Multimodal Intervention to Improve Functional Status in Hypertensive Older Adults: A Pilot Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Procedures
2.2. Participants
2.3. Randomization
2.4. Pharmacological Intervention
2.5. Exercise Intervention
2.6. Retention, Adherence, and Safety
2.7. Assessments
2.8. Statistical Analyses
3. Results
3.1. Participant Recruitment and Randomization
3.2. Retention
3.3. Adherence and Safety
3.4. Participant Characteristics
3.5. Gait Speed
3.6. Blood Pressure, Body Composition, and Exercise Capacity
3.7. Clinical Metabolic Profiles
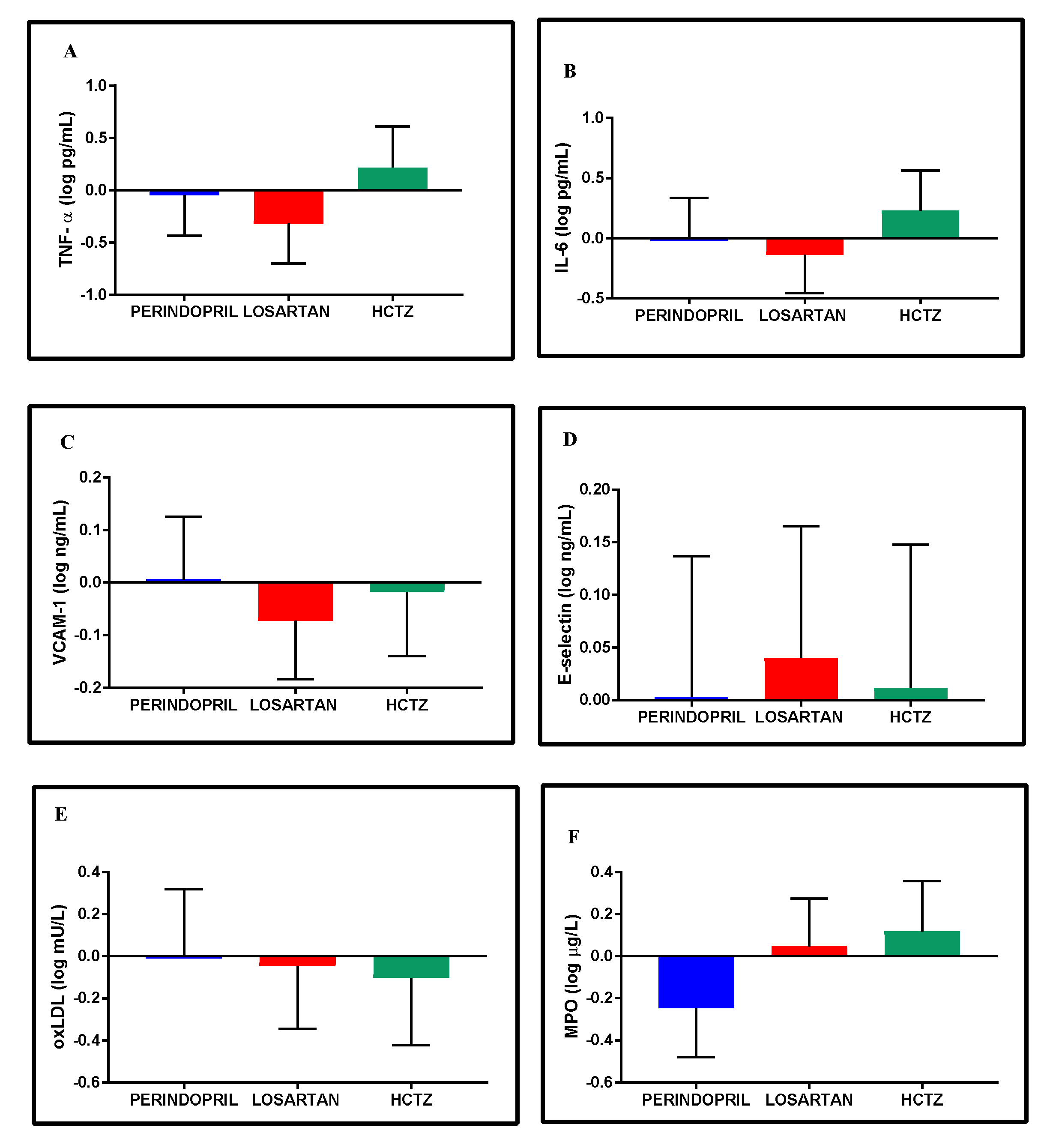
3.8. Inflammatory and Oxidative Stress Biomarkers
3.9. Exercise Mode
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muszalik, M.; Dijkstra, A.; Kędziora-Kornatowska, K.; Zielińska-Więczkowska, H.; Kornatowski, T. Independence of elderly patients with arterial hypertension in fulfilling their needs, in the aspect of functional assessment and quality of life (QoL). Arch. Gerontol. Geriatr. 2011, 52, e204–e209. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Simonsick, E.M.; Naydeck, B.L.; Boudreau, R.M.; Kritchevsky, S.B.; Nevitt, M.C.; Pahor, M.; Satterfield, S.; Brach, J.S.; Studenski, S.A.; et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 2006, 295, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Olson, M.B.; Kip, K.; Kelsey, S.F.; Johnson, B.D.; Mark, D.B.; Reis, S.E.; Mankad, S.; Rogers, W.J.; Pohost, G.M.; et al. The value of estimated functional capacity in estimating outcome: Results from the NHBLI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006, 47, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- McGinn, A.P.; Kaplan, R.C.; Verghese, J.; Rosenbaum, D.M.; Psaty, B.M.; Baird, A.E.; Lynch, J.K.; Wolf, P.A.; Kooperberg, C.; Larson, J.C.; et al. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke 2008, 39, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Eisenberg, M.J.; Morin, J.-F.; Bergman, H.; Monette, J.; Noiseux, N.; Perrault, L.P.; Alexander, K.P.; Langlois, Y.; Dendukuri, N.; et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 2010, 56, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Dumurgier, J.; Elbaz, A.; Ducimetiere, P.; Tavernier, B.; Alperovitch, A.; Tzourio, C. Slow walking speed and cardiovascular death in well functioning older adults: Prospective cohort study. BMJ 2009, 339, b4460. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2018, 48, 16–31. [Google Scholar] [CrossRef]
- Peel, N.M.; Kuys, S.S.; Klein, K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 68, 39–46. [Google Scholar] [CrossRef]
- Rosano, C.; Longstreth, W.R.B.; Taylor, C.A.; Du, Y.; Kuller, L.H.; Newman, A.B. High Blood Pressure Accelerates Gait Slowing in Well- Functioning Older Adults over 18-Years of Follow-Up. J. Am. Geriatr. Soc. 2011, 59, 390–397. [Google Scholar] [CrossRef]
