Respiratory Variations in Electrocardiographic R-Wave Amplitude during Acute Hypovolemia Induced by Inferior Vena Cava Clamping in Patients Undergoing Liver Transplantation
Abstract
1. Introduction
2. Methods
2.1. Study Population and Data Analysis
2.2. Statistical Analysis
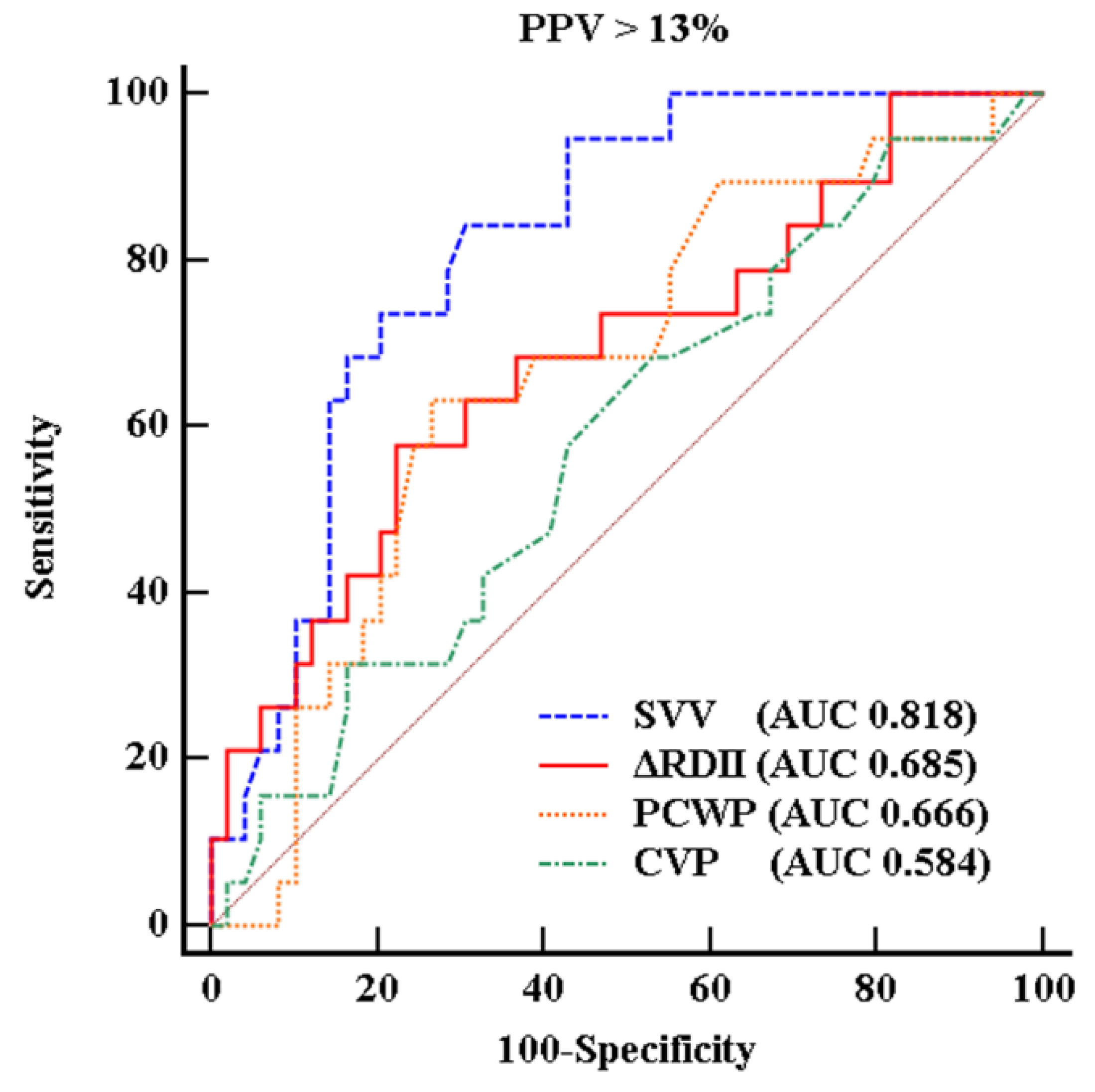
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Michard, F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005, 103, 419–428. [Google Scholar] [CrossRef]
- Michard, F.; Boussat, S.; Chemla, D.; Anguel, N.; Mercat, A.; Lecarpentier, Y.; Richard, C.; Pinsky, M.R.; Teboul, J.L. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am. J. Respir. Crit. Care Med. 2000, 162, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.K.; Senn, A.; Weibel, L.; Zollinger, A. Assessment of stroke volume variation for prediction of fluid responsiveness using the modified FloTrac and PiCCOplus system. Crit. Care 2008, 12. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R. Using ventilation-induced aortic pressure and flow variation to diagnose preload responsiveness. Intensive Care Med. 2004, 30, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Guerin, L.; Jozwiak, M.; Bataille, A.; Julien, F.; Richard, C.; Teboul, J.L. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br. J. Anaesth. 2013, 110, 207–213. [Google Scholar] [CrossRef]
- Huang, L.; Critchley, L.A.; Zhang, J. Major upper abdominal surgery alters the calibration of bioreactance cardiac output readings, the NICOM, when comparisons are made against suprasternal and esophageal doppler intraoperatively. Anesth. Analg. 2015, 121, 936–945. [Google Scholar] [CrossRef]
- Kwon, C.H.; Kim, S.H. Intraoperative management of critical arrhythmia. Korean J. Anesthesiol. 2017, 70, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.A. A theoretical analysis of intracavitary blood mass influence on the heart-lead relationship. Circ. Res. 1956, 4, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.R.; Gorcsan, J., 3rd; Gasior, T.A.; Mandarino, W.A.; Deneault, L.G.; Hattler, B.G.; Kunig, H. Changes in electrocardiographic morphology reflect instantaneous changes in left ventricular volume and output in cardiac surgery patients. Am. J. Cardiol. 1995, 76, 667–674. [Google Scholar] [CrossRef]
- Feldman, T.; Borow, K.M.; Neumann, A.; Lang, R.M.; Childers, R.W. Relation of electrocardiographic R-wave amplitude to changes in left ventricular chamber size and position in normal subjects. Am. J. Cardiol. 1985, 55, 1168–1174. [Google Scholar] [CrossRef]
