disorders of apolipoprotein B metabolism. Nat. Rev. Endocrinol. 2016, 12, 467–484. [Google Scholar] [CrossRef] [PubMed]
- The challenges of measuring Lp(a): A fight against Hydra? Atherosclerosis 2019, 289, 181–183. [CrossRef] [PubMed] [Green Version]
- Dati, F.; Tate, J.R.; Marcovina, S.M.; Steinmetz, A.; International Federation of Clinical Chemistry and Laboratory Medicine; IFCC Working Group for Lipoprotein(a) Assay Standardization. First WHO/IFCC International Reference Reagent for Lipoprotein(a) for Immunoassay—Lp(a) SRM 2B. Clin. Chem. Lab. Med. 2004, 42, 670–676. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Albers, J.J.; Scanu, A.M.; Kennedy, H.; Giaculli, F.; Berg, K.; Couderc, R.; Dati, F.; Rifai, N.; Sakurabayashi, I.; et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin. Chem. 2000, 46, 1956–1967. [Google Scholar]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef]
- Scharnagl, H.; Stojakovic, T.; Dieplinger, B.; Dieplinger, H.; Erhart, G.; Kostner, G.M.; Herrmann, M.; März, W.; Grammer, T.B. Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis 2019, 289, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Marcovina, S.M.; Zhang, Z.H.; Gaur, V.P.; Albers, J.J. Identification of 34 apolipoprotein(a) isoforms: Differential expression of apolipoprotein(a) alleles between American blacks and whites. Biochem. Biophys. Res. Commun. 1993, 191, 1192–1196. [Google Scholar] [CrossRef]
- Lassman, M.E.; McLaughlin, T.M.; Zhou, H.; Pan, Y.; Marcovina, S.M.; Laterza, O.; Roddy, T.P. Simultaneous quantitation and size characterization of apolipoprotein(a) by ultra-performance liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.L.; Watts, G.F. Is Lipoprotein(a) Ready for Prime-Time Use in the Clinic? Cardiol. Clin. 2018, 36, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Albers, J.J. Lipoprotein (a) measurements for clinical application. J. Lipid Res. 2016, 57, 526–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Fazio, S.; Ferdinand, K.C.; Ginsberg, H.N.; Koschinsky, M.L.; Marcovina, S.M.; Moriarty, P.M.; Rader, D.J.; Remaley, A.T.; Reyes-Soffer, G.; et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 177–192. [Google Scholar] [CrossRef]
- Ellis, K.L.; Chakraborty, A.; Moses, E.K.; Watts, G.F. To test, or not to test: That is the question for the future of lipoprotein(a). Expert Rev. Cardiovasc. Ther. 2019, 17, 241–250. [Google Scholar] [CrossRef]
- Takami, S.; Yamashita, S.; Kihara, S.; Ishigami, M.; Takemura, K.; Kume, N.; Kita, T.; Matsuzawa, Y. Lipoprotein(a) enhances the expression of intercellular adhesion molecule-1 in cultured human umbilical vein endothelial cells. Circulation 1998, 97, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.; Jung, Y.; Koschinsky, M.L. Apolipoprotein(a), through its strong lysine-binding site in KIV(10’), mediates increased endothelial cell contraction and permeability via a Rho/Rho kinase/MYPT1-dependent pathway. J. Biol. Chem. 2008, 283, 30503–30512. [Google Scholar] [CrossRef] [Green Version]
- Bouchareb, R.; Mahmut, A.; Nsaibia, M.J.; Boulanger, M.C.; Dahou, A.; Lépine, J.L.; Laflamme, M.H.; Hadji, F.; Couture, C.; Trahan, S.; et al. Autotaxin Derived From Lipoprotein(a) and Valve Interstitial Cells Promotes Inflammation and Mineralization of the Aortic Valve. Circulation 2015, 132, 677–690. [Google Scholar] [CrossRef]
- Riches, K.; Porter, K.E. Lipoprotein(a): Cellular Effects and Molecular Mechanisms. Cholesterol 2012, 2012, 923289. [Google Scholar] [CrossRef] [Green Version]