- Balzi, D.; Lauretani, F.; Barchielli, A.; Ferrucci, L.; Bandinelli, S.; Buiatti, E.; Milaneschi, Y.; Guralnik, J.M. Risk factors for disability in older persons over 3-year follow-up. Age Ageing 2009, 39, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Dumurgier, J.; Elbaz, A.; Dufouil, C.; Tavernier, B.; Tzourio, C. Hypertension and lower walking speed in the elderly: The Three-City study. J. Hypertens. 2010, 28, 1506. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Manini, T.M.; Hsu, F.-C.; Cesari, M.; Anton, S.D.; Nayfield, S.; Stafford, R.S.; Church, T.S.; Pahor, M.; Carter, C.S. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J. Am. Geriatr. Soc. 2012, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, I.; Lackland, D.T.; Cupples, L.A.; Lipsitz, L.A. Association between concurrent and remote blood pressure and disability in older adults. Hypertension 2007, 50, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Anton, S.D.; Bavry, A.A.; Carter, C.S.; Daniels, M.J.; Pahor, M. Multi-modal intervention to reduce cardiovascular risk among hypertensive older adults: Design of a randomized clinical trial. Contemp. Clin. Trials 2015, 43, 237–242. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs/Population Division. World Population Ageing; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011; pp. 2–14. [Google Scholar]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, T.A.; Cohen, A.J. Medical care expenditures for selected circulatory diseases: Opportunities for reducing national health expenditures. Med. Care 1999, 37, 994–1012. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.B.; Lee-McMullen, B.; Phelan, D.; Gilkes, J.; Carter, C.S.; Buford, T.W. The renin-angiotensin system and prevention of age-related functional decline: Where are we now? Age (Dordr) 2015, 37, 9753. [Google Scholar] [CrossRef]
- McAlister, F.A. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are beneficial in normotensive atherosclerotic patients: A collaborative meta-analysis of randomized trials. Eur. Heart J. 2012, 33, 505–514. [Google Scholar] [CrossRef]
- Cranney, A. Is there a new role for angiotensin-converting-enzyme inhibitors in elderly patients? CMAJ 2007, 177, 891–892. [Google Scholar] [CrossRef]
- Sumukadas, D.; Struthers, A.D.; McMurdo, M.E.T. Sarcopenia—A potential target for Angiotensin-converting enzyme inhibition? Gerontology 2006, 52, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Onder, G.; Kritchevsky, S.B.; Pahor, M. Angiotensin-converting enzyme inhibition intervention in elderly persons: Effects on body composition and physical performance. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Sica, D. Are There Pleiotropic Effects of Antihypertensive Medications or Is It All About the Blood Pressure in the Patient with Diabetes and Hypertension? J. Clin. Hypertens. 2011, 13, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin converting- enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Marzetti, E.; Leeuwenburgh, C.; Manini, T.; Foster, T.C.; Groban, L.; Scarpace, P.J.; Morgan, D. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2012, 67, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; von Haehling, S.; Anker, S.D. Are we closer to having drugs to treat muscle wasting disease? J. Cachexia Sarcopenia Muscle 2014, 5, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif. Tissue Int. 2016, 98, 319–333. [Google Scholar] [CrossRef]