- Daniels, S.; Iskandrian, A.S.; Hakki, A.H.; Kane, S.A.; Bemis, C.E.; Horowitz, L.N.; Greenspan, A.M.; Segal, B.L. Correlation between changes in R wave amplitude and left ventricular volume induced by rapid atrial pacing. Am. Heart J. 1984, 107, 711–717. [Google Scholar] [CrossRef]
- Cannesson, M.; Keller, G.; Desebbe, O.; Lehot, J.J. Relations between respiratory changes in R-wave amplitude and arterial pulse pressure in mechanically ventilated patients. J. Clin. Monit. Comput. 2010, 24, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Lorne, E.; Mahjoub, Y.; Guinot, P.G.; Fournier, Y.; Detave, M.; Pila, C.; Ben Ammar, A.; Labont, B.; Zogheib, E.; Dupont, H. Respiratory variations of R-wave amplitude in lead II are correlated with stroke volume variations evaluated by transesophageal Doppler echocardiography. J. Cardiothorac. Vasc. Anesth. 2012, 26, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Garutti Martinez, I.; Olmedilla, L.; Perez-Pena, J.M.; Zaballos, M.; Sanz, J.; Vigil, M.D.; Navia, J. Response to clamping of the inferior vena cava as a factor for predicting postreperfusion syndrome during liver transplantation. Anesth. Analg. 1997, 84, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Hwang, G.S. Cardiovascular dysfunction and liver transplantation. Korean J. Anesthesiol. 2018, 71, 85–91. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, Y.J.; Kim, J.W.; Song, J.G.; Hwang, G.S. Prediction of fluid responsiveness by a non-invasive respiratory systolic time interval variation using heart sound signals in recipients undergoing liver transplantation. Transplant. Proc. 2017, 49, 1082–1086. [Google Scholar] [CrossRef]
- Kim, S.H.; Moon, Y.J.; Lee, S.; Jeong, S.M.; Song, J.G.; Hwang, G.S. Atrioventricular conduction disturbances immediately after hepatic graft reperfusion and their outcomes in patients undergoing liver transplantation. Liver Transpl. 2016, 22, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hwang, G.S.; Kim, S.O.; Kim, Y.K. Is stroke volume variation a useful preload index in liver transplant recipients? A retrospective analysis. Int. J. Med. Sci. 2013, 10, 751–757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, H.M.; Park, S.K.; Moon, Y.J.; Kim, J.W.; Kim, S.K.; Sang, B.H.; Seo, D.K.; Yoo, B.W.; Hwang, G.S. Arrhythmogenic potential develops rapidly at graft reperfusion before the start of hypotension during living-donor liver transplantation. Korean J. Anesthesiol. 2016, 69, 37–43. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, J.G.; Park, J.H.; Kim, J.W.; Park, Y.S.; Hwang, G.S. Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients. Anesth. Analg. 2013, 116, 94–100. [Google Scholar] [CrossRef]
