The Effect of Aspirin on Preventing Vascular Access Dysfunction in Incident Hemodialysis Patients: A Prospective Cohort Study in Korean Clinical Research Centers for End-Stage Renal Disease (CRC for ESRD)
Abstract
1. Introduction
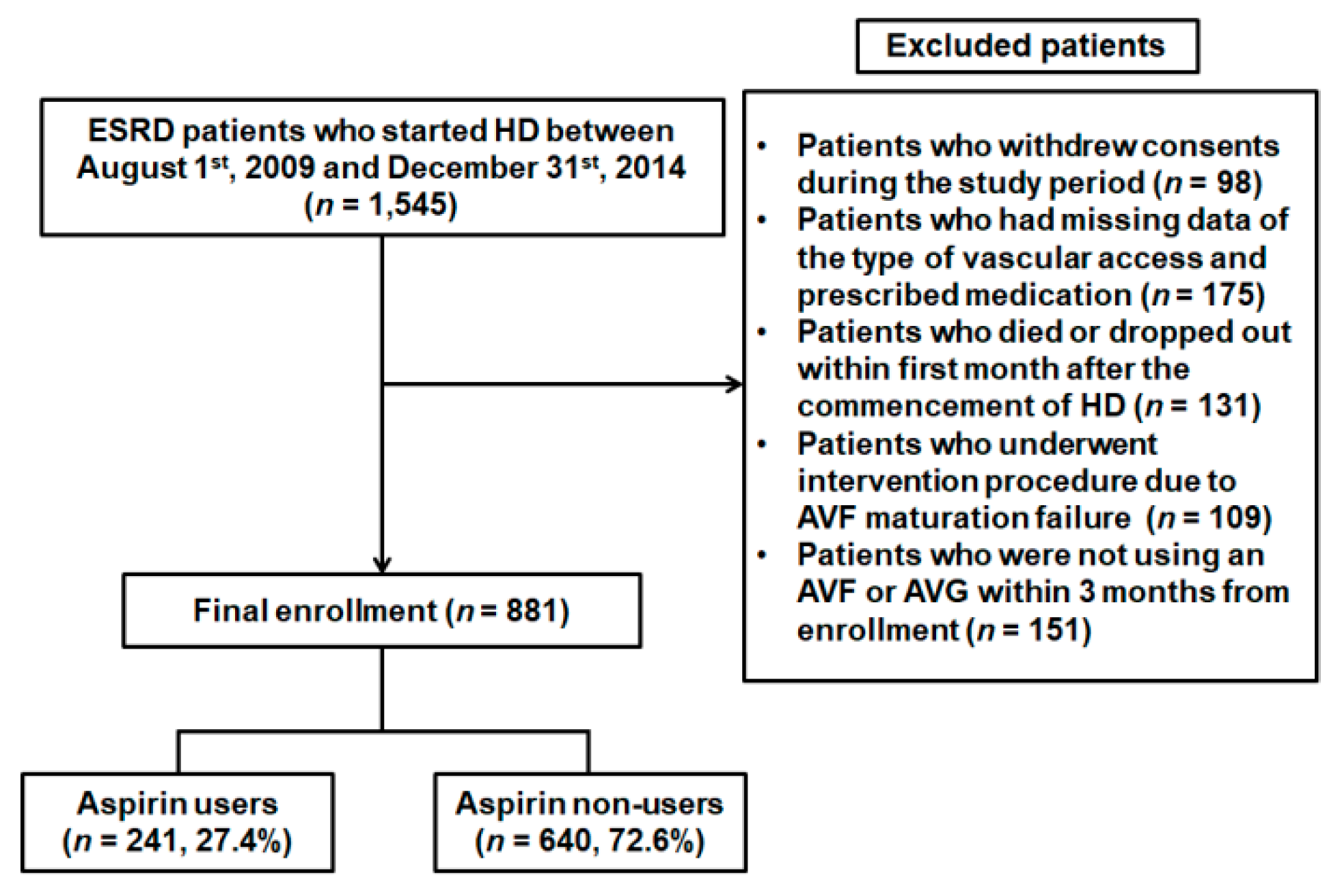
2. Materials and Methods
2.1. Subjects
2.2. Data Collection
2.3. Outcome Measurement
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
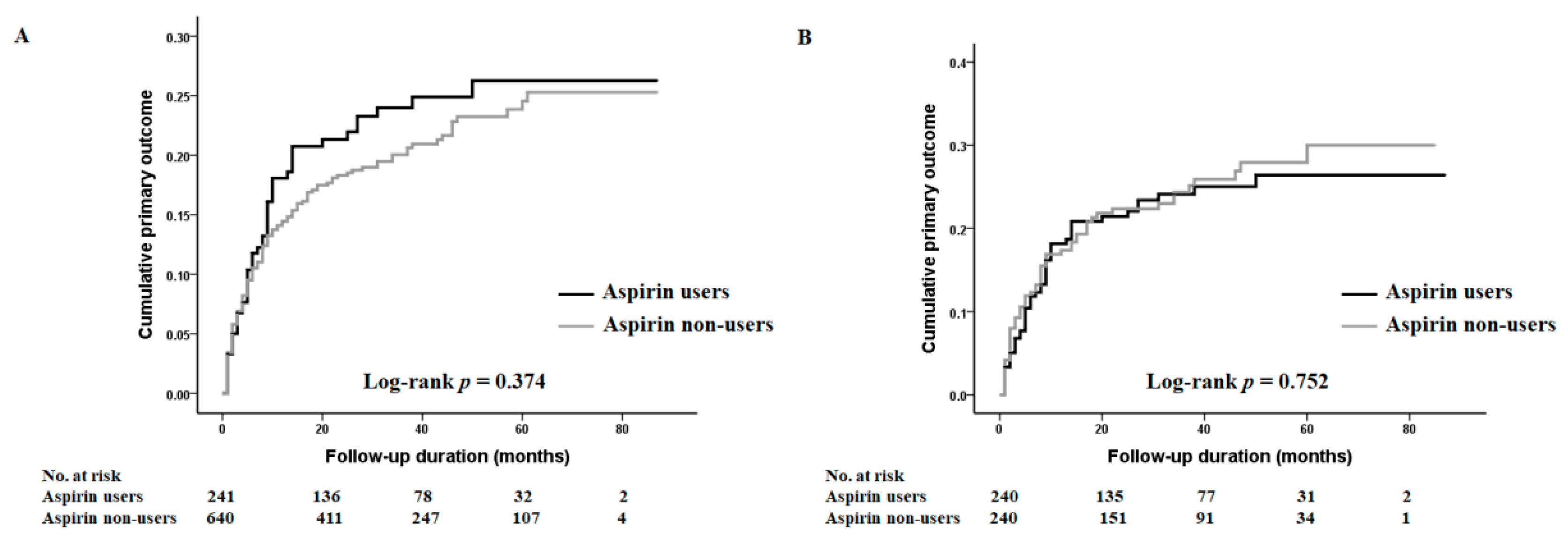
3.2. The Association between Aspirin Use and the Incidence of Vascular Access Failure
3.3. The Effect of Aspirin Usage on Secondary Vascular Access Failure
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hicks, C.W.; Canner, J.K.; Arhuidese, I.; Zarkowsky, D.S.; Qazi, U.; Reifsnyder, T.; Black, J.H., 3rd; Malas, M.B. Mortality benefits of different hemodialysis access types are age dependent. J. Vasc. Surg. 2015, 61, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Malas, M.B.; Canner, J.K.; Hicks, C.W.; Arhuidese, I.J.; Zarkowsky, D.S.; Qazi, U.; Schneider, E.B.; Black, J.H., 3rd; Segev, D.L.; Freischlag, J.A. Trends in incident hemodialysis access and mortality. JAMA Surg. 2015, 150, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Moist, L.M.; Lok, C.E. Incident dialysis access in patients with end-stage kidney disease: What needs to be improved. Semin. Nephrol. 2017, 37, 151–158. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Ashraff, S.; Carline, T. Maturation of arteriovenous fistula: Analysis of key factors. Kidney Res. Clin. Pract. 2017, 36, 318–328. [Google Scholar] [CrossRef]