- Spira, D.; Walston, J.; Buchmann, N.; Nikolov, J.; Demuth, I.; Steinhagen-Thiessen, E.; Eckardt, R.; Norman, K. Angiotensin-Converting Enzyme Inhibitors and Parameters of Sarcopenia: Relation to Muscle Mass, Strength and Function: Data from the Berlin Aging Study-II (BASE-II). Drugs Aging 2016, 33, 829–837. [Google Scholar] [CrossRef]
- Sumukadas, D.; Band, M.; Miller, S.; Cvoro, V.; Witham, M.; Struthers, A.; McConnachie, A.; Lloyd, S.M.; McMurdo, M. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J. Gerontol. A. Biol. Sci. Med. Sci. 2014, 69, 736–743. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.J.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.J.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M. Sedentary behavior research network (SBRN)–Terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.L.; Mills, K.M.; King, A.C.; Haskell, W.L.; Gillis, D.; Ritter, P.L. CHAMPS Physical Activity Questionnaire for Older Adults: Outcomes for interventions. Med. Sci. Sport. Exerc. 2001, 33, 1126–1141. [Google Scholar] [CrossRef]
- Rolland, Y.M.; Cesari, M.; Miller, M.E.; Penninx, B.W.; Atkinson, H.H.; Pahor, M. Reliability of the 400-M Usual-Pace Walk Test as an Assessment of Mobility Limitation in Older Adults. J. Am. Geriatr. Soc. 2004, 52, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. American College of Sports Medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. American College of Sports Medicine Guidelines for Exercise Testing and Prescription, 7th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1988. [Google Scholar]
- Jakicic, J.M.; Marcus, M.; Gallagher, K.I.; Randall, C.; Thomas, E.; Goss, F.L.; Robertson, R.J. Evaluation of the Sense Wear Pro Armband to assess energy expenditure during exercise. Med. Sci. Sports Exerc. 2004, 36, 897–904. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Blackwell, T.L.; Cauley, J.A.; Ensrud, K.E.; Dam, T.T.; Harrison, S.L.; Peters, K.W.; Mackey, D.C. Objective assessment of activity, energy expenditure, and functional limitations in older men: The osteoporotic fractures in men study. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2013, 68, 1518–1524. [Google Scholar] [CrossRef]
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Horne, E.; Lancaster, G.A.; Matson, R.; Cooper, A.; Ness, A.; Leary, S. Pilot trials in physical activity journals: A review of reporting and editorial policy. Pilot Feasibility Stud. 2018, 4, 125. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; O’Cathain, A.; Altman, D.; Bretz, F.; et al. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Choo, P.W.; Rand, C.S.; Inui, T.S.; Lee, M.L.; Cain, E.; Cordeiro-Breault, M.; Canning, C.; Platt, R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med. Care 1999, 37, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.C. Frailty, Aging, and Cardiac Surgery Outcomes: The Stopwatch Tells the Story. J. Am. Coll. Cardiol. 