- Alian, A.A.; Galante, N.J.; Stachenfeld, N.S.; Silverman, D.G.; Shelley, K.H. Impact of central hypovolemia on photoplethysmographic waveform parameters in healthy volunteers part 2: Frequency domain analysis. J. Clin. Monit. Comput. 2011, 25, 387–396. [Google Scholar] [CrossRef]
- Soltner, C.; Dantec, R.; Lebreton, F.; Huntzinger, J.; Beydon, L. Changes in R-Wave amplitude in DII lead is less sensitive than pulse pressure variation to detect changes in stroke volume after fluid challenge in ICU patients postoperatively to cardiac surgery. J. Clin. Monit. Comput. 2010, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Giraud, R.; Siegenthaler, N.; Morel, D.R.; Romand, J.A.; Brochard, L.; Bendjelid, K. Respiratory change in ECG-wave amplitude is a reliable parameter to estimate intravascular volume status. J. Clin. Monit. Comput. 2013, 27, 107–111. [Google Scholar] [CrossRef]
- Giraud, R.; Siegenthaler, N.; Bendjelid, K. Pulse pressure variation, stroke volume variation and dynamic arterial elastance. Crit. Care 2011, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, A.J.; Vasquez, C.J.; Fuenmayor, A.M.; Winterdaal, D.M.; Rodriguez, D. Hemodialysis changes the QRS amplitude in the electrocardiogram. Int J. Cardiol. 1993, 41, 141–145. [Google Scholar] [CrossRef]
- McManus, J.G.; Convertino, V.A.; Cooke, W.H.; Ludwig, D.A.; Holcomb, J.B. R-wave amplitude in lead II of an electrocardiograph correlates with central hypovolemia in human beings. Acad. Emerg. Med. 2006, 13, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Rinehart, J.; Canales, C.; Cannesson, M. Comparison of automated vs. manual determination of the respiratory variations in the EKG R wave amplitude for the prediction of fluid responsiveness during surgery. J. Comput. Surg. 2014, 1, 5. [Google Scholar] [CrossRef]
- Krenn, C.G.; Hoda, R.; Nikolic, A.; Greher, M.; Plochl, W.; Chevtchik, O.O.; Steltzer, H. Assessment of ventricular contractile function during orthotopic liver transplantation. Transpl. Int. 2004, 17, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Renner, J.; Meybohm, P.; Hanss, R.; Gruenewald, M.; Scholz, J.; Bein, B. Effects of norepinephrine on dynamic variables of fluid responsiveness during hemorrhage and after resuscitation in a pediatric porcine model. Paediatr. Anaesth. 2009, 19, 688–694. [Google Scholar] [CrossRef]
- Giraud, R.; Siegenthaler, N.; Arroyo, D.; Bendjelid, K. Impact of epinephrine and norepinephrine on two dynamic indices in a porcine hemorrhagic shock model. J. Trauma Acute Care Surg. 2014, 77, 564–569. [Google Scholar] [CrossRef]