- Tsironis, L.D.; Mitsios, J.V.; Milionis, H.J.; Elisaf, M.; Tselepis, A.D. Effect of lipoprotein (a) on platelet activation induced by platelet-activating factor: Role of apolipoprotein (a) and endogenous PAF-acetylhydrolase. Cardiovasc. Res. 2004, 63, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Boffa, M.B.; Koschinsky, M.L. Lipoprotein (a): Truly a direct prothrombotic factor in cardiovascular disease? J. Lipid Res. 2016, 57, 745–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; Goldberg, R.; et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018. [Google Scholar] [CrossRef]
- Anderson, T.J.; Gregoire, J.; Pearson, G.J.; Barry, A.R.; Couture, P.; Dawes, M.; Francis, G.A.; Genest, J., Jr.; Grover, S.; Gupta, M.; et al. 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2016, 32, 1263–1282. [Google Scholar] [CrossRef] [PubMed]
- Stefanutti, C.; Julius, U.; Watts, G.F.; Harada-Shiba, M.; Cossu, M.; Schettler, V.J.; De Silvestro, G.; Soran, H.; Van Lennep, J.R.; Pisciotta, L.; et al. Toward an international consensus-Integrating lipoprotein apheresis and new lipid-lowering drugs. J. Clin. Lipidol. 2017, 11, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V. (EAS) TTFftmodotESoCEaEAS. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019. [Google Scholar] [CrossRef]
- Cegla, J.; Neely, R.D.G.; France, M.; Ferns, G.; Byrne, C.D.; Halcox, J.; Datta, D.; Capps, N.; Shoulders, C.; Qureshi, N.; et al. HEART UK Medical, Scientific and Research Committee. HEART UK consensus statement on Lipoprotin(a): A call to action. Atherosclerosis 2019, in press. [Google Scholar] [CrossRef] [Green Version]
- Afshar, M.; Pilote, L.; Dufresne, L.; Engert, J.C.; Thanassoulis, G. Lipoprotein(a) Interactions With Low-Density Lipoprotein Cholesterol and Other Cardiovascular Risk Factors in Premature Acute Coronary Syndrome (ACS). J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Chieng, D.; Pang, J.; Ellis, K.L.; Hillis, G.S.; Watts, G.F.; Schultz, C.J. Elevated lipoprotein(a) and low-density lipoprotein cholesterol as predictors of the severity and complexity of angiographic lesions in patients with premature coronary artery disease. J. Clin. Lipidol. 2018, 12, 1019–1026. [Google Scholar] [CrossRef]
- Ye, Z.; Haycock, P.C.; Gurdasani, D.; Pomilla, C.; Boekholdt, S.M.; Tsimikas, S.; Khaw, K.T.; Wareham, N.J.; Sandhu, M.S.; Forouhi, N.G. The association between circulating lipoprotein(a) and type 2 diabetes: Is it causal? Diabetes 2014, 63, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Zawacki, A.W.; Dodge, A.; Woo, K.M.; Ralphe, J.C.; Peterson, A.L. In pediatric familial hypercholesterolemia, lipoprotein(a) is more predictive than LDL-C for early onset of cardiovascular disease in family members. J. Clin. Lipidol. 2018, 12, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.L.; Perez de Isla, L.; Alonso, R.; Fuentes, F.; Watts, G.F.; Mata, P. Value of Measuring Lipoprotein(a) During Cascade Testing for Familial Hypercholesterolemia. J. Am. Coll. Cardiol. 2019, 73, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.C.; Haynes, R.; Baigent, C. The role of lipoprotein (a) in chronic kidney disease. J. Lipid Res. 2018, 59, 577–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, A.; Damrauer, S.M.; Anderson, A.H.; Xie, D.; Budoff, M.J.; Go, A.S.; He, J.; Lash, J.P.; Ojo, A.; Post, W.S.; et al. Lipoprotein(a) and Risk of Myocardial Infarction and Death in Chronic Kidney Disease: Findings From the CRIC Study (Chronic Renal Insufficiency Cohort). Arterioscler. Thromb. Vasc. Biol 2017, 37, 1971–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Bajaj, S.; Virk, H.; Bikkina, M.; Shamoon, F. Rapid Progression of Coronary Atherosclerosis: A Review. Thrombosis 2015, 2015, 634983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, N.R.; Mora, S.; Ridker, P.M. Lipoprotein(a) and Cardiovascular Risk Prediction Among Women. J. Am. Coll. Cardiol. 2018, 72, 287–296. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.A.; Pineda, A.L.; Wasserman, S.M.; Ceska, R.; Ezhov, M.V.; et al. Lipoprotein(a), PCSK9 inhibition and cardiovascular risk: Insights from the FOURIER trial. Circulation 2018. [Google Scholar] [CrossRef]
