Beneficial Effect of Exercise on Cognitive Function during Peripheral Arterial Disease: Potential Involvement of Myokines and Microglial Anti-Inflammatory Phenotype Enhancement
Abstract
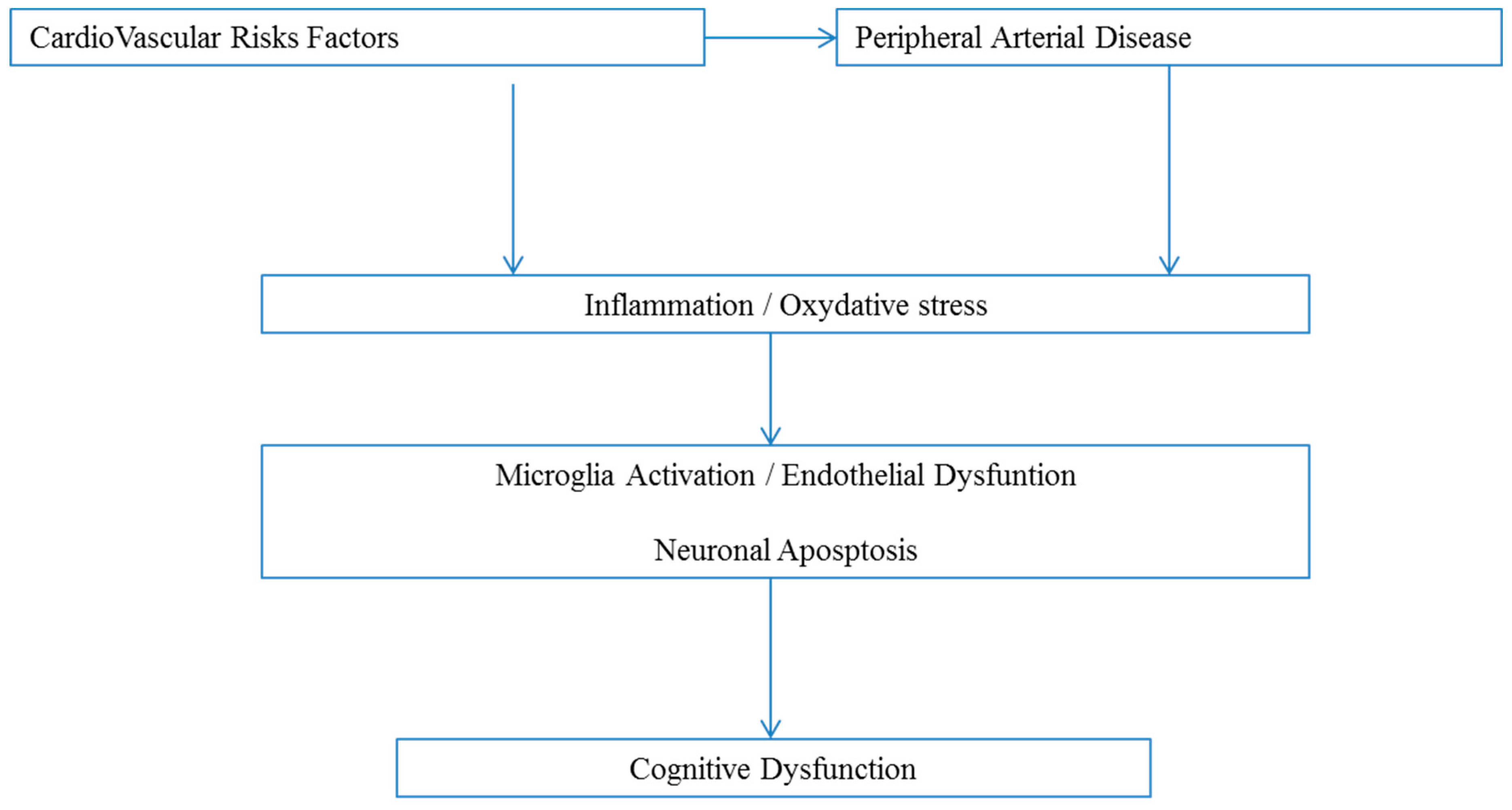
:1. Introduction
2. Cognitive Function in Patients with Peripheral Arterial Disease
2.1. Main Clinical Data
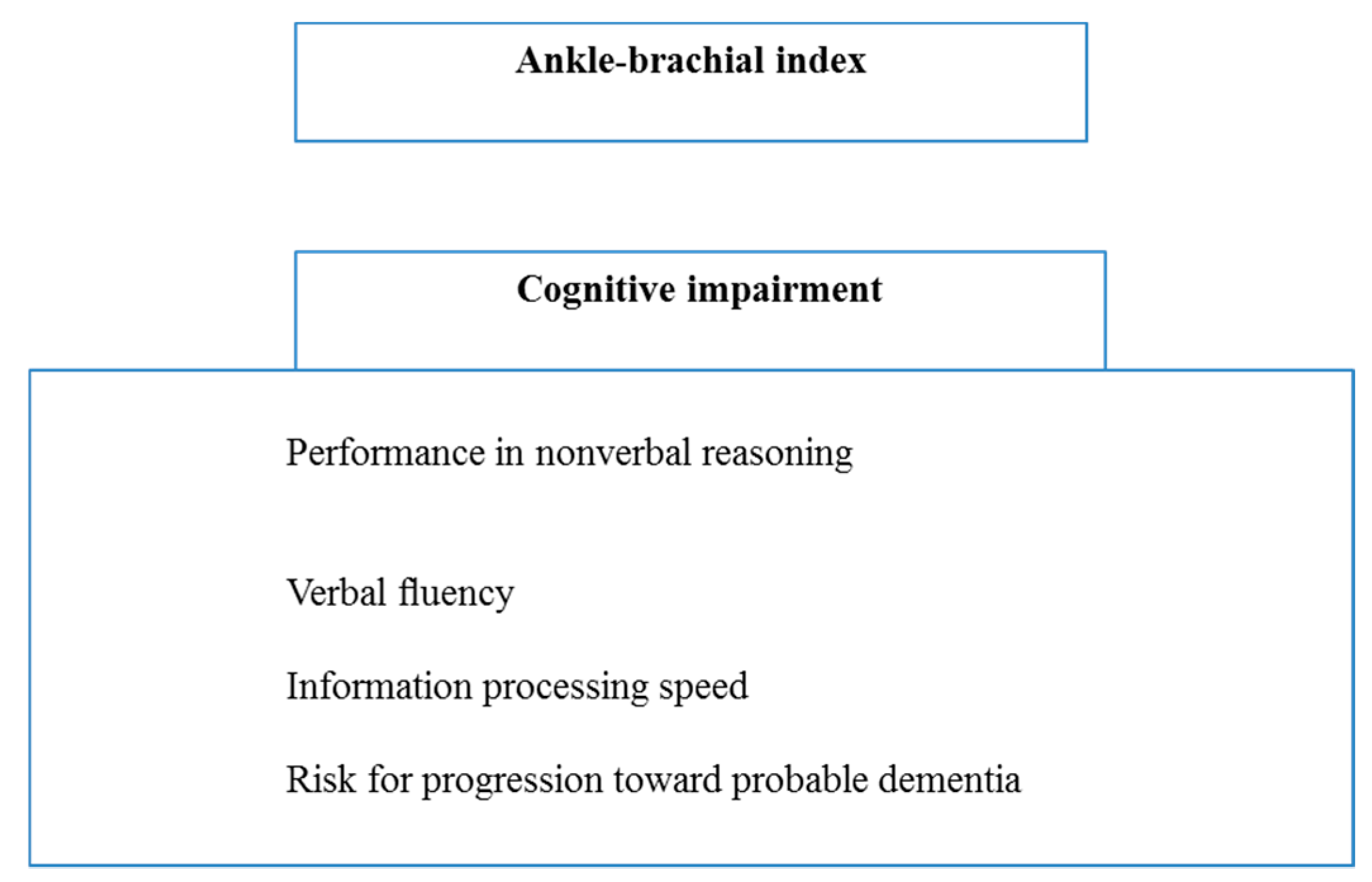
2.2. The Ankle-Brachial Index as an Indicator of Cognitive Dysfunction in PAD
3. Implications of Myokines in the Beneficial Effects of Exercise on Brain Function in Peripheral Arterial Disease Patients
3.1. Involvement of Myokines in Exercise-Induced Protection of the Cognitive Function
3.2. Microglial Anti-Inflammatory Phenotype and Exercise
4. Exercise Characteristics and Improvement in Cognitive Function
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramos, R.; Quesada, M.; Solanas, P.; Subirana, I.; Sala, J.; Vila, J.; Masiá, R.; Cerezo, C.; Elosua, R.; Grau, M.; et al. Prevalence of symptomatic and asymptomatic peripheral arterial disease and the value of the ankle-brachial index to stratify cardiovascular risk. Eur. J. Vasc. Endovasc. Surg. 2009, 3, 305–311. [Google Scholar] [CrossRef]
- Alzamora, M.T.; Forés, R.; Baena-Díez, J.M.; Pera, G.; Toran, P.; Sorribes, M.; Vicheto, M.; Reina, M.D.; Sancho, A.; Albaladejo, C.; et al. The peripheral arterial disease study (PERART/ARTPER): Prevalence and risk factors in the general population. BMC Public Health 2010, 10, 38. [Google Scholar] [CrossRef]
- Felix-Redondo, F.J.; Fernandez-Berges, D.; Grau, M.; Baena-Diez, J.M.; Mostaza, J.M.; Vila, J. Prevalence and clinical characteristics of peripheral arterial disease in the study population Hermex. Rev. Esp. Cardiol. 2012, 65, 726–733. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group; Bell, K.; Caporusso, J.; Durand-Zaleski, I.; et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef]
- Reeve, T.E.; Ur, R.; Craven, T.E.; Kaan, J.H.; Goldman, M.P.; Edwards, M.S.; Hurie, J.B.; Velazquez-Ramirez, G.; Corriere, M.A. Grip strength measurement for frailty assessment in patients with vascular disease and associations with comorbidity, cardiac risk, and sarcopenia. J. Vasc. Surg. 2018, 67, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, D.A.; Puttick, M.; Libertiny, G.; Hicks, R.C.J.; Earby, L.E.; Richards, T. Supervised exercise training for intermittent claudication: Lasting benefit at three years. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 322–326. [Google Scholar] [CrossRef]
- Fakhry, F.; van de Luijtgaarden, K.M.; Bax, L.; den Hoed, P.T.; Hunink, M.G.M.; Rouwet, E.V.; Spronk, S. Supervised walking therapy in patients with intermittent claudication. J. Vasc. Surg. 2012, 56, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Yokoyama, N.; Tamori, Y.; Akutsu, K.; Hashimoto, H.; Takeshita, S. Patients with peripheral artery disease who complete 12-week supervised exercise training program show reduced cardiovascular mortality and morbidity. Circ. J. 2009, 73, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Nead, K.T.; Olin, J.W.; Myers, J.; Cooke, J.P.; Leeper, N.J. Effect of physical activity assessment on prognostication for peripheral artery disease and mortality. Mayo Clin. Proc. 2015, 90, 339–345. [Google Scholar] [CrossRef]