- Bossert, T.; Gummert, J.F.; Bittner, H.B.; Barten, M.; Walther, T.; Falk, V.; Mohr, F.W. Swan-Ganz catheter-induced severe complications in cardiac surgery: Right ventricular perforation, knotting, and rupture of a pulmonary artery. J. Card. Surg. 2006, 21, 292–295. [Google Scholar] [CrossRef]
- Michard, F. Smartphones and e-tablets in perioperative medicine. Korean J. Anesthesiol. 2017, 70, 493–499. [Google Scholar] [CrossRef]
- Sayadi, O.; Shamsollahi, M.B. Model-based fiducial points extraction for baseline wandered electrocardiograms. IEEE Trans. Biomed. Eng. 2008, 55, 347–351. [Google Scholar] [CrossRef]
- Kroeker, C.A.; Shrive, N.G.; Belenkie, I.; Tyberg, J.V. Pericardium modulates left and right ventricular stroke volumes to compensate for sudden changes in atrial volume. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2247–H2254. [Google Scholar] [CrossRef]


| Characteristics | |
|---|---|
| Patient characteristics and comorbidities | |
| Sex, Male (%) | 23 (67.6) |
| Age (years) | 52.7 ± 9.5 |
| Weight (kg) | 64.6 ± 10.7 |
| Height (cm) | 166.5 ± 7.8 |
| BMI (kg/m2) | 23.3 ± 3.4 |
| Cardiovascular disease a | 4 (11.4) |
| Hypertension | 5 (14.2) |
| Diabetes mellitus | 12 (34.3) |
| Child-Pugh score | 8.4 ± 2.3 |
| MELD b score | 15.5 ± 6.9 |
| Disease | |
| Viral hepatitis-related ESLD c | 21 (61.7) |
| Alcoholic cirrhosis | 6 (17.6) |
| Others | 7 (20.6) |
| Drug | |
| Beta-blocker | 11 (32.4) |
| Furosemide/spironolactone | 21(61.8)/18 (52.9) |
| Echocardiographic findings | |
| Left ventricle mass index (g/m2) | 89.4 ± 20.2 |
| Left ventricular ejection fraction (%) | 65.0 ± 5.5 |
| Left ventricle internal dimension at end diastole (mm) | 50.8 ± 8.2 |
| Left ventricular posterior wall thickness at end diastole (mm) | 8.7 ± 1.1 |
| Interventricular septal dimension at end diastole (mm) | 8.4 ± 1.8 |
| E/E’ ratio d | 9.0 ± 2.4 |
| Intraoperative norepinephrine use (%) | 31 (91.2) |
| Before | After | p-Value | |
|---|---|---|---|
| MAP (mmHg) | 88 ± 13 | 78 ± 9 | <0.001 |
| Heart rate (bpm) | 84 ± 12 | 87 ± 14 | 0.007 |
| CVP (mmHg) | 11.6 ± 3.4 | 10.7 ± 3.7 | 0.197 |
| PCWP (mmHg) | 19.9 ± 5.0 | 16.3 ± 4.5 | 0.001 |
| COSG (L/min) | 7.0 ± 2.0 | 6.2 ± 2.0 | <0.001 |
| SVSG (mL/beat) | 86 ± 27 | 77 ± 28 | <0.001 |
| COFT (L/min) | 6.4 ± 1.7 | 5.8 ± 1.7 | 0.001 |
| SVFT (mL/beat) | 77 ± 24 | 69 ± 26 | 0.011 |
| SVV (%) | 6.1 ± 3.3 | 9.7 ± 4.9 | <0.001 |
| PPV (%) | 7.0 ± 4.4 | 11.3 ± 5.6 | <0.001 |
| ΔRDII (%) | 11.1 ± 6.1 | 14.2 ± 7.8 | 0.002 |
| Frequency analysis Area under curve (mVolt•√Hz) | |||
| Respiratory frequency | 0.092 (0.018, 0.218) | 0.139 (0.025, 0.314) | 0.016 |