2010, 56, 1677–1678. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; McBurnie, M.A.; Bittner, V.; Tracy, R.P.; McNamara, R.; Arnold, A.; Newman, A.B. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003, 123, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Steele, B. Timed walking tests of exercise capacity in chronic cardiopulmonary illness. J. Cardiopulm. Rehabil. 1996, 16, 25–33. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Waeber, B.; Parati, G.; Staessen, J.; Myers, M.G. Clinical review Blood pressure measuring devices: Recommendations of the European Society of Hypertension. BMJ 2001, 322, 531–536. [Google Scholar] [CrossRef]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Applied Longitudinal Analysis, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. arXiv, 2014; arXiv:1406.5823. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkings: Philadelphia, PA, USA, 2014; ISBN 9781609136055. [Google Scholar]
- Charlesworth, C.J.; Smit, E.; Lee, D.S.H.; Alramadhan, F.; Odden, M.C. Polypharmacy Among Adults Aged 65 Years and Older in the United States: 1988–2010. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 989–995. [Google Scholar] [CrossRef]
- Pahor, M.; Guralnik, J.M.; Ambrosius, W.T.; Blair, S.; Bonds, D.E.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Groessl, E.J.; et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE Study randomized clinical trial. J. Am. Med. Dir. Assoc. 2014, 311, 2387–2396. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Atknson, H.H.; Penninx, B.W.; Bari, M.D.; Tracy, R.P.; Pahor, M. Angiotensin converting enzyme inhibition and novel cardiovascular risk biomarkers. Am. Heart J. 2009, 157, 334.e1–334.e8. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Woodsa, A.J.; Ashizawac, T.; Barba, D.; Buford, T.W.; Carter, C.S.; Clark, D.J.; Cohen, R.A.; Corbett, D.B.; Cruz-Almeida, Y.; et al. Successful Aging: Advancing the Science of Physical Independence in Older Adults. Ageing Res. Rev. 2015, 24, 304–327. [Google Scholar] [CrossRef] [PubMed]
- Rajadhyaksha, V. Conducting feasibilities in clinical trials: An investment to ensure a good study. Perspect. Clin. Res. 2010, 1, 190–193. [Google Scholar]
- El-Kotob, R.; Giangregorio, L.M. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud. 2018, 4, 137. [Google Scholar] [CrossRef] [PubMed]
- Katula, J.A.; Kritchevsky, S.B.; Guralnik, J.M.; Glynn, N.W.; Pruitt, L.; Wallace, K.; Walkup, M.P.; Hsu, F.-C.; Studenski, S.A.; Gill, T.M.; et al. Lifestyle Interventions and Independence for Elders pilot study: Recruitment and baseline characteristics. J. Am. Geriatr. Soc. 2007, 55, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar] [CrossRef] [PubMed]
- Cryer, M.J.; Horani, T.; Dipette, D.J. Diabetes and Hypertension: A Comparative Review of Current Guidelines. J. Clin. Hypertens. 2016, 18, 95–100. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 48–759. [Google Scholar] [CrossRef]