- Joseph, A.-M.; Adhihetty, P.J.; Leeuwenburgh, C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol. (Lond.) 2016, 594, 5105–5123. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Laverny, G.; Paradis, S.; Schlagowski, A.I.; Charles, A.L.; Singh, F.; Zoll, J.; Thaveau, F.; Lonsdorfer, E.; Dufour, S.; et al. Moderate Exercise Allows for shorter Recovery Time in Critical Limb Ischemia. Front Physiol. 2017, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.D.; Reed, A.B.; Leuenberger, U.A.; Sinoway, L.I. Physiology in Medicine : Peripheral arterial disease. J. Appl. Physiol. 2013, 115, 1219–1226. [Google Scholar] [CrossRef]
- Hung, C.L.; Tseng, J.W.; Chao, H.H.; Hung, T.M.; Wang, H.S. Effect of Acute Exercise Mode on Serum Brain-Derived Neurotrophic Factor (BDNF) and Task Switching Performance. J. Clin. Med. 2018, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Törpel, A.; Herold, F.; Hamacher, D.; Müller, N.G.; Schega, L. Strengthening the Brain-Is Resistance Training with Blood Flow Restriction an Effective Strategy for Cognitive Improvement? J. Clin. Med. 2018, 7, 337. [Google Scholar] [CrossRef]
- Zhang, L.; Chopp, M.; Zhang, Y.; Xiong, Y.; Li, C.; Sadry, N.; Rhaleb, I.; Lu, M.; Zhang, Z.G. Diabetes Mellitus Impairs Cognitive Function in Middle-Aged Rats and Neurological Recovery in Middle-Aged Rats After Stroke. Stroke 2016, 47, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef]
- Moonga, I.; Niccolini, F.; Wilson, H.; Pagano, G.; Politis, M. Hypertension is associated with worse cognitive function and hippocampal hypometabolism in Alzheimer’s disease. Eur. J. Neurol. 2017, 24, 1173–1182. [Google Scholar] [CrossRef]
- Dik, M.G.; Jonker, C.; Comijs, H.C.; Deeg, D.J.; Kok, A.; Yaffe, K.; Penninx, B.W. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007, 30, 2655–2660. [Google Scholar] [CrossRef]
- Snyder, H.M.; Corriveau, R.A.; Craft, S.; Faber, J.E.; Greenberg, S.M.; Knopman, D.; Lamb, B.T.; Montine, T.J.; Nedergaard, M.; Schaffer, C.B.; et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015, 11, 710–717. [Google Scholar] [CrossRef]
- Leardini-Tristão, M.; Borges, J.P.; Freitas, F.; Rangel, R.; Daliry, A.; Tibiriçá, E.; Estato, V. The impact of early aerobic exercise on brain microvascular alterations induced by cerebral hypoperfusion. Brain Res. 2017, 1657, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Obadia, N.; Lessa, M.A.; Daliry, A.; Silvares, R.R.; Gomes, F.; Tibiriçá, E.; Estato, V. Cerebral microvascular dysfunction in metabolic syndrome is exacerbated by ischemia-reperfusion injury. BMC Neurosci. 2017, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Charles, A.L.; Chamaraux-Tran, T.N.; Bouitbir, J.; Meyer, A.; Zoll, J.; Schneider, F.; Geny, B. Oxidative stress precedes skeletal muscle mitochondrial dysfunction during experimental aortic cross-clamping but is not associated with early lung, heart, brain, liver, or kidney mitochondrial impairment. J. Vasc. Surg. 2014, 60, 1043–1051. [Google Scholar] [CrossRef]