- Ravani, P.; Palmer, S.C.; Oliver, M.J.; Quinn, R.R.; MacRae, J.M.; Tai, D.J.; Pannu, N.I.; Thomas, C.; Hemmelgarn, B.R.; Craig, J.C.; et al. Associations between hemodialysis access type and clinical outcomes: A systematic review. J. Am. Soc. Nephrol. JASN 2013, 24, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaishi, A.A.; Liu, A.R.; Lok, C.E.; Zhang, J.C.; Moist, L.M. Complications of the arteriovenous fistula: A systematic review. J. Am. Soc. Nephrol. JASN 2017, 28, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002, 324, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed]
- Rothwell, P.M.; Algra, A.; Chen, Z.; Diener, H.C.; Norrving, B.; Mehta, Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: Time-course analysis of randomised trials. Lancet 2016, 388, 365–375. [Google Scholar] [CrossRef]
- Sood, M.M.; Larkina, M.; Thumma, J.R.; Tentori, F.; Gillespie, B.W.; Fukuhara, S.; Mendelssohn, D.C.; Chan, K.; de Sequera, P.; Komenda, P.; et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: Results from the dopps. Kidney Int. 2013, 84, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.; Bragg-Gresham, J.L.; Piera, L.; Akizawa, T.; Asano, Y.; Mason, N.; Gillespie, B.W.; Young, E.W. Aspirin prescription and outcomes in hemodialysis patients: The dialysis outcomes and practice patterns study (dopps). Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2007, 50, 602–611. [Google Scholar] [CrossRef]
- Grosser, N.; Schroder, H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cgmp pathway. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Kata, D.; Foldesi, I.; Feher, L.Z.; Hackler, L., Jr.; Puskas, L.G.; Gulya, K. A novel pleiotropic effect of aspirin: Beneficial regulation of pro- and anti-inflammatory mechanisms in microglial cells. Brain Res. Bull. 2017, 132, 61–74. [Google Scholar] [CrossRef]