- Chennamsetty, I.; Kostner, K.M.; Claudel, T.; Vinod, M.; Frank, S.; Weiss, T.S.; Trauner, M.; Kostner, G.M. Nicotinic acid inhibits hepatic APOA gene expression: Studies in humans and in transgenic mice. J. Lipid Res. 2012, 53, 2405–2412. [Google Scholar] [CrossRef] [Green Version]
- Clofibrate and niacin in coronary heart disease. JAMA J. Am. Med. Assoc. 1975, 231, 360–381. [CrossRef]
- Tuck, C.H.; Holleran, S.; Berglund, L. Hormonal regulation of lipoprotein(a) levels: Effects of estrogen replacement therapy on lipoprotein(a) and acute phase reactants in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1822–1829. [Google Scholar] [CrossRef]
- Henriksson, P.; Angelin, B.; Berglund, L. Hormonal regulation of serum Lp (a) levels. Opposite effects after estrogen treatment and orchidectomy in males with prostatic carcinoma. J. Clin. Investig. 1992, 89, 1166–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahebkar, A.; Serban, M.C.; Penson, P.; Gurban, C.; Ursoniu, S.; Toth, P.P.; Jones, S.R.; Lippi, G.; Kotani, K.; Kostner, K.; et al. The Effects of Tamoxifen on Plasma Lipoprotein(a) Concentrations: Systematic Review and Meta Anal. Drugs 2017, 77, 1187–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momtazi-Borojeni, A.A.; Katsiki, N.; Pirro, M.; Banach, M.; Rasadi, K.A.; Sahebkar, A. Dietary natural products as emerging lipoprotein(a)-lowering agents. J. Cell Physiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, B.R.; Richter, Y.; Nagel, D.; Heigl, F.; Vogt, A.; Roeseler, E.; Parhofer, K.; Ramlow, W.; Koch, M.; Utermann, G.; et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat. Clin. Pract. Cardiovasc Med. 2009, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Leebmann, J.; Roeseler, E.; Julius, U.; Heigl, F.; Spitthoever, R.; Heutling, D.; Breitenberger, P.; Maerz, W.; Lehmacher, W.; Heibges, A.; et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: Prospective observational multicenter study. Circulation 2013, 128, 2567–2576. [Google Scholar] [CrossRef] [Green Version]
- Roeseler, E.; Julius, U.; Heigl, F.; Spitthoever, R.; Heutling, D.; Breitenberger, P.; Leebmann, J.; Lehmacher, W.; Kamstrup, P.R.; Nordestgaard, B.G.; et al. Lipoprotein Apheresis for Lipoprotein(a)-Associated Cardiovascular Disease: Prospective 5 Years of Follow-Up and Apolipoprotein(a) Characterization. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2019–2027. [Google Scholar] [CrossRef] [Green Version]
- Thompson, G.; Parhofer, K.G. Current Role of Lipoprotein Apheresis. Curr. Atheroscler. Rep. 2019, 21, 26. [Google Scholar]
- van Capelleveen, J.C.; van der Valk, F.M.; Stroes, E.S. Current therapies for lowering lipoprotein (a). J. Lipid Res. 2016, 57, 1612–1618. [Google Scholar] [CrossRef] [Green Version]
- Stiekema, L.C.A.; Stroes, E.S.G.; Verweij, S.L.; Kassahun, H.; Chen, L.; Wasserman, S.M.; Sabatine, M.S.; Mani, V.; Fayad, Z.A. Persistent arterial wall inflammation in patients with elevated lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur. Heart J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Ray, K.K.; Vallejo-Vaz, A.J.; Ginsberg, H.N.; Davidson, M.H.; Louie, M.J.; Bujas-Bobanovic, M.; Minini, P.; Eckel, R.H.; Cannon, C.P. Lipoprotein(a) reductions from PCSK9 inhibition and major adverse cardiovascular events: Pooled analysis of alirocumab phase 3 trials. Atherosclerosis 2019. [Google Scholar] [CrossRef] [Green Version]
- Warden, B.A.; Minnier, J.; Watts, G.F.; Fazio, S.; Shapiro, M.D. Impact of PCSK9 inhibitors on plasma lipoprotein(a) concentrations with or without a background of niacin therapy. J. Clin. Lipidol. 2019. [Google Scholar] [CrossRef]