- Karimi, N.; Haghani, M.; Noorafshan, A.; Moosavi, S.M.S. Structural and functional disorders of hippocampus following ischemia/reperfusion in lower limbs and kidneys. Neuroscience 2017, 358, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Li, J.; Yang, S.B.; Mo, L.Q.; Hu, J.H.; Yuan, W.L. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012, 1432, 36–45. [Google Scholar] [CrossRef]
- Rafnsson, S.B.; Deary, I.J.; Fowkes, F.G. Peripheral arterial disease and cognitive function. Vasc. Med. 2009, 14, 51–61. [Google Scholar] [CrossRef]
- Waldstein, S.R.; Tankard, C.F.; Maier, K.J.; Pelletier, J.R.; Snow, J.; Gardner, A.W.; Macko, R.; Katzel, L.I. Peripheral arterial disease and cognitive function. Psychosom. Med. 2003, 65, 757–763. [Google Scholar] [CrossRef]
- Gardner, A.W.; Waldstein, S.R.; Montgomery, P.S.; Zhao, Y.D. Effect of cognitive status on exercise performance and quality of life in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2016, 63, 98–104. [Google Scholar] [CrossRef]
- Cavalcante, B.R.; Germano-Soares, A.H.; Gerage, A.M.; Leicht, A.; Tassitano, R.M.; Bortolotti, H.; de Mello Franco, F.G.; Wolosker, N.; Cucato, G.G.; Ritti-Dias, R.M. Association between physical activity and walking capacity with cognitive function in peripheral artery disease patients. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 672–678. [Google Scholar] [CrossRef]
- Mangiafico, R.A.; Sarnataro, F.; Mangiafico, M.; Fiore, C.E. Impaired cognitive performance in asymptomatic peripheral arterial disease: Relation to C-reactive protein and D-dimer levels. Age Ageing 2006, 35, 60–65. [Google Scholar] [CrossRef]
- Williams, R.M.; Turner, A.P.; Green, M.; Norvell, D.C.; Henderson, A.W.; Hakimi, K.N.; Blake, D.J.; Czerniecki, J.M. Changes in cognitive function from presurgery to 4 months postsurgery in individuals undergoing dysvascular amputation. Arch. Phys. Med. Rehabil. 2014, 95, 663–669. [Google Scholar] [CrossRef]
- Phillips, N.A.; Mate-Kole, C.C.; Kirby, R.L. Neuropsychological function in peripheral vascular disease amputee patients. Arch. Phys. Med. Rehabil. 1993, 74, 1309–1314. [Google Scholar] [CrossRef]
- Hart, C.R.; Layec, G.; Trinity, J.D.; Kwon, O.S.; Zhao, J.; Reese, V.R.; Gifford, J.R.; Richardson, R.S. Increased skeletal muscle mitochondrial free radical production in peripheral arterial disease despite preserved mitochondrial respiratory capacity. Exp. Physiol. 2018, 103, 838–850. [Google Scholar] [CrossRef]