- Liu, S.-H.; Eaton, C.B.; Driban, J.B.; McAlindon, T.E.; Lapane, K.L. Comparison of self-report and objective measures of physical activity in US adults with osteoarthritis. Rheumatol. Int. 2016, 36, 1355–1364. [Google Scholar] [CrossRef]
- Thyregod, M.; Bodtger, U. Coherence between self-reported and objectively measured physical activity in patients with chronic obstructive lung disease: A systematic review. Int. J. COPD 2016, 11, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Keidar, S.; Heinrich, R.; Kaplan, M.; Hayek, T.; Aviram, M. Angiotensin II Administration to Atherosclerotic Mice Increases Macrophage Uptake of Oxidized LDL A Possible Role for Interleukin-6. Arter. Thromb. Vasc. Biol. 2001, 21, 1464–1469. [Google Scholar] [CrossRef]
- Hayek, T.; Attias, J.; Coleman, R.; Brodsky, S.; Smith, J.; Breslow, J.L.; Keidar, S. The angiotensin-converting enzyme inhibitor, fosinopril, and the angiotensin II receptor antagonist, losartan, inhibit LDL oxidation and attenuate atherosclerosis independent of lowering blood pressure in apolipoprotein E deficient mice. Cardiovasc. Res. 1999, 44, 579–587. [Google Scholar] [CrossRef]
- Sivasubramaniam, S.; Kumarasamy, B. Pleiotropic Effects of Losartan in Hypertensive Patients with Dyslipidemia. J. Clin. Diagn. Res. 2017, 11, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Adams-huet, B.; Vega, G.L.; Toto, R.D. Effect of losartan and spironolactone on triglyceride-rich lipoproteins in diabetic nephropathy. J. Investig. Med. 2016, 64, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Ragonesi, P.D.; Mugellini, A.; Ciccarelli, L.; Fogari, R. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: A randomized, double-blind, placebo-controlled 12-month study. Hypertens. Res. 2004, 27, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.C.; Pershadsingh, H.A.; Ho, C.I.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M.A.; Kurtz, T.W. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertens (Dallas, Tex. 1979) 2004, 43, 993–1002. [Google Scholar] [CrossRef]
- Morgan, A.E.; Mooney, K.M.; Wilkinson, S.J.; Pickles, N.A.; Mc Auley, M.T. Cholesterol metabolism: A review of how ageing disrupts the biological mechanisms responsible for its regulation. Ageing Res. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Scott, D.; Trbojevic, T.; Skinner, E.; Clark, R.A.; Levinger, P. Associations of calf inter- and intra-muscular adipose tissue with cardiometabolic health and physical function in community-dwelling older adults. J. Musculoskelet. Neuronal Interact. 2015, 15, 350–357. [Google Scholar]
- Bennett, J.A.; Riegel, B.; Bittner, V.; Nichols, J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung 2002, 31, 262–270. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975. [Google Scholar] [CrossRef]





| Outcomes | Total (n = 31) | Perindopril (n = 10) | Losartan (n = 13) | HCTZ (n = 8) |
|---|---|---|---|---|
| Female, % | 61.3 | 50.0 | 69.2 | 62.5 |
| Age, years | 70.6 (6.1) | 72.9 (7.2) | 71.0 (6.2) | 67.1 (2.2) |