- Tsimikas, S.; Viney, N.J.; Hughes, S.G.; Singleton, W.; Graham, M.J.; Baker, B.F.; Burkey, J.L.; Yang, Q.; Marcovina, S.M.; Geary, R.S.; et al. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015, 386, 1472–1483. [Google Scholar] [CrossRef]
- Viney, N.J.; van Capelleveen, J.C.; Geary, R.S.; Xia, S.; Tami, J.A.; Yu, R.Z.; Marcovina, S.M.; Hughes, S.G.; Graham, M.J.; Crooke, R.M.; et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016, 388, 2239–2253. [Google Scholar] [CrossRef]
- Mencarelli, A.; Fiorucci, S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med 2010, 14, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennamsetty, I.; Claudel, T.; Kostner, K.M.; Baghdasaryan, A.; Kratky, D.; Levak-Frank, S.; Frank, S.; Gonzalez, F.J.; Trauner, M.; Kostner, G.M. Farnesoid X receptor represses hepatic human APOA gene expression. J. Clin. Investig. 2011, 121, 3724–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanassoulis, G. Screening for High Lipoprotein(a). Circulation 2019, 139, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, V.S.; Flynn, P.D.; Raphael, C.E.; Newsome, S.; Khan, T.; Ali, A.; Halliday, B.; Studer Bruengger, A.; Malley, T.; Sharma, P.; et al. Lipoprotein(a) in patients with aortic stenosis: Insights from cardiovascular magnetic resonance. PLoS ONE 2017, 12, e0181077. [Google Scholar] [CrossRef] [Green Version]


| Canadian Cardiovascular Society 2016 [104] | Mighty Medic Group 2017 [105] | AHA/ACC Group 2018 [103] | NLA 2019 [88] | EAS/ESC 2019 [106] | HEART-UK [107] | |
|---|---|---|---|---|---|---|
| Recommended measurement population | Intermediate Framingham risk score or family history of premature CAD | Intermediate/high risk CVD patients with premature CVD, FH, or family history of premature CVD without elevated LDL-c or recurrent CVD with statin therapy | Family history of premature ASCVD or personal history of ASCVD not explained by major risk factors | Family or personal history of premature ASCVD, primary severe hypercholesterolaemia (LDL-c ≥ 190 mg/dL), or FH. | High CVD risk or strong family history of premature ASCVD, including premature CVD, FH, recurrent CVD despite optimal LLT, or ≥5% 10-year CVD risk. * Plasma levels should be measured at least once in a person’s life for risk stratification. | A personal or family history of premature ASCVD; 1 relative with Lp(a) > 200 nmol/L, FH, or genetic dyslipidaemia; CAVD; or increased 10-year risk of CVD |
| Risk threshold | >30 mg/dL | >30 mg/dL or >45 nmol/L | ≥50 mg/dL or ≥125 nmol/L | ≥50 mg/dL or ≥100 nmol/L * population dependent | State a level >180 mg/dL or >430 nmol/L equivalent to heterozygous FH | 32–90 nmol/L, minor; 90–200 nmol/L, moderate; 200–400 nmol/L, high; >400 nmol/L, very high |
| Recommended treatment | Consideration of elevated Lp(a) in shared decision making | Niacin or, if refractory, selective apheresis. | None specified | None specified | None specified | Reduce overall ASCVD risk, control hyperlipidaemia, and consider apheresis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, N.C.; Kostner, K.M.; Sullivan, D.R.; Nestel, P.; Watts, G.F. Molecular, Population, and Clinical Aspects of Lipoprotein(a): A Bridge Too Far? J. Clin. Med. 2019, 8, 2073. https://doi.org/10.3390/jcm8122073
Ward NC, Kostner KM, Sullivan DR, Nestel P, Watts GF. Molecular, Population, and Clinical Aspects of Lipoprotein(a): A Bridge Too Far? Journal of Clinical Medicine. 2019; 8(12):2073. https://doi.org/10.3390/jcm8122073
Chicago/Turabian StyleWard, Natalie C., Karam M. Kostner, David R. Sullivan, Paul Nestel, and Gerald F. Watts. 2019. "Molecular, Population, and Clinical Aspects of Lipoprotein(a): A Bridge Too Far?" Journal of Clinical Medicine 8, no. 12: 2073. https://doi.org/10.3390/jcm8122073