- Price, J.F.; McDowell, S.; Whiteman, M.C.; Deary, I.J.; Stewart, M.C.; Fowkes, F.G. Ankle brachial index as a predictor of cognitive impairment in the general population: Ten-year follow-up of the Edinburgh Artery Study. J. Am. Geriat. Soc. 2006, 54, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Lynn, H.; Wong, S.Y.; Hong, A.; Tang, Y.N.; Lau, W.Y.; Lau, E.; Orwoll, E.; Kwok, T.C. Correlates for a low ankle-brachial index in elderly Chinese. Atherosclerosis 2006, 186, 360–366. [Google Scholar] [CrossRef]
- Espeland, M.A.; Newman, A.B.; Sink, K.; Gill, T.M.; King, A.C.; Miller, M.E.; Guralnik, J.; Katula, J.; Church, T.; Manini, T.; et al. Associations Between Ankle-Brachial Index and Cognitive Function: Results From the Lifestyle Interventions and Independence for Elders Trial. J. Am. Med. Dir. Assoc. 2015, 16, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jiang, R.; Su, Z.; Jia, J.; Zhang, N.; Wu, J.; Chen, S.; Zhao, X. A low ankle-brachial index is associated with cognitive impairment: The APAC study. Atherosclerosis 2016, 255, 90–95. [Google Scholar] [CrossRef]
- Shaik, M.A.; Venketasubramanian, N.; Cheng, C.Y.; Wong, T.Y.; Vrooman, H.; Ikram, M.K.; Hilal, S.; Chen, C. Ankle brachial index, MRI markers and cognition: The Epidemiology of Dementia in Singapore study. Atherosclerosis 2017, 263, 272–277. [Google Scholar] [CrossRef]
- Hong, J.B.; Leonards, C.O.; Endres, M.; Siegerink, B.; Liman, T.G. Ankle-Brachial Index and Recurrent Stroke Risk: Meta-Analysis. Stroke 2016, 47, 317–322. [Google Scholar] [CrossRef]
- Stewart, K.J.; Hiatt, W.R.; Regensteiner, J.G.; Hirsch, A.T. Exercise training for claudication. N. Engl. J. Med. 2002, 347, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar]
- Steensberg, A.; van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Klarlund Pedersen, B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Akerstrom, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Cechetti, F.; Worm, P.V.; Elsner, V.R.; Bertoldi, K.; Sanches, E.; Ben, J.; Siqueira, I.R.; Netto, C.A. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol. Learn. Mem. 2012, 97, 90–96. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Zhang, L.; Huang, C.; Xiu, Y.; Wang, F.; Zhou, C.; Luo, Y.; Xiao, Q.; Tang, Y. Effects of long-term exercise on spatial learning, memory ability, and cortical capillaries in aged rats. Med. Sci. Monit. 2015, 21, 945–954. [Google Scholar]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef]
- Kim, O.Y.; Song, J. The Role of Irisin in Alzheimer’s Disease. J. Clin. Med. 2018, 7, 407. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W. Can irisin be a linker between physical activity and brain function? Biomol. Concepts 2016, 7, 253–258. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.H.; Song, F.; Sun, Y. Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediators Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivoandin vitrostudies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Atherton, P.J.; Phillips, B.E. Greek goddess or Greek myth: The effects of exercise on irisin/FNDC5 in humans. J. Physiol. 2013, 591, 5267–5268. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.A.; Fernández-Real, J.M.; Mantzoros, C. Irisin in humans: Recent advances and questions for future research. Metabolism 2014, 63, 178–180. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Küster, O.C.; Laptinskaya, D.; Fissler, P.; Schnack, C.; Zügel, M.; Nold, V.; Thurm, F.; Pleiner, S.; Karabatsiakis, A.; von Einem, B.; et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J. Alzheimers Dis. 2017, 59, 1097–1111. [Google Scholar] [CrossRef]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018. [Google Scholar] [CrossRef]
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Progress in cardiovascular diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Gruol, D.L. IL-6 regulation of synaptic function in the CNS. Neuropharmacology 2015, 96, 42–54. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 and its receptor: Ten years later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef]
- Eskes, C.; Honegger, P.; Juillerat-Jeanneret, L.; Monnet-Tschudi, F. Microglial reaction induced by noncytotoxic methylmercury treatment leads to neuroprotection via interactions with astrocytes and IL-6 release. Glia 2002, 37, 43–52. [Google Scholar] [CrossRef]
- Krady, J.K.; Lin, H.W.; Liberto, C.M.; Basu, A.; Kremlev, S.G.; Levison, S.W. Ciliary neurotrophic factor and interleukin-6 differentially activate microglia. J. Neurosci. Res. 2008, 86, 1538–1547. [Google Scholar] [CrossRef]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a mental cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef]
- Ma, S.H.; Zhuang, Q.X.; Shen, W.X.; Peng, Y.P.; Qiu, Y.H. Interleukin-6 reduces NMDAR-mediated cytosolic Ca(2)(+) overload and neuronal death via JAK/CaN signaling. Cell Calcium. 2015, 58, 286–295. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Jia, X.; Wang, Q.; Li, Y.; Hu, M.; Tian, L.; Yang, J.; Xing, W.; Zhang, W.; et al. Neuroprotection by IFN-gamma via astrocyte-secreted IL-6 in acute neuroinflammation. Oncotarget 2017, 8, 40065–40078. [Google Scholar]
- Gmiat, A.; Micielska, K.; Kozłowska, M.; Flis, D.J.; Smaruj, M.; Kujach, S.; Jaworska, J.; Lipińska, P.; Ziemann, E. The impact of a single bout of high intensity circuit training on myokines’ concentrations and cognitive functions in women of different age. Physiol. Behav. 2017, 179, 290–297. [Google Scholar] [CrossRef]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef]
- Rodgers, B.D.; Garikipati, D.K. Clinical, agricultural, and evolutionary biology of myostatin: A comparative review. Endocr. Rev. 2008, 29, 513–534. [Google Scholar] [CrossRef]
- Feldman, B.J.; Streeper, R.S.; Farese, R.V., Jr.; Yamamoto, K.R. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc. Natl. Acad. Sci. USA 2006, 103, 15675–15680. [Google Scholar] [CrossRef]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, F.Y.; Hsiao, Y.H. Myostatin Is Associated With Cognitive Decline in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 1984–1991. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Green, H.F.; Treacy, E.; Keohane, A.K.; Sullivan, A.M.; O’Keeffe, G.W.; Nolan, Y.M. A role for interleukin-1beta in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol. Cell Neurosci. 2012, 49, 311–321. [Google Scholar] [CrossRef]