- Brahmbhatt, A.; Remuzzi, A.; Franzoni, M.; Misra, S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016, 89, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Misra, S. New insights into dialysis vascular access: Molecular targets in arteriovenous fistula and arteriovenous graft failure and their potential to improve vascular access outcomes. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.C.; Da Silva, A. Medical adjuvant treatment to increase patency of arteriovenous fistulae and grafts. Cochrane Database Syst. Rev. 2015, 7, Cd002786. [Google Scholar] [CrossRef]
- Tanner, N.C.; da Silva, A.F. Medical adjuvant treatment to improve the patency of arteriovenous fistulae and grafts: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2016, 52, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Di Micco, L.; Razavian, M.; Craig, J.C.; Ravani, P.; Perkovic, V.; Tognoni, G.; Graziano, G.; Jardine, M.; Pellegrini, F.; et al. Antiplatelet therapy to prevent hemodialysis vascular access failure: Systematic review and meta-analysis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2013, 61, 112–122. [Google Scholar] [CrossRef]
- Yun, T.; Ko, Y.E.; Kim, S.J.; Kang, D.H.; Choi, K.B.; Oh, H.J.; Ryu, D.R. The additional benefit of weighted subjective global assessment (sga) for the predictability of mortality in incident peritoneal dialysis patients: A prospective study. Medicine 2017, 96, e8421. [Google Scholar] [CrossRef]
- The Clinical Research Center for ESRD. Available online: http://webdb.crc-esrd.co.kr (accessed on 3 May 2019).