| MMSE | 27.8 (1.5) | 27.9 (1.3) | 27.8 (1.7) | 27.6 (1.5) |
| Race, Caucasian, % | 67.7 | 80.0 | 69.2 | 50.0 |
| Education, college graduate, % | 54.8 | 60.0 | 53.8 | 50.0 |
| Measurements | ||||
| Weight, kg | 91.3 (14.3) | 88.6 (14.8) | 93.6 (16.4) | 91.0 (10.9) |
| Body mass index, kg/m2 | 33.4 (6.0) | 31.7 (6.6) | 34.5 (6.4) | 33.8 (4.5) |
| Total body fat mass, kg | 38.5 (11.4) | 37.1(12.2) | 40.3 (12.6) | 37.6 (9.2) |
| Total lean mass, kg | 48.0 (7.4) | 47.2 (7.7) | 48.5 (8.8) | 48.1 (5.2) |
| Systolic blood pressure, mmHg | 133.8 (15.3) | 133.2 (10.9) | 134.8 (14.5) | 132.9 (22.2) |
| Diastolic blood pressure, mmHg | 81.1 (9.1) | 79.3 (4.6) | 81.2 (10.0) | 83.1 (12.3) |
| Total cholesterol, mg/dL | 187.1 (37.3) | 187.6 (29.6) | 193.2 (43.9) | 176.4 (36.3) |
| HDL cholesterol, mg/dL | 58.4 (14.0) | 63.2 (10.4) | 56.2 (15.6) | 55.9 (15.5) |
| LDL cholesterol, mg/dL | 105.0 (31.3) | 100.1 (27.5) | 111.5 (37.6) | 100.6 (26.0) |
| Triglycerides, mg/dL | 122.1 (50.5) | 121.5 (49.6) | 134.9 (58.4) | 101.9 (33.7) |
| Fasting glucose, mg/dL | 95.7 (9.5) | 93.9 (6.7) | 96.4 (12.1) | 96.8 (8.4) |
| Creatinine, mg/dL | 0.9 (0.2) | 0.9 (0.1) | 1.0 (0.2) | 0.9 (0.2) |
| BUN/Creatinine ratio, mg/dL | 20.2 (6.3) | 20.7 (4.5) | 19.1 (6.9) | 21.2 (7.5) |
| Functional Measures | ||||
| 6-min walk distance, m | 394.8 (80.9) | 392.5 (72.2) | 388.1 (97.8) | 408.8 (68.2) |
| SPPB | 10.5 (1.3) | 10.1 (1.5) | 10.4 (1.4) | 11.1 (0.8) |
| CHAMPS, min/week | 51.0 (51.1) | 46.0 (48.4) | 50.8 (46.2) | 57.5 (66.5) |
| 4-m gait speed, m/s | 0.97 (0.15) | 0.95 (0.17) | 0.98 (0.14) | 0.99 (0.13) |
| Energy Expenditure | ||||
| Energy expenditure, cal/day | 2267 (352) | 2279 (375) | 2492 (647) | 2162 (269) |
| Low PA, min/day | 283.0 (190.1) | 317.6 (171.8) | 442.8 (226.4) | 236.2 (137.4) |
| Moderate or higher PA, min/day | 46.9 (30.0) | 55.0 (30.5) | 72.8 (30.2) | 41.2 (22.7) |
| Dietary intake | ||||
| Total intake, kcal/day | 1737 (569) | 1938 (508) | 1624 (725) | 1655 (304) |
| Protein, gr/day | 68.9 (21.4) | 73.9 (17.7) | 63.6 (26.7) | 70.8 (17.2) |
| Carbohydrate, gr/day | 215.9 (67.8) | 240.7 (64.6) | 206.3 (77.4) | 199.0 (54.5) |
| Sugar, gr/day | 77.8 (30.0) | 78.1 (25.5) | 79.5 (35.9) | 74.6 (29.4) |
| Fat, gr/day | 69.2 (33.4) | 74.9 (31.3) | 62.5 (42.9) | 72.2 (17.7) |
| Vitamin D, μg | 2.76 (4.74) | 2.79 (3.14) | 3.61 (6.90) | 1.37 (1.18) |
| Medication | ||||
| Total medication, n | 3.7 (1.6) | 3.8 (1.8) | 3.9 (1.7) | 3.4 (1.5) |
| Antidyslipidemic medication, % | 45.2 | 50.0 | 30.8 | 62.5 |
| ACEs, % | 32.3 | 50.0 | 30.8 | 12.5 |
| ARA, % | 22.6 | 10.0 | 23.1 | 37.5 |
| Outcomes | Perindopril | Losartan | HCTZ | |||
|---|---|---|---|---|---|---|
| Aerobic | Concurrent | Aerobic | Concurrent | Aerobic | Concurrent | |
| Baseline-8 Weeks | 8–24 Weeks | Baseline-8 Weeks | 8–24 Weeks | Baseline-8 Weeks | 8–24 Weeks | |
| 6-min walk distance, m | 45.8 (19.1, 72.6) | −27.1 (−55.9, 1.6) | 67.5 (44.7, 90.3) | 2.3 (−23.2, 27.8) | 40.0 (8.9, 63.2) | 19.6 (−10.6, 49.7) |