- Michelucci, A.; Heurtaux, T.; Grandbarbe, L.; Morga, E.; Heuschling, P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009, 210, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Littlefield, A.M.; Setti, S.E.; Priester, C.; Kohman, R.A. Voluntary exercise attenuates LPS-induced reductions in neurogenesis and increases microglia expression of a proneurogenic phenotype in aged mice. J. Neuroinflammation 2015, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Kohman, R.A.; DeYoung, E.K.; Bhattacharya, T.K.; Peterson, L.N.; Rhodes, J.S. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav. Immun. 2012, 26, 803–810. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, L.; Pan, X.; Zheng, H.; Chen, X.; Li, L.; Luo, J.; Hu, X. Physical Exercise Improves Cognitive Function Together with Microglia Phenotype Modulation and Remyelination in Chronic Cerebral Hypoperfusion. Front. Cell. Neurosci. 2017, 11, 404. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Tucker, D.; Wang, R.; Ahmed, M.E.; Brann, D.; Zhang, Q. Treadmill; Exercise Exerts Neuroprotection and Regulates Microglial Polarization and Oxidative Stress in a Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. J. Alzheimers Dis. 2017, 56, 1469–1484. [Google Scholar] [CrossRef]
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275. [Google Scholar] [CrossRef]
- McDermott, M.M. Exercise training for intermittent claudication. J. Vasc. Surg. 2017, 66, 1612–1620. [Google Scholar] [CrossRef]
- Golledge, J.; Singh, T.P.; Alahakoon, C.; Pinchbeck, J.; Yip, L.; Moxon, J.V.; Morris, D.R. Meta-analysis of clinical trials examining the benefit of structured home exercise in patients with peripheral artery disease. Br. J. Surg. 2019, 106, 319–331. [Google Scholar] [CrossRef]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S. Changes in vascular and inflammatory biomarkers after exercise rehabilitation in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2019. [Google Scholar] [CrossRef]
- Ritti-Dias, R.M.; Correia, M.A.; Andrade-Lima, A.; Cucato, G.G. Exercise as a therapeutic approach to improve blood pressure in patients with peripheral arterial disease: Current literature and future directions. Expert Rev. Cardiovasc. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Parmenter, B.J.; Dieberg, G.; Smart, N.A. Exercise training for management of peripheral arterial disease: A systematic review and meta-analysis. Sports Med. 2015, 45, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.; Albines, D.; Hopkins-Rosseel, D. The Effectiveness of Exercise on Cognitive Performance in Individuals with Known Vascular Disease: A Systematic Review. J. Clin. Med. 2019, 8, 294. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. The Role of Sex in Memory Function: Considerations and Recommendations in the Context of Exercise. J. Clin. Med. 2018, 7, 132. [Google Scholar] [CrossRef] [PubMed]