- Tonelli, M.; Klarenbach, S.; Jindal, K.; Manns, B. Economic implications of screening strategies in arteriovenous fistulae. Kidney Int. 2006, 69, 2219–2226. [Google Scholar] [CrossRef][Green Version]
- Wong, V.; Ward, R.; Taylor, J.; Selvakumar, S.; How, T.V.; Bakran, A. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 1996, 12, 207–213. [Google Scholar] [CrossRef]
- Gagliardi, G.M.; Rossi, S.; Condino, F.; Mancuso, D.; Greco, F.; Tenuta, R.; Savino, O.; Bonofiglio, R.; Domma, F.; Latorre, G. Malnutrition, infection and arteriovenous fistula failure: Is there a link? J. Vasc. Access 2011, 12, 57–62. [Google Scholar] [CrossRef]
- Smith, G.E.; Gohil, R.; Chetter, I.C. Factors affecting the patency of arteriovenous fistulas for dialysis access. J. Vasc. Surg. 2012, 55, 849–855. [Google Scholar] [CrossRef]
- Aitken, E.; Jackson, A.; Kong, C.; Coats, P.; Kingsmore, D. Renal function, uraemia and early arteriovenous fistula failure. BMC Nephrol. 2014, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Stolic, R.V.; Trajkovic, G.Z.; Kostic, M.M.; Mihailovic, B.; Jovanovic, A.N.; Lazic, B.D.; Matijasevic, I.R.; Jaksic, M.D.; Mirkovic, Z.M.; Smilic, T.N.; et al. Factors affecting the patency of arteriovenous fistulas for hemodialysis: Single center experience. Hemodial. Int. 2018, 22, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Elder, S.J.; Bragg-Gresham, J.L.; Pisoni, R.L.; Yamazaki, S.; Akizawa, T.; Jadoul, M.; Hugh, R.C.; Port, F.K.; Fukuhara, S. Consistent aspirin use associated with improved arteriovenous fistula survival among incident hemodialysis patients in the dialysis outcomes and practice patterns study. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.S.; Beck, G.J.; Dember, L.M.; Vazquez, M.A.; Greenberg, A.; Delmez, J.A.; Allon, M.; Himmelfarb, J.; Hu, B.; Greene, T.; et al. Use of aspirin associates with longer primary patency of hemodialysis grafts. J. Am. Soc. Nephrol. JASN 2011, 22, 773–781. [Google Scholar] [CrossRef]
- Locham, S.; Beaulieu, R.J.; Dakour-Aridi, H.; Nejim, B.; Malas, M.B. Role of antiplatelet therapy in the durability of hemodialysis access. J. Nephrol. 2018, 31, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Joseph Lo, Z.; Tay, W.M.; Lee, Q.; Chua, J.L.; Tan, G.W.; Chandrasekar, S.; Narayanan, S. Predictors of radio-cephalic arteriovenous fistulae patency in an asian population. J. Vasc. Access 2016, 17, 411–416. [Google Scholar] [PubMed]
- Hsu, Y.H.; Yen, Y.C.; Lin, Y.C.; Sung, L.C. Antiplatelet agents maintain arteriovenous fistula and graft function in patients receiving hemodialysis: A nationwide case-control study. PLoS ONE 2018, 13, e0206011. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, R.; Barker-Finkel, J.; Allo, M. Statin therapy is not associated with improved vascular access outcomes. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1447–1450. [Google Scholar] [CrossRef]