| SBP, mmHg | 5.0 (−4.4, 14.5) | 1.8 (−11.7, 15.2) | 8.9 (0.8, 17.0) | −5.1 (−16.9, 6.7) | −2.3 (−11.9, 7.3) | 0.5 (−12.9, 13.9) |
| DBP, mmHg | 1.1 (−3.8, 6.1) | 1.4 (−7.7, 10.4) | 3.4 (−0.8, 7.6) | 3.1 (−11.0, 4.9) | 2.4 (−2.6, 7.4) | 3.4 (−12.4, 5.7) |
| Total cholesterol, mg/dL | −11.7 (−27,9, 4.6) | 9.8 (−11.5, 40.0) | −8.3 (−22.3, 5.6) | 16.2 (−2.5, 34.9) | −7.3 (−24.6, 10.1) | 3.3 (−18.9, 25.5) |
| HDL cholesterol, mg/dL | −0.3 (−4.9, 4.4) | 4.8 (−0.4, 9.9) | 2.1 (−1.9, 6.2) | −0.2 (−4.8, 4.4) | −0.3 (−5.1, 4.6) | 2.3 (−3.2, 7.7) |
| LDL cholesterol, mg/dL | −9.8 (−25.7, 6.0) | 3.4 (−16.4, 23.2) | −9.1 (−22.5, 4.5) | 11.5 (−6.0, 28.9) | −7.4 (−24.1, 9.3) | 2.6 (−18.1, 23.4) |
| Triglycerides, mg/dL | −22.0 (−48.8, 4.8) | 9.5 (−25.2, 44.2) | −6.7 (−29.6, 16.2) | 22.8 (−7.7, 53.3) | 3.7 (−25.7, 33.1) | −1.3 (−37.6, 35.0) |
| Fasting glucose, mg/dL | 1.0 (−3.5, 5.4) | −1.3 (−8.4, 5.9) | −3.6 (−7.3, 0.2) | 0.4 (−5.8, 6.7) | −1.2 (−5.6, 3.3) | 4.4 (−2.7, 11.5) |
| hsCRP, log mg/L | 0.2 (−0.2, 0.7) | −0.5 (−1.1, 0.2) | −0.1 (−0.5, 0.3) | 0.1(−0.5, 0.6) | −0.1 (−0.6, 0.3) | 0.0 (−0.7, 0.7) |
| TNF-α, log pg/mL | 0.2 (−0.2, 0.6) | −0.3 (−0.8, 0.2) | −0.0 (−0.4, 0.3) | −0.3 (−0.8, 0.2) | 0.1 (−0.3, 0.5) | 0.1 (−0.4, 0.6) |
| IL-6, log pg/mL | 0.0 (−0.3, 0.4) | −0.0 (−0.6, 0.6) | −0.0 (−0.3, 0.3) | −0.1 (−0.6, 0.4) | 0.3 (0.0, 0.7) | −0.1 (−0.7. 0.4) |
| VCAM-1, log ng/mL | 0.0 (−0.1, 0.2) | −0.0 (−0.2, 0.1) | −0.1 (−0.2, −0.0) | 0.0 (−0.1, 0.2) | −0.0 (−0.2, 0.1) | 0.0 (−0.2, 0.2) |
| E-selectin, log ng/mL | 0.1 (−0.1, 0.2) | −0.1 (−0.2, 0.1) | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.2) | −0.1 (−0.3, 0.0) | 0.1 (−0.0, 0.3) |
| oxLDL, log mU/L | −0.3 (−0.6, 0.0) | 0.3 (−0.2, 0.8) | −0.2 (−0.5, 0.1) | 0.2 (−0.3, 0.6) | −0.1 (−0.4, 0.2) | 0.0 (−0.5, 0.5) |
| MPO, log μg/L | −0.1 (−0.3, 0.2) | −0.2 (−0.4, 0.1) | −0.1 (−0.4, 0.1) | 0.2 (−0.1, 0.4) | −0.1 (−0.3, 0.2) | 0.2 (−0.1, 0.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, L.C.; Jaeger, B.C.; Anton, S.D.; Bavry, A.A.; Handberg, E.M.; Gardner, A.K.; Harper, S.A.; Roberts, L.M.; Sandesara, B.; Carter, C.S.; et al. Multimodal Intervention to Improve Functional Status in Hypertensive Older Adults: A Pilot Randomized Controlled Trial. J. Clin. Med. 2019, 8, 196. https://doi.org/10.3390/jcm8020196
Baptista LC, Jaeger BC, Anton SD, Bavry AA, Handberg EM, Gardner AK, Harper SA, Roberts LM, Sandesara B, Carter CS, et al. Multimodal Intervention to Improve Functional Status in Hypertensive Older Adults: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine. 2019; 8(2):196. https://doi.org/10.3390/jcm8020196
Chicago/Turabian StyleBaptista, Liliana C., Byron C. Jaeger, Stephen D. Anton, Anthony A. Bavry, Eileen M. Handberg, Anna K. Gardner, Sara A. Harper, Lisa M. Roberts, Bhanuprasad Sandesara, Christy S. Carter, and et al. 2019. "Multimodal Intervention to Improve Functional Status in Hypertensive Older Adults: A Pilot Randomized Controlled Trial" Journal of Clinical Medicine 8, no. 2: 196. https://doi.org/10.3390/jcm8020196