| Reference | Study Sample | Total Sample Size and SEX | Mean Age | Cognitive Measures | Cognitive Results |
|---|---|---|---|---|---|
| Phillips et al., 1993 [32] (Arch Phys Med Rehabil.) | Patients with lower-extremity amputations secondary to PAD and healthy volunteers | 37: PAD group (4 women and 10 men) and healthy volunteers (9 women and 5 men) | 67.4 ± 14.8 and 69.9 ± 9.3 | Learning and memory (WAIS-R Digit Symbol subtest, WMS-R); language and verbal ability; visuoperceptual organization and constructional abilities; problem solving (MCST), abstract reasoning, and concept formation; social judgement and sequential reasoning; psychomotor function. | The PAD amputee patients performed more poorly than controls (p < 0.002, one-tailed) on the WAIS-R Digit Symbol subtest and obtained fewer categories on the MSCT than did the controls. There were trends (p < 0.01, one-tailed) toward lower patient scores on a number of other neuropsychological tests including the WAIS-R Vocabulary, Arithmetic, Similarities, and picture arrangement subtests, oral fluency (COWAT, orthographic condition), and the copy administration of the ROCF. |
| Waldstein et al., 2003 [27] (Psychosomatic Medicine) | PAD, stroke, hypertensive and normotensive patients | 107: Normotensive group (7 women and 16 men), hypertensive group (5 women and 15 men), PAD group (10 women and 28 men) and stroke group (6 women and 20 men) | 66.3 ± 5.8 70.0 ± 5.7 69.8 ± 7.0 62.3 ± 8.1 | Tests for verbal memory (WMS-R) non verbal memory attention, was evaluated by recall of geometric figures using the Visual Reproductions subscale of the WMS-R. Trail Making Test Parts A and B and the Stroop Color-Word Test for perceptuo-motor speed and executive functions. Motor speed and manual dexterity were examined with the Grooved Pegboard test. | PAD patients performed more poorly than normotensive patients in tests of non verbal memory, verbal working memory (p < 0.002), perceptuomotor speed, attention and mental flexibility and motor speed and manual dexterity (p < 0.00001) and compared to hypertensive patients in verbal memory (p < 0.002), verbal working memory perceptuomotor speed, attention and mental flexibility. Stroke<PAD<Hypertensive<Normotensive |
| Mangiafico et al., 2006 [30] (Age and Ageing). | Asymptomatic PAD (APAD) - stage I | 328: APAD group (42 women and 122 men) and Control group (44 women and 120 men) | 70.0 ± 3.4 and 70.3 ± 3.7 | Cognitive domains of attention and verbal working memory (Digit Span Forward and Backward), perceptuomotor speed, attention and mental flexibility (Trail Making Test), visuoconstructive skills and visual memory ROCF Copy and ROCF Delayed Recall and the global cognitive functioning (MMSE). | Patients with APAD scored significantly worse (p < 0.0001) than control subjects on five cognitive tests: Digit Span Backward, Trail Making A, Trail Making B, ROCF Copy and ROCF Delayed Recall |