- Dixon, B.S.; Beck, G.J.; Vazquez, M.A.; Greenberg, A.; Delmez, J.A.; Allon, M.; Dember, L.M.; Himmelfarb, J.; Gassman, J.J.; Greene, T.; et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N. Engl. J. Med. 2009, 360, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Murley, A.; Wijewardane, A.; Wilmink, T.; Baharani, J. Should patients be on antithrombotic medication for their first arteriovenous fistulae? J. Vasc. Access 2016, 17, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Irish, A.B.; Viecelli, A.K.; Hawley, C.M.; Hooi, L.S.; Pascoe, E.M.; Paul-Brent, P.A.; Badve, S.V.; Mori, T.A.; Cass, A.; Kerr, P.G.; et al. Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yevzlin, A.S.; Conley, E.L.; Sanchez, R.J.; Young, H.N.; Becker, B.N. Vascular access outcomes and medication use: A usrds study. Semin. Dial. 2006, 19, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Sreedhara, R.; Himmelfarb, J.; Lazarus, J.M.; Hakim, R.M. Anti-platelet therapy in graft thrombosis: Results of a prospective, randomized, double-blind study. Kidney Int. 1994, 45, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Birch, N.; Fillaus, J.; Florescu, M.C. The effect of statin therapy on the formation of arteriovenous fistula stenoses and the rate of reoccurrence of previously treated stenoses. Hemodial. Int. Int. Symp. Home Hemodial. 2013, 17, 586–593. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 881) | Aspirin Users (n = 241) | Aspirin Non-Users (n = 640) | p-Value |
|---|---|---|---|---|
| Age, years | 57.9 ± 13.4 | 62.6 ± 11.3 | 56.2 ± 13.7 | <0.001 |
| Male, n (%) | 562 (63.8%) | 161 (66.8%) | 401 (62.7%) | 0.253 |
| Body mass index, kg/m2 | 23.2 ± 3.5 | 23.2 ± 3.3 | 23.2 ± 3.5 | 0.815 |
| Comorbid diseases, n (%) | ||||
| Diabetes mellitus | 540 (61.3%) | 176 (73.0%) | 364 (56.9%) | <0.001 |
| Coronary arterial disease | 126 (14.3%) | 81 (33.6%) | 45 (7.0%) | <0.001 |
| Peripheral arterial disease | 88 (10.0%) | 40 (16.6%) | 48 (7.5%) | <0.001 |
| Cerebrovascular accident | 27 (3.1%) | 12 (5.0%) | 15 (2.3%) | 0.043 |
| Smoking, n (%) | ||||
| Non-smoker | 468 (53.1%) | 113 (46.9%) | 355 (55.5%) | 0.023 |
| Ex- or Current smoker | 413 (46.9%) | 128 (53.1%) | 285 (44.5%) | |
| Biochemical parameters | ||||
| Hemoglobin, g/dL | 8.9 ± 1.6 | 9.1 ± 1.6 | 8.8 ± 1.6 | 0.073 |
| Albumin, g/dL | 3.4 ± 0.6 | 3.4 ± 0.6 | 3.4 ± 0.6 | 0.781 |
| Blood urea nitrogen, mg/dL | 87.2 ± 37.7 | 86.4 ± 35.0 | 88.5 ± 38.6 | 0.122 |
| Creatinine, mg/dL | 8.9 ± 4.6 | 8.7 ± 3.3 | 9.0 ± 4.9 | 0.085 |
| Total cholesterol, mg/dL | 155.2 ± 47.8 | 146.5 ± 43.1 | 158.4 ± 49.1 | 0.002 |
| Triglyceride, mg/dL | 124.7 ± 76.7 | 121.1 ± 71.3 | 126.1 ± 78.7 | 0.460 |
| Arteriovenous fistula, n (%) | 714 (81.0%) | 183 (75.9%) | 531 (83.0%) | 0.018 |
| Vascular access failure event (primary outcome) ‡, n (%) | 180 (20.4%) | 52 (21.6%) | 128 (20.0%) | 0.605 |
| Follow-up duration, months † | 30 (11–50) | 26 (8–47) | 31 (12–51) | 0.033 |
| Incidence rate, person-year | 0.077 | 0.091 | 0.073 | 0.207 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age, per 1 year | 1.01 (1.00–1.02) | 0.031 | 1.01 (1.00–1.02) | 0.332 |
| Female vs. male | 1.60 (1.20–2.15) | 0.002 | 1.68 (1.25–2.25) | 0.001 |
| Body mass index, per 1 kg/m2 | 0.99 (0.95–1.04) | 0.779 | - | - |
| DM vs. non-DM | 1.88 (1.35–2.62) | <0.001 | 1.71 (1.21–2.41) | 0.002 |
| Pre-existing CAD vs. non-CAD | 1.41 (0.97–2.06) | 0.072 | 1.33 (0.88–2.01) | 0.179 |
| Pre-existing PAD vs. non-PAD | 1.84 (1.24–2.74) | 0.003 | 1.45 (0.95–2.21) | 0.086 |
| Pre-existing CVA vs. non-CVA | 1.79 (0.91–3.49) | 0.090 | 1.73 (0.87–3.43) | 0.118 |
| Smoking | ||||
| Non-smoker | 1.00 (reference) | - | - | - |