| Cardiac frequency | 1.670 (0.700, 2.750) | 2.013 (1.059, 3.542) | 0.001 |
| Norepinephrine < 0.1 μg/kg/min or None | Norepinephrine ≥ 0.1 μg/kg/min | |||||
|---|---|---|---|---|---|---|
| n = 26 | n = 8 | |||||
| Before | After | p-Value | Before | After | p-Value | |
| COSG (L/min) | 6.8 (5.8;7.3) | 5.7 (4.8;6.7) | 0.002 | 6.5 (5.8;8.5) | 5.5 (5.8;8.5) | 0.014 |
| SVSG (mL/beat) | 84.0 (66.5;112.0) | 76.0 (60.8;93.3) | 0.003 | 78.5 (57.3;101.5) | 57.5 (48.0;96.0) | 0.042 |
| COFT (L/min) | 6.4 (4.6;8.3) | 5.9 (4.0;7.4) | 0.003 | 6.4 (5.5;7.6) | 6.4 (5.1;6.9) | 0.061 |
| SVFT (mL/beat) | 76.5 (58.8;98.8) | 66.5 (46.5;86.8) | 0.013 | 65.5 (56.5;99.0) | 64.0 (48.5;96.6) | 0.400 |
| CVP (mmHg) | 12.0 (9.9;14.3) | 10.7 (8.7;12.3) | 0.153 | 10.8 (6.2;13.8) | 10.4 (8.4;13.3) | 0.917 |
| PCWP (mmHg) | 19.5 (15.8;22.6) | 14.8 (12.6;18.7) | 0.000 | 20.4 (15.8;26.2) | 20.0 (14.8;25.0) | 0.236 |
| SVV (%) | 4.7 (3.2;7.6) | 8.9 (5.9;11.0) | 0.000 | 6.9 (3.9;13.0) | 13.3 (6.0;15.3) | 0.107 |
| PPV (%) | 5.6 (3.5;8.8) | 10.2 (7.4;14.9) | 0.000 | 6.3(3.2;12.8) | 11.8 (5.0;14.9) | 0.234 |
| ΔRDII (%) | 10.9 (5.6;17.8) | 13.5 (8.4;19.1) | 0.032 | 12.3(5.5;16.7) | 14.1(6.7;28.8) | 0.093 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Kim, S.-H.; Park, Y.-S.; Thiele, R.H.; Shin, W.-J.; Hwang, G.-S. Respiratory Variations in Electrocardiographic R-Wave Amplitude during Acute Hypovolemia Induced by Inferior Vena Cava Clamping in Patients Undergoing Liver Transplantation. J. Clin. Med. 2019, 8, 717. https://doi.org/10.3390/jcm8050717
Park H-S, Kim S-H, Park Y-S, Thiele RH, Shin W-J, Hwang G-S. Respiratory Variations in Electrocardiographic R-Wave Amplitude during Acute Hypovolemia Induced by Inferior Vena Cava Clamping in Patients Undergoing Liver Transplantation. Journal of Clinical Medicine. 2019; 8(5):717. https://doi.org/10.3390/jcm8050717
Chicago/Turabian StylePark, Hee-Sun, Sung-Hoon Kim, Yong-Seok Park, Robert H. Thiele, Won-Jung Shin, and Gyu-Sam Hwang. 2019. "Respiratory Variations in Electrocardiographic R-Wave Amplitude during Acute Hypovolemia Induced by Inferior Vena Cava Clamping in Patients Undergoing Liver Transplantation" Journal of Clinical Medicine 8, no. 5: 717. https://doi.org/10.3390/jcm8050717
APA StylePark, H.-S., Kim, S.-H., Park, Y.-S., Thiele, R. H., Shin, W.-J., & Hwang, G.-S. (2019). Respiratory Variations in Electrocardiographic R-Wave Amplitude during Acute Hypovolemia Induced by Inferior Vena Cava Clamping in Patients Undergoing Liver Transplantation. Journal of Clinical Medicine, 8(5), 717. https://doi.org/10.3390/jcm8050717