| Williams et al., 2014 [31] (Arch Phys Med Rehab.) | PAD or DM patients with lower extremity amputation. | 87: Presurgicaly (1 woman and 28 men) and postsurgicaly (6 women and 52 men) | 63 ± 10 and 62 ± 8 | Neuropsychological Test Score: executive function (semantic fluency), auditory-verbal learning (list learning), and verbal memory (list recall) | Improvement in overall performance between presurgery and 6 weeks (p = 0.03) and presurgery and 4 months (p = 0.06), but no differences between 6 weeks and 4 months after amputation. |
| Gardner et al., 2016 [28] (Journal of Vascular Surgery) | Symptomatic PAD: Patients with a perfect MMSE score of 30 points and patients with score < 30 points. | 246: PAD patients with score of 30 (65 women and 58 men) and PAD patients with score <30 (61 women and 62 men) | 64 ± 10 and 65 ± 11 | MMSE questionnaire | Lower cognitive screening scores were associated with greater ambulatory impairment. Worse cognitive status was associated with lower scores in multiple dimensions of health-related QoL; The group with lower MMSE scores had a lower education level (p < 0.01), a greater prevalence of CAD (p = 0.02), (p = 0.01), and arthritis (p < 0.01), and took more medications for diabetes (p < 0.01) |
| Cavalcante et al., 2018 [29] (Eur. J. Vasc. Endovasc. Surg.) | Symptomatic PAD (intermittent claudication in one or two legs, stage) | 130: 29 women and 101 men | 67 ± 8 | Cognitive function; global, memory, executive function and attention by MoCA test | 86% of patients were classified as probably having a cognitive impairment; Greater memory performance was associated with greater moderate to vigorous physical activity leaves (p = 0.044) and walking capacity (p = 0.033) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leardini-Tristao, M.; Charles, A.-L.; Lejay, A.; Pizzimenti, M.; Meyer, A.; Estato, V.; Tibiriçá, E.; Andres, E.; Geny, B. Beneficial Effect of Exercise on Cognitive Function during Peripheral Arterial Disease: Potential Involvement of Myokines and Microglial Anti-Inflammatory Phenotype Enhancement. J. Clin. Med. 2019, 8, 653. https://doi.org/10.3390/jcm8050653
Leardini-Tristao M, Charles A-L, Lejay A, Pizzimenti M, Meyer A, Estato V, Tibiriçá E, Andres E, Geny B. Beneficial Effect of Exercise on Cognitive Function during Peripheral Arterial Disease: Potential Involvement of Myokines and Microglial Anti-Inflammatory Phenotype Enhancement. Journal of Clinical Medicine. 2019; 8(5):653. https://doi.org/10.3390/jcm8050653
Chicago/Turabian StyleLeardini-Tristao, Marina, Anne-Laure Charles, Anne Lejay, Mégane Pizzimenti, Alain Meyer, Vanessa Estato, Eduardo Tibiriçá, Emmanuel Andres, and Bernard Geny. 2019. "Beneficial Effect of Exercise on Cognitive Function during Peripheral Arterial Disease: Potential Involvement of Myokines and Microglial Anti-Inflammatory Phenotype Enhancement" Journal of Clinical Medicine 8, no. 5: 653. https://doi.org/10.3390/jcm8050653