| Ex- or Current smoker | 0.78 (0.58–1.05) | 0.101 | - | - |
| Total cholesterol, per 1 mg/dL | 1.00 (0.99–1.01) | 0.114 | - | - |
| Triglyceride, per 1 mg/dL | 1.00 (0.99–1.00) | 0.106 | - | - |
| AVG vs. AVF | 1.77 (1.28–2.46) | 0.001 | 1.55 (1.11–2.16) | 0.010 |
| Aspirin users vs. Aspirin non-users | 1.16 (0.84–1.60) | 0.378 | 0.89 (0.62–1.27) | 0.510 |
| Subgroup | Aspirin Users vs. Aspirin Non-Users | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Gender ‡ | ||
| Male | 0.89 (0.55–1.45) | 0.643 |
| Female | 0.86 (0.51–1.46) | 0.571 |
| Diabetes mellitus § | ||
| Yes | 0.94 (0.63–1.40) | 0.767 |
| No | 0.92 (0.51–1.67) | 0.408 |
| The type of vascular access ¶ | ||
| AVF | 0.83 (0.54–1.28) | 0.392 |
| AVG | 1.11 (0.59–2.08) | 0.750 |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age, per 1 year | 1.00 (0.98–1.02) | 0.914 | - | - |
| Female vs. male | 1.69 (1.16–2.45) | 0.007 | 1.27 (0.78–2.09) | 0.340 |
| Body mass index, per 1 kg/m2 | 0.99 (0.94–1.05) | 0.775 | - | - |
| DM vs. non-DM | 1.61 (1.01–2.56) | 0.047 | 1.46 (0.91–2.36) | 0.117 |
| Pre-existing CAD vs. non-CAD | 1.15 (0.74–1.78) | 0.542 | 1.14 (0.70–1.85) | 0.595 |
| Pre-existing PAD vs. non-PAD | 1.84 (1.15–2.97) | 0.012 | 1.79 (1.06–3.05) | 0.031 |
| Pre-existing CVA vs. non-CVA | 1.93 (0.94–3.96) | 0.074 | 1.75 (0.84–3.66) | 0.139 |
| Smoking | ||||
| Non-smoker | 1.00 (reference) | - | 1.00 (reference) | - |
| Ex- or Current smoker | 0.76 (0.40–1.12) | 0.135 | - | - |
| Total cholesterol, per 1 mg/dL | 1.00 (0.99–1.01) | 0.250 | - | - |
| Triglyceride, per 1 mg/dL | 1.00 (0.98–1.02) | 0.193 | - | - |
| AVG vs. AVF | 1.59 (1.05–2.41) | 0.029 | 1.58 (1.03–2.42) | 0.037 |
| Aspirin users vs. Aspirin non-users | 0.94 (0.65–1.37) | 0.754 | 0.84 (0.56–1.24) | 0.376 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.H.; Oh, H.J.; Kim, Y.S.; Kim, Y.-L.; Chang, J.H.; Ryu, D.-R. The Effect of Aspirin on Preventing Vascular Access Dysfunction in Incident Hemodialysis Patients: A Prospective Cohort Study in Korean Clinical Research Centers for End-Stage Renal Disease (CRC for ESRD). J. Clin. Med. 2019, 8, 677. https://doi.org/10.3390/jcm8050677
Kim CH, Oh HJ, Kim YS, Kim Y-L, Chang JH, Ryu D-R. The Effect of Aspirin on Preventing Vascular Access Dysfunction in Incident Hemodialysis Patients: A Prospective Cohort Study in Korean Clinical Research Centers for End-Stage Renal Disease (CRC for ESRD). Journal of Clinical Medicine. 2019; 8(5):677. https://doi.org/10.3390/jcm8050677
Chicago/Turabian StyleKim, Chan Ho, Hyung Jung Oh, Yon Su Kim, Yong-Lim Kim, Jae Hyun Chang, and Dong-Ryeol Ryu. 2019. "The Effect of Aspirin on Preventing Vascular Access Dysfunction in Incident Hemodialysis Patients: A Prospective Cohort Study in Korean Clinical Research Centers for End-Stage Renal Disease (CRC for ESRD)" Journal of Clinical Medicine 8, no. 5: 677. https://doi.org/10.3390/jcm8050677
APA StyleKim, C. H., Oh, H. J., Kim, Y. S., Kim, Y.-L., Chang, J. H., & Ryu, D.-R. (2019). The Effect of Aspirin on Preventing Vascular Access Dysfunction in Incident Hemodialysis Patients: A Prospective Cohort Study in Korean Clinical Research Centers for End-Stage Renal Disease (CRC for ESRD). Journal of Clinical Medicine, 8(5), 677. https://doi.org/10.3390/jcm8050677